Thyroid nodules (TN) are a prevalent pathology that can generate morbidity, in which case the traditional treatment is usually surgery.
ObjectiveTo analyse the efficacy of radiofrequency ablation (RFA) treatment as a therapeutic alternative in the combined clinical, morphological, and functional control of predominantly solid, benign and clinically relevant TNs in patients not subsidiary to surgery.
Materials and methodsA descriptive, retrospective, case series study was carried out to assess the efficacy and safety of the use of RFA. According to medical criteria, the selected patients underwent a clinical, ultrasound, and biochemical assessment prior to the procedure and then after the procedure at 1, 3, 6, and 12 months and then every 6–12 months according to medical criteria.
ResultsA total of 100 RFA were performed on 83 patients with 85 TNs of ≥2.5 cm with an initial volume (IV) of 21.48 ± 15.89 ml. After a mean of 1.17 RFA sessions per TN, the volume decreased progressively and significantly (p < 0.01 for all times compared to the initial value), with a mean volume reduction rate (VRR) in relation to the IV of 54.43 ± 19.56% at 1-month follow-up; 67.69 ± 17% at 3 months; 70.38 ± 15.46% at 6 months; 70.67 ± 17.27% at 12 months and 70.24 ± 17.7% at the last follow-up. 88% of the patients followed up >6 months achieved the combined objective of a volume reduction rate of more than 50% of the VI, thyroid normal function and absence of clinic; and in all of these, it was maintained until the final follow-up date. Acute complications (mostly mild and all transient) were reported in 9 of the 100 RFA performed.
ConclusionOur findings validate in our setting the efficacy and safety of RFA in predominantly large and solid TNs, and add undescribed information to position the technique more favourably as a therapeutic alternative.
Los nódulos tiroideos (NT) son una patología prevalente que puede generar morbilidad, en cuyo caso el tratamiento tradicional suele ser cirugía.
ObjetivoAnalizar la eficacia del tratamiento con ablación por radiofrecuencia (ARF) como alternativa terapéutica en el control combinado clínico, morfológico y funcional de los NT predominantemente sólidos, benignos y clínicamente relevantes en pacientes no subsidiarios de cirugía.
Materiales y métodosSe realizó un estudio descriptivo, retrospectivo, de serie de casos, para valorar la eficacia y seguridad del uso de ARF. A los pacientes seleccionados se les realizó una evaluación clínica, ecográfica y bioquímica previa al procedimiento y posterior al mismo al mes 1, 3, 6 y 12 y luego cada 6–12 meses según criterio médico.
ResultadosSe realizaron un total de 100 ARF, en 83 pacientes, con 85 N T de ≥2,5 cm con un volumen inicial (VI) de 21.48 ± 15.89 ml. Tras una media de 1.17 sesiones de ARF por NT, el volumen disminuyó progresiva y significativamente (p < 0.01 para todos los tiempos frente al valor inicial), con una tasa de reducción de volumen (TRV) promedio respecto al VI de 54.43 ± 19.56% al mes de seguimiento; 67.69 ± 17% a los 3 meses; 70.38 ± 15.46% a los 6 meses; 70.67 ± 17.27% a los 12 meses y 70.24 ± 17.7% en el último seguimiento. El 88% de los pacientes seguidos >6 meses alcanzó el objetivo combinado de tasa de reducción de volumen de más de 50% del VI, normofunción tiroidea y ausencia de clínica; y en todos estos se mantuvo hasta la fecha final de seguimiento. En 9 de las 100 ARF realizadas se reportaron complicaciones agudas (en su mayoría leves y todas transitorias).
ConclusiónEstos hallazgos validan en nuestro medio la eficacia y seguridad de la ARF en NT predominantemente sólidos de gran tamaño y añaden información no descrita para posicionarla más favorablemente como alternativa terapéutica.
