Thyroid dysfunction is a common problem in pregnant women. It is usually of an autoimmune origin, with Hashimotós thyroiditis and Graveś disease being the most common conditions. Although hormonal changes and transplacental antibody transfer may occur, specific neonatal screening has not been shown to be useful.
Patients and methodA prospective study of newborns of women with autoimmune thyroid disease born at a level III university hospital (November 2013–December 2016). Neonates were selected during their stay at the maternity. Babies with perinatal asphyxia were excluded. Data were collected from the clinical histories of mothers and newborns.
ResultsA total of 191 neonates were included. Ninety percent of mothers had been diagnosed with autoimmune hypothyroidism. Only 5.8% of newborns had some laboratory disorder, consisting of slightly increased thyroid-stimulating hormone levels, which returned to normal at the age of one month and did not correlate to thyroid peroxidase antibody levels. Transient hyperthyrotropinemia was diagnosed in one newborn and required thyroxin treatment during the first year of life. Among newborns from mothers with Graveś disease, 36.8% had some abnormal laboratory value during the first 7 days of life, but there were no cases of hyperthyroidism and only one of transient hyperthyrotropinemia.
ConclusionsExperience at our hospital in screening of newborns from hypothyroid mothers reveals a high number of laboratory controls with a poor diagnostic yield. No relationship was found between thyroid peroxidase antibody levels and thyroid dysfunction. We support the recommendations to continue testing serum thyroid-stimulating hormone and FT4 levels at 48 h of life in newborns of mothers with autoimmune hypothyroidism.
La patología tiroidea es un problema frecuente en las mujeres embarazadas. Su etiología suele ser autoinmune siendo la tiroiditis de Hashimoto y la enfermedad de Graves las entidades principales. A pesar de las posibles alteraciones hormonales y del paso transplacentario de anticuerpos estimulantes, la realización de un cribado neonatal específico no ha demostrado su utilidad en el neonato.
Pacientes y métodosEstudio prospectivo (noviembre de 2013-diciembre de 2016) de los hijos de madre con patología tiroidea autoinmune nacidos en un hospital universitario nivel III. Los recién nacidos se seleccionaron en maternidad. Se excluyeron los casos de asfixia perinatal. Los datos se recogieron de las historias clínicas de madres y recién nacidos.
ResultadosSe incluyeron 191 recién nacidos. El 90% de las madres estaban diagnosticadas de hipotiroidismo autoinmune, de cuyos hijos solo un 5,8% tuvo alguna alteración analítica tratándose de leves ascensos de tirotropina, con normalización al mes de vida, no correlacionándose con los niveles de anti-TPO. Se diagnosticó una hipertirotropinemia transitoria que precisó tratamiento durante el primer año de vida. El 36,8% de los hijos de madre con Graves tuvo algún control analítico alterado en los primeros 7 días de vida, no diagnosticándose ningún caso de hipertiroidismo y solo una hipertirotropinemia transitoria.
ConclusionesLa experiencia de nuestro centro en el manejo del cribado neonatal tiroideo muestra un elevado número de controles analíticos con un escaso rendimiento diagnóstico. No encontramos relación entre niveles de anticuerpos anti-TPO y disfunción tiroidea. Apoyamos las recomendaciones de mantener el cribado neonatal universal como única exploración en los hijos de madre hipotiroidea.
Pregnancy is characterized by many physiological changes, including modifications of thyroid function, and hypothyroidism has been observed in up to 2.5% of all pregnant women.1 Thyroid disease is the second leading cause of endocrine disorders in women of childbearing age, and is a common problem in pregnant women (10–20%).2 The underlying cause is usually autoimmune, with Graves’ disease and Hashimoto’s thyroiditis being the main conditions. It is not exceptional for both diseases to occur successively in the same patient. This may indicate the existence of a shared etiopathogenesis.3
Pregnant women with clinical or subclinical hypothyroidism are at an increased risk of suffering miscarriage, anemia, arterial hypertension, diabetes, placental abruption, fetal death and prematurity, low birth weight, respiratory distress, the need for admission to intensive care, etc.4 This decrease in thyroid hormone levels in early pregnancy can result in irreversible fetal developmental defects, since thyroid hormones have profound effects upon the development of vertebrate species, affecting almost all the tissues and particularly the brain.5,6 Thyroid hormone deficiency affects the main brain development processes, particularly neuronal migration, synaptogenesis and myelinization.7 A number of studies have correlated maternal hypothyroidism to the poorer neuropsychological development of these children.8,9
Autoimmune thyroiditis is the most common cause of hypothyroidism during pregnancy, with the presence of antithyroid antibodies being a characteristic finding. Antibodies targeted to thyroid peroxidase (anti-TPO) and/or thyroglobulin (Tg-Ab) have been identified in up to 8–14% of all pregnant women, depending on the series.10,11 However, the