Thyroid nodules (TNs) are a common clinical finding, and more than 50% of the global adult population currently has at least one TN. They are more prevalent in women and are closely related to age.1
In total, 75–85% of non-hyperfunctioning or 'cold' TNs are benign. They are predominantly solid, and asymptomatic and can be managed by ultrasound observation and regular clinical follow-up. However, up to 66% of these show significant growth, causing some compressive signs or symptoms.2 In these cases, surgery has been the treatment of choice to date. Although it is a safe procedure available at the vast majority of centres, it does pose several drawbacks: complications can occur in 1%–10% of cases, including at centres staffed by specialist surgeons,3–6 all total thyroidectomies and most hemithyroidectomies result in hypothyroidism,7 it is dependent on the surgical waiting list of each centre or region, and it usually requires general anaesthesia and hospitalisation (therefore entailing a high cost). Moreover, surgery may not be appropriate for patients at high surgical risk or for those unwilling to undergo it.1,8
Some 5–10% of predominantly solid TNs are hyperfunctioning, and radioactive iodine (radioiodine, I131) is the treatment of choice for most of these cases. Studies following treatment with I131 show normalisation of thyroid function in 75−90% of patients in the first few months and a TN volume reduction of 35–40% within the first year.9–11 However, the individual morphological response is very difficult to predict as TN size is maintained in up to 50% of patients and continues growing in 25%, while fewer than half show improvement in compressive symptoms. In addition, the risk of permanent hypothyroidism (clinical or subclinical) after treatment with I131 is estimated to be in the range of 11–73%.12–14 In TNs with recurrent or persistent hyperthyroidism after I131 or requiring rapid control, as well as those contraindicated for I131 or with associated compressive symptoms, the only treatment option to date has also been surgery.1,8,15,16
For these reasons, the last two decades have seen the development and optimisation of various image-guided and minimally-invasive techniques currently considered useful options in certain TN cases. One of these alternatives is radiofrequency ablation (RFA). This is ideally conducted on an outpatient basis with local anaesthesia via a transisthmic approach using self-cooling monopolar or bipolar electrodes with an active straight tip and employing the 'moving-shot' technique.8,16
RFA can induce a rapid, significant and long-lasting reduction in TN volume. The volume reduction rate (VRR), whether in one or several RFA sessions, varies from site to site between 51% and 97.9% at the end of follow-up (typically 70–80% after 6–12 months, which is when TN maximum reduction to be sustained over time is usually reached). It has been reported that treatment success (TS), defined as a >50% decrease in the baseline volume (BV), is achieved in up to 91% of TNs treated with RFA. The published results on symptomatic control and quality of life are similar to surgery, with fewer complications and most euthyroid patients retaining normal thyroid gland function.8,16,17
The variability in VRR and TS results has been attributed to various factors, particularly the baseline volume and the composition of the thyroid nodule.8,15,18–20 Most studies published to date have included TN with a significant cystic component and/or small pre-procedural volume17,19–26 with both parameters favouring efficacy results.
Despite the plethora of publications on RFA in TN, there are very studies that have assessed the efficacy of the technique specifically in predominantly solid (>50%) and large (mean BV > 20 cc) TN series, in addition to the fact that most do not specify how many patients achieve TS. Furthermore, no study has so far jointly analysed the efficacy of the technique on the simultaneous clinical, morphological and functional control of each case.
The main aim of this study was to analyse the medium-to-long-term safety and efficacy of treatment with RFA (in routine clinical practice) on the comprehensive control (clinical, morphological and functional) of predominantly solid, large and clinically-significant TNs in patients treated at our centre. Differences in the attainment of these objectives according to the BV of these TNs and our team's learning curve (LC) were also analysed. Finally, the optimisation capacity of our results is briefly explored in the discussion, and they are contextualised, taking into account the reproducibility of publications both within our setting and beyond.
MethodStudy design and patient selectionA descriptive, retrospective, case series study was conducted that analysed the results of adult patients with predominantly solid, benign and clinically-significant TNs ≥2.5 cm treated with RFA since the technique was added to our centre’s portfolio of services (July 2014) until January 2019, in accordance with our routine clinical practice protocol (Table 1). These patients accounted for all TN patients treated with RFA at our centre when this paper began to be drafted.
Inclusion/exclusion criteria for radiofrequency ablation of benign thyroid nodules at our centre.