potential effect of these antibodies upon thyroid function in the newborn is not clear. A review of the literature reveals studies identifying no clear relationship between anti-TPO antibodies and congenital hypothyroidism,12,13 as well as studies describing an increased prevalence of transient congenital hypothyroidism in children born to mothers with autoimmune thyroid disease.14 An association between the presence of anti-TPO antibodies and altered neuropsychological development in childhood has also been described.15 It seems that in the presence of congenital hypothyroidism risk, the latter is greater between 2–4 weeks of life. In most cases, spontaneous thyroid-stimulating hormone (TSH) normalization occurs in the first month of life.16
Hyperthyroidism in pregnancy occurs in 0.1−0.4% of all pregnant women, and Graves’ disease is by far the most common underlying cause. Graves’ disease is less common than Hashimoto’s thyroiditis, but has important potential effects upon the fetus and newborn infant.17 The transplacental transmission of long-acting TSH receptor stimulating antibodies (anti-TSHR or TRAb) increases gradually in the course of pregnancy, particularly in the last trimester; as a result, fetal hypothyroidism is more frequent towards the end of pregnancy. This transmission of antibodies can lead to fetal and neonatal hyperthyroidism. In these cases it is clear that the risk of hyperthyroidism is conditioned by antibody titers, disease activity during pregnancy, and a history of radioiodine treatment. Exceptionally, newborn infants may develop transient central hypothyroidism. It should be noted that TRAb may be present in the mother even years after she has received radioiodine or has undergone thyroidectomy. There is, therefore, a continuing risk to a fetus or newborn.11
Considering the high incidence of autoimmune thyroid disease among pregnant women, at the start of the present study we decided to adopt a follow-up protocol for both the offspring of mothers with thyroiditis and the offspring of mothers with Graves’ disease, and to evaluate the results obtained.
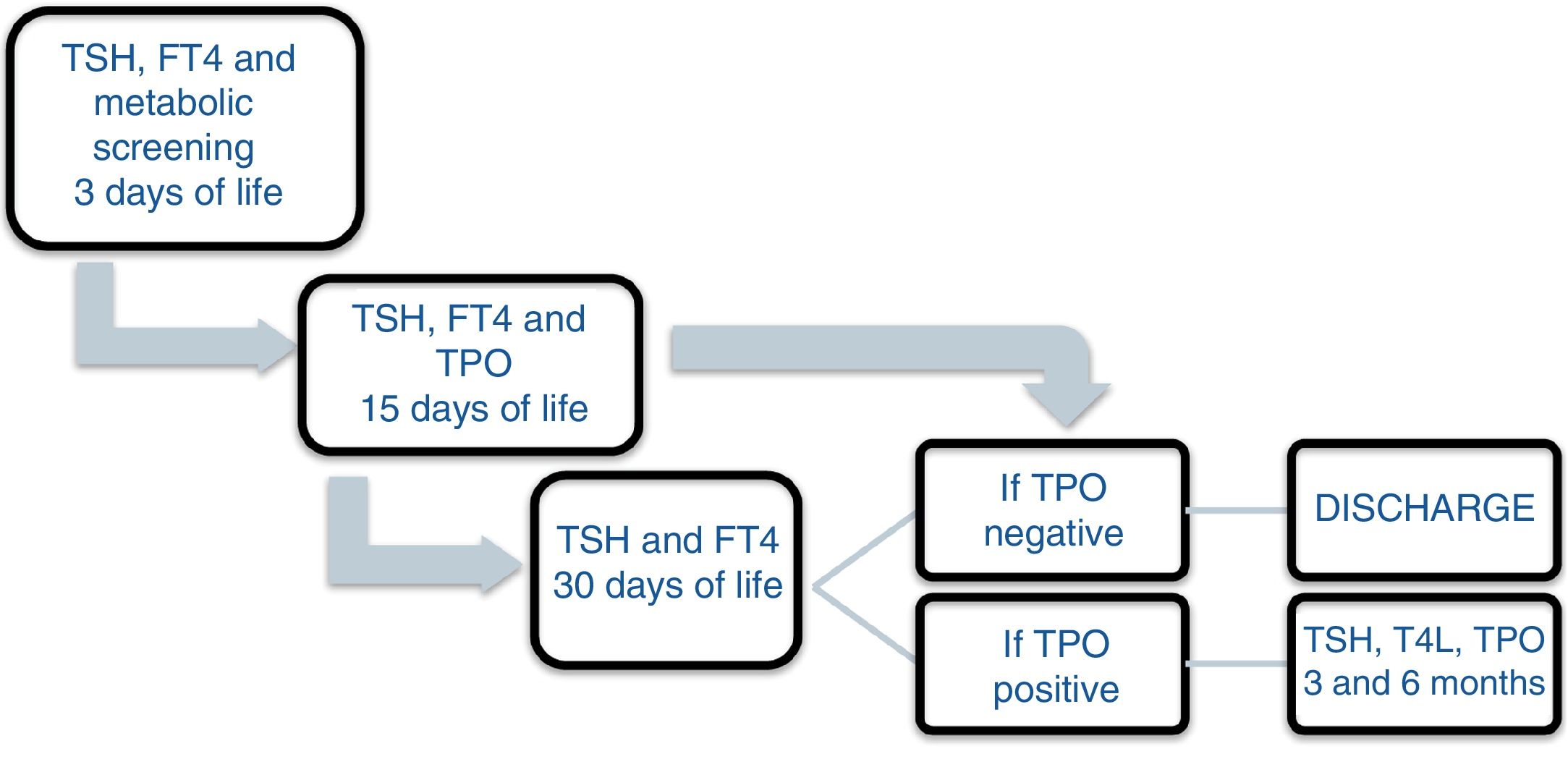
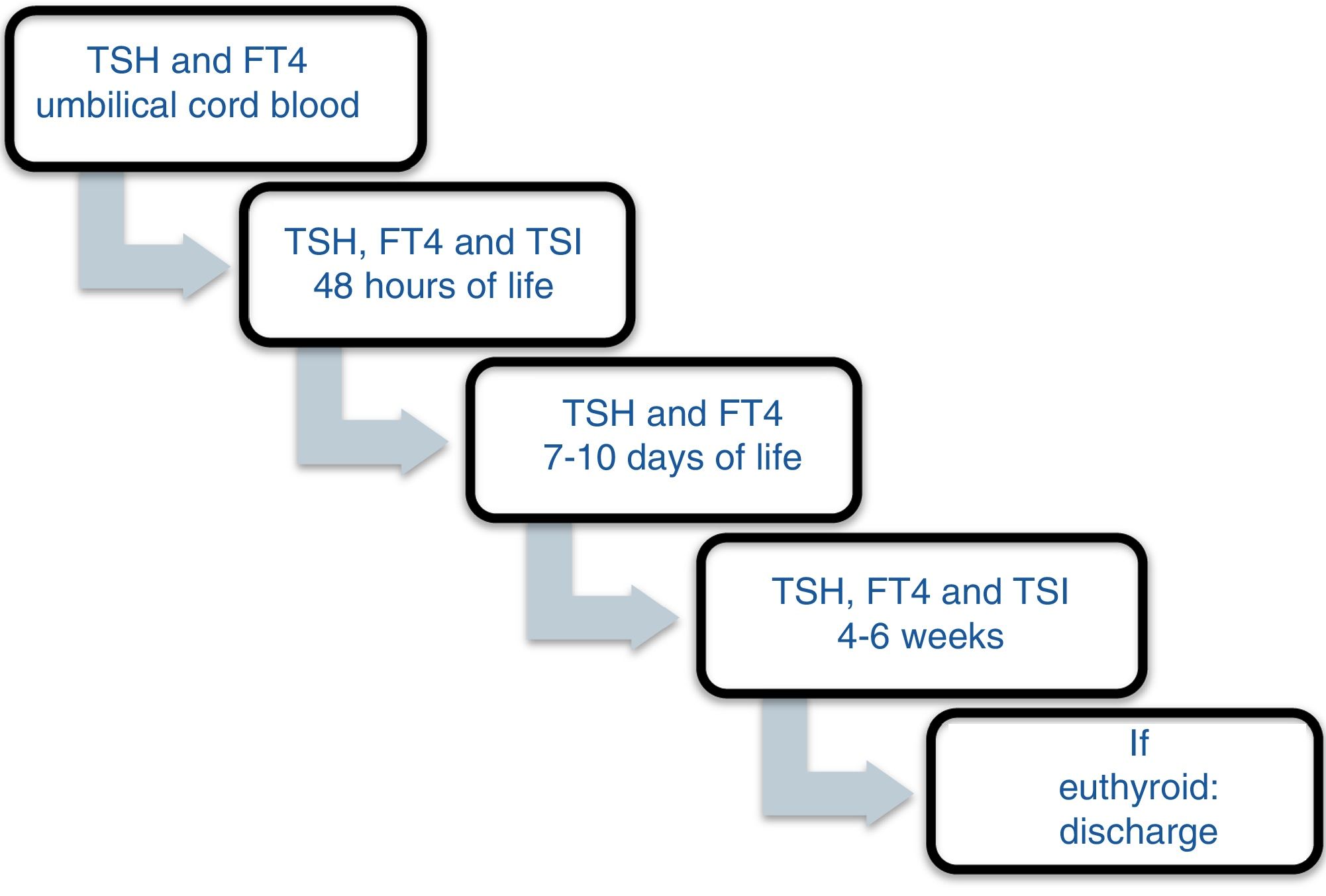
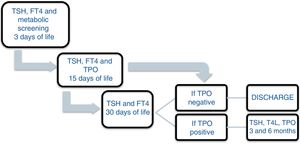
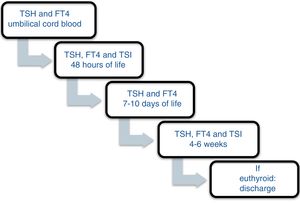
Patients and methodsA prospective study was carried out between November 2013 and December 2016 involving the offspring of mothers with autoimmune thyroid disease born in a tertiary university hospital. In October 2013, a protocol was agreed with the Department of Pediatric Endocrinology to monitor the children of mothers with autoimmune thyroid disease. Differentiated follow-up was established for children born to hyperthyroid mothers and children born to mothers with autoimmune hypothyroidism (Figs. 1 and 2). The protocol was amended during the follow-up period because the Institute of Molecular Biology and Genetics recommended the discontinuation of laboratory testing at 15 days of life because it coincided with the second metabolic screening test which the Institute performed on the children of mothers with thyroid disease.
At our hospital, early detection tests for congenital diseases are systematically performed between 48−72 h of life, and are referred to the Institute of Molecular Biology and Genetics, TSH being measured with a cut-off point of 10 mU/l. In the hospital laboratory, the TSH, FT4 and anti-TPO levels were measured by paramagnetic particle chemiluminescent immunoassay (DxI 800 Beckman Coulter).
All the mothers had been followed-up on at the endocrinology and/or obstetrics clinics, and thyroid function was determined at least once. The children were selected from the maternity ward. Cases of perinatal asphyxia were excluded. The data were collected from the case histories of the mothers and newborn infants at the time of the first control after infant discharge, with the laboratory results obtained on each visit being recorded. In relation to the mother, we documented the diagnosis, the time of diagnosis, thyroid function and treatment during pregnancy. The epidemiological data of the children were analyzed: gestational age, birth weight (BW), the Apgar score at minutes 1 and 5, systematic metabolic screening test results, and laboratory test results during follow-up: TSH, T4, T3, anti-TPO, thyroid-stimulating immunoglobulin (TSI), the number of laboratory controls performed, the need for referral to the endocrinology clinic, and age and diagnosis at discharge. The children remained under follow-up for as long as they proved positive for anti-TPO antibodies. If at one month of life the antibodies proved negative and the hormones were within normal ranges, the patients were discharged.
The SPSS version 17 statistical package was used for data analysis. Categorical variables were reported as absolute and relative frequencies. The normal distribution of quantitative variables was analyzed by the Kolmogorov Smirnov test. Variables exhibiting a normal distribution were reported as the mean and standard deviation (SD), while those with a non-normal distribution were presented as the median and interquartile range (IQR). Quantitative variables with a normal distribution were compared with the Student t-test, while the Mann–Whitney U-test was used in the case of variables with a non-normal distribution. The relationship between quantitative variables was explored using bivariate Pearson correlation analysis and scatter plots.
ResultsDuring the study period, a total of 191 children born to mothers with autoimmune thyroid disease were enrolled. A total of 10.5% of the women were diagnosed with hyperthyroidism and 89.5% with autoimmune hypothyroidism. In turn, 39.3% were diagnosed during pregnancy, with hypothyroidism by far being the most common diagnosis (94.7%). A total of 85.8% of the mothers with autoimmune thyroiditis and 57.9% of those with Graves’ disease received treatment during pregnancy.
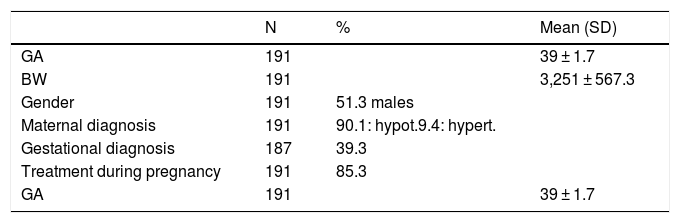
With regard to the newborn infants, the mean gestational age was 39 weeks (39 ± 1.71), and 91.6% were full term births. The mean BW was 3251 ± 567.3 g. A little over half of the infants (51.3%) were males (Table 1).
General characteristics of the 191 newborn infants and their mothers.
| N | % | Mean (SD) | |
|---|---|---|---|
| GA | 191 | 39 ± 1.7 | |
| BW | 191 | 3,251 ± 567.3 | |
| Gender | 191 | 51.3 males | |
| Maternal diagnosis | 191 | 90.1: hypot.9.4: hypert. | |
| Gestational diagnosis | 187 | 39.3 | |
| Treatment during pregnancy | 191 | 85.3 | |
| GA | 191 | 39 ± 1.7 |
SD: standard deviation; GA: gestational age; hypert: hyperthyroidism; hypot: hypothyroidism; BW: birth weight.
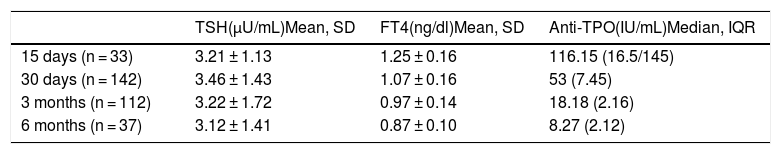
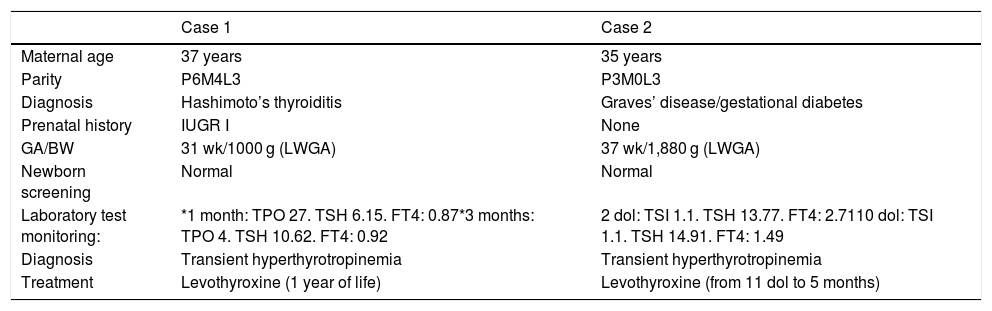
A total of 94.2% of the offspring of women with hypothyroidism had laboratory values within normal ranges or with nonsignificant alterations (Table 2). Only 5.8% exhibited significant alterations during follow-up. Most alterations consisted of mild TSH elevation, with values in all cases < 10 µU/mL, except for one patient with a maximum of 12.95 µU/mL (the reference values used indicate that TSH should be < 6.5 µU/mL in infants under one month of age). In 81.1% of the cases, these alterations were found to have disappeared at the next control (one month of age). No laboratory test alterations were recorded at the control performed at one month of life (n = 143). At three months of follow-up, one patient required referral to the endocrinology clinic (case 1, Table 3), being diagnosed with transient hyperthyrotropinemia requiring levothyroxine therapy during the first year of life. The remaining patients had normal thyroid function. After 6 months, TSH and FT4 levels were seen to be within normal ranges in all patients. On selecting only the full term infants, no cases of hypothyroidism were observed among the offspring of mothers with thyroiditis. Compliance over follow-up was 83.1%.
Laboratory test results of the offspring of mothers with autoimmune thyroiditis.
| TSH(µU/mL)Mean, SD | FT4(ng/dl)Mean, SD | Anti-TPO(IU/mL)Median, IQR | |
|---|---|---|---|
| 15 days (n = 33) | 3.21 ± 1.13 | 1.25 ± 0.16 | 116.15 (16.5/145) |
| 30 days (n = 142) | 3.46 ± 1.43 | 1.07 ± 0.16 | 53 (7.45) |
| 3 months (n = 112) | 3.22 ± 1.72 | 0.97 ± 0.14 | 18.18 (2.16) |
| 6 months (n = 37) | 3.12 ± 1.41 | 0.87 ± 0.10 | 8.27 (2.12) |
Anti-TPO: thyroid peroxidase antibodies; SD: standard deviation; IQR: interquartile range.
Description of the cases that received treatment.
| Case 1 | Case 2 | |
|---|---|---|
| Maternal age | 37 years | 35 years |
| Parity | P6M4L3 | P3M0L3 |
| Diagnosis | Hashimoto’s thyroiditis | Graves’ disease/gestational diabetes |
| Prenatal history | IUGR I | None |
| GA/BW | 31 wk/1000 g (LWGA) | 37 wk/1,880 g (LWGA) |
| Newborn screening | Normal | Normal |
| Laboratory test monitoring: | *1 month: TPO 27. TSH 6.15. FT4: 0.87*3 months: TPO 4. TSH 10.62. FT4: 0.92 | 2 dol: TSI 1.1. TSH 13.77. FT4: 2.7110 dol: TSI 1.1. TSH 14.91. FT4: 1.49 |
| Diagnosis | Transient hyperthyrotropinemia | Transient hyperthyrotropinemia |
| Treatment | Levothyroxine (1 year of life) | Levothyroxine (from 11 dol to 5 months) |
Anti-TPO: thyroid peroxidase antibodies (IU/mL); LWGA: low weight for gestational age; IUGR I: intrauterine growth restriction type I; dol: days of life; GA: gestational age; BW: birth weight; TSH: thyroid stimulating hormone (µU/mL); FT4: free thyroxine (ng/dl); TSI: thyroid stimulating immunoglobulin (IU/mL).
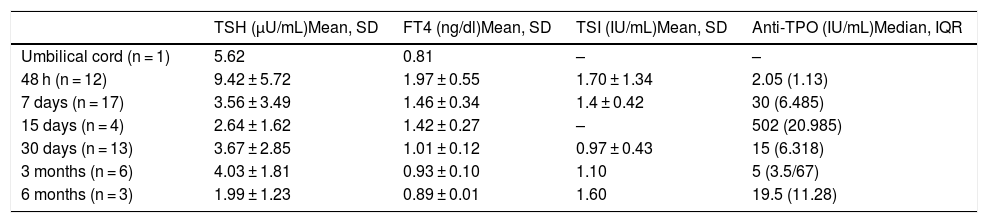
Of the offspring of mothers with Graves’ disease, 36.8% showed relevant alterations in the laboratory controls (Table 4). All the laboratory test alterations were observed in the first 7 days of life (60% in the first 48 h), and all involved TSH increments > 10 µU/mL. Eighty percent had normal thyroid function at one month of life. A child born to a hyperthyroid mother was referred to the endocrinology clinic due to pathological TSH elevation at 48 h after birth (case 2, Table 3), being diagnosed with transient hyperthyrotropinemia (treatment with levothyroxine was required for 5 months). Of note is the fact that follow-up of the children of hyperthyroid mothers was irregular. Thus, TSH and FT4 in umbilical cord blood was measured in only one patient. This figure increased to 68.4% at 48 h and 89.5% at 7 days, before decreasing back to 68.4% at one month of life. No cases of hyperthyroidism were detected.
Laboratory test results of the offspring of mothers with Graves’ disease.
| TSH (µU/mL)Mean, SD | FT4 (ng/dl)Mean, SD | TSI (IU/mL)Mean, SD | Anti-TPO (IU/mL)Median, IQR | |
|---|---|---|---|---|
| Umbilical cord (n = 1) | 5.62 | 0.81 | – | – |
| 48 h (n = 12) | 9.42 ± 5.72 | 1.97 ± 0.55 | 1.70 ± 1.34 | 2.05 (1.13) |
| 7 days (n = 17) | 3.56 ± 3.49 | 1.46 ± 0.34 | 1.4 ± 0.42 | 30 (6.485) |
| 15 days (n = 4) | 2.64 ± 1.62 | 1.42 ± 0.27 | – | 502 (20.985) |
| 30 days (n = 13) | 3.67 ± 2.85 | 1.01 ± 0.12 | 0.97 ± 0.43 | 15 (6.318) |
| 3 months (n = 6) | 4.03 ± 1.81 | 0.93 ± 0.10 | 1.10 | 5 (3.5/67) |
| 6 months (n = 3) | 1.99 ± 1.23 | 0.89 ± 0.01 | 1.60 | 19.5 (11.28) |
Anti-TPO: thyroid peroxidase antibodies; SD: standard deviation; IQR: interquartile range; TSI: thyroid stimulating immunoglobulin.
The TPO antibody levels at first sampling were not significantly related to other laboratory test alterations, though the TPO levels were slightly higher in this group (median 20 versus 27). The anti-TPO titers were positive in 87.5% of the children subjected to laboratory testing after 15 days, while 81.1% were positive after one month, and 55.8% after three months. At 6 months, 44.7% of the children remained positive. The thyroid-stimulating immunoglobulin (TSI) titers were negative in 94.7% of the children born to mothers with hyperthyroidism, and proved positive in only one patient. Unlike in other studies, no correlation was found between the antibody titers and birth weight.18
A total of 388 laboratory controls were performed (339 children with hypothyroid mothers and 49 children with hyperthyroid mothers) during the follow-up of these patients, with an average of 2.1 ± 1 controls per patient. Children born to mothers with hyperthyroidism underwent more laboratory controls (mean = 3 ± 1.10 versus 2 ± 0.98), the maximum number of controls being 6 per patient. Mean age at discharge was 3.41 ± 3.9 months for children of hyperthyroid mothers versus 3.1 ± 2.18 for children of hypothyroid mothers.
DiscussionThe follow-up of children born to mothers with autoimmune thyroiditis is a controversial issue. In this regard, the literature contains studies both in favor13 and against the need for such follow-up.11,12,14–16 Despite the possible hormonal disorders and transplacental transmission of stimulating antibodies, specific newborn infant screening has not been shown to be necessary.
In our study, most offspring of mothers with Hashimoto’s thyroiditis had normal control values. In agreement with previous reports,10 the most frequently observed alterations were transient TSH elevations with normal T4 levels in the first month of life. In principle, these increases were not related to shorter gestational age or to lower birth weight, and we likewise observed no statistically significant association with the anti-TPO titers. We only documented one case of persistent hyperthyrotropinemia (0.58%) that required replacement therapy with levothyroxine; however, as this was a premature patient, it cannot be ruled out that the condition was influenced by prematurity. No laboratory test alterations were recorded after one month of age despite positive TPO levels even up to 6 months of age.
The percentage of patients with laboratory test alterations was greater in the group of children of mothers with Graves’ disease, and the number of patients requiring replacement therapy was also greater (0.58% versus 5.2%). Of note is the fact that the disorder most commonly found in these patients was TSH elevation, and no cases of hyperthyroidism were recorded.
Based on our results and according to the most recent publications in this field,11 it could be affirmed that our follow-up protocol for children of mothers with thyroiditis is inefficient. It appears to be excessive to be performing an average of three laboratory controls per patient when no alterations have been observed beyond 15 days of life. The exception was the case of a premature patient in which hyperthyrotropinemia was detected at one month of corrected age.
The need to determine anti-TPO titers is not clear, as it does not appear to be correlated to an increased risk of congenital hypothyroidism. As regards the offspring of mothers with Graves’ disease, close monitoring is required due to the possibility of their developing hyperthyroidism and hypothyroidism, as occurred in our study. The measurement of TRAb in pregnancy or umbilical cord blood is very useful for identifying newborn infants at high or low risk of developing hyperthyroidism.11
The follow-up rate of the offspring of mothers with hypothyroidism was 74%, while follow-up of the offspring of hyperthyroid mothers was irregular, with important variations between controls.
Our study has a number of limitations, since it reflects the experience of a single center with acceptable but not optimum follow-up. In addition, while the sample size was considerable, it may not have been sufficient to establish statistical significance for relevant variables such as antibody titers and their relation to altered thyroid function. Nevertheless, the findings are supported by the new recommendations of the Spanish Society of Pediatric Endocrinology (Sociedad Española de Endocrinología Pediátrica), which do not advocate thyroid evaluation different from systematic neonatal screening of infants born to mothers with hypothyroidism treated with thyroxin.11
We consider this recommendation to be very appropriate, on the one hand with a view to improving the management of healthcare resources (which are limited), and on the other (and no less importantly) with the aim of minimizing the psychological impact upon the family due to the conducting of tests on the child that have been shown to be unnecessary. In turn, it would be advisable to involve the parents in scientifically warranted screening programs for various diseases, with a view to improving adherence.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Marín Urueña SI, Infante López ME, Samaniego Fernández CM, Montejo Vicente MM, Escribano García C, Izquierdo Caballero R, et al. Seguimiento del hijo de madre con patología tiroidea autoinmune. ¿Qué no debemos cribar? Endocrinol Diabetes Nutr. 2020;67:172–178.