| Inclusion criteria |
|---|
| 1. ≥ 18 years of age |
| 2. Understanding and signing the informed consent (approved by the centre's ethic's committee) prior to the procedure |
| 3. 1 or 2 'dominant/target', predominantly solid TNs: |
| - ≥2.5-cm maximum diameter; |
| - benign (confirmed by two cytological results obtained by ultrasound-guided fine-needle aspiration and reported as such according to the Bethesda criteria); |
| - clinically significant, either due to: |
| manifesting at least one symptom (dyspnoea, dysphagia, dysphonia, cough or sensation of foreign body) and or compressive sign, or clinical (positive Pemberton's sign), radiological (extrinsic compression when the food bolus passes through oesophageal-gastroduodenal transit or compression and significant narrowing of the tracheal lumen on chest X-ray/CT) or spirometric (pattern suggestive of extrathoracic upper airway compression); |
| significant growth (>50% of the volume and/or 20% of at least two diameters compared to the baseline size in at least the last two consecutive ultrasound scans) and/or being hyperfunctioning (confirmed by Tc-99 m thyroid scintigraphy or I-123)and causing hyperthyroidism (clinical/subclinical). |
| 4. Not being eligible for conventional treatment(s) established as per current national and international consensus clinical guidelines (surgery and/or treatment with I-131, depending on the case) because they meet any of the following criteria: |
| - High surgical risk |
| - Any potential post-treatment hypothyroidism would give rise to complications or hinder the management of comorbidities/concomitant situations (for example: patients with psychiatric disorders, plans to have a baby in the following year, ischaemic heart disease/heart failure with depressed LVEF, etc.) |
| - Any comorbidity that complicates optimal replacement therapy with levothyroxine if necessary (for example: chronic atrophic gastritis, relapsing/recurrent or persistent H. pylori infection, gastrectomised patients, chronic malabsorption disorder, permanent or frequent gastrointestinal intolerance, etc.) |
| - They are unwilling to undergo conventional treatment(s) |
| Exclusion criteria |
|---|
| Evidence of clear ultrasound signs of suspected malignancy in the nodule to be treated or in any of the patient's TNs |
| Fine-needle aspiration consistent with malignancy in any of the patient's TNs |
| History of prior neck radiotherapy |
| First-line family history of differentiated thyroid cancer (2), medullary carcinoma of thyroid or multiple endocrine neoplasias |
| Confirmed pregnancy at the time of treatment |
| Carrier of implantable electronic medical devices |
| Uncorrectable moderate/severe coagulopathy or thrombopenia |
| Permanent paralysis of the contralateral vocal cord (either by history or by confirmation after pre-procedural clinical assessment) |
| Presence of large, coarse calcifications or eggshell calcifications that prevent the needle being inserted and/or significantly limit its visibility in a significant proportion of the nodule |
| Presence of significant intrathoracic growth of the nodule to be treated, which is unfeasible from a technical point of view (not even assuming a possible partial reduction in the size of the nodule and possible re-treatment of the residual nodule in a second session) |
| In the case of MNGs, in the opinion of the doctor who is going to perform the procedure, the treatment of the target nodule(s) does not significantly affect the morbidity generated by it or its clinical course, generally due to the persistence of relevant residual nodular disease. |
The study complies with the basic principles of the World Medical Association’s Declaration of Helsinki (2013). Given the study design (non-interventional and retrospective), the informed consent of the patients included was not required, given that they were not interviewed and the duly anonymised data collected came from the patients' medical records.
Pre-procedural assessmentBefore treatment, the clinical history of all patients was carefully analysed with a detailed description of demographic and clinical variables, as well as paraclinical findings attributable to TN/multinodular goitre (MNG) or findings relevant for their management [gender, age, thyroid-stimulating hormone (TSH) before treatment, autoimmune thyroid disease diagnosed before treatment: yes/no, pre-treatment hypothyroidism or hyperthyroidism: yes/no, single TN/MNG, location of the TN treated, initial maximum diameter in mm, BV in ml, a manifestation of pre-treatment compressive symptoms/signs, following the criteria stipulated in the inclusion criteria: yes/no].
A neck ultrasound and lab tests were subsequently performed. If any abnormal thyroid function parameters were observed, RFA was only conducted after taking pharmacological therapeutic measures, if indicated, and confirming their normalisation per the recommendations stipulated in the current international consensus guidelines and our centre's routine clinical practice protocols. At the same time, the treatment variables relevant to the subsequent analysis of the results [procedure order number, treatment indication(s), procedure duration in minutes, maximum power used (in W), complete ablation: yes/no, need for pre-procedural analgesia and/or intravenous corticosteroids: yes/no, periprocedural acute complications: yes (specify)/no] were recorded.
TNs were classified according to BV as medium (≤12 ml), large (12−30 ml) and very large (>30 ml), in accordance with the literature.19,23,27–34
Radiofrequency ablationThe procedures were conducted by two specialists with more than four years of proven experience in interventional procedures for cervical conditions and previously trained on RFA in TNs. RFA was performed on an outpatient basis using local anaesthesia (together with conscious sedation for the first 10 cases) and in accordance with the Korean Society of Thyroid Radiology's 2012 consensus recommendations for RFA34 subsequently broadly endorsed by international consensus.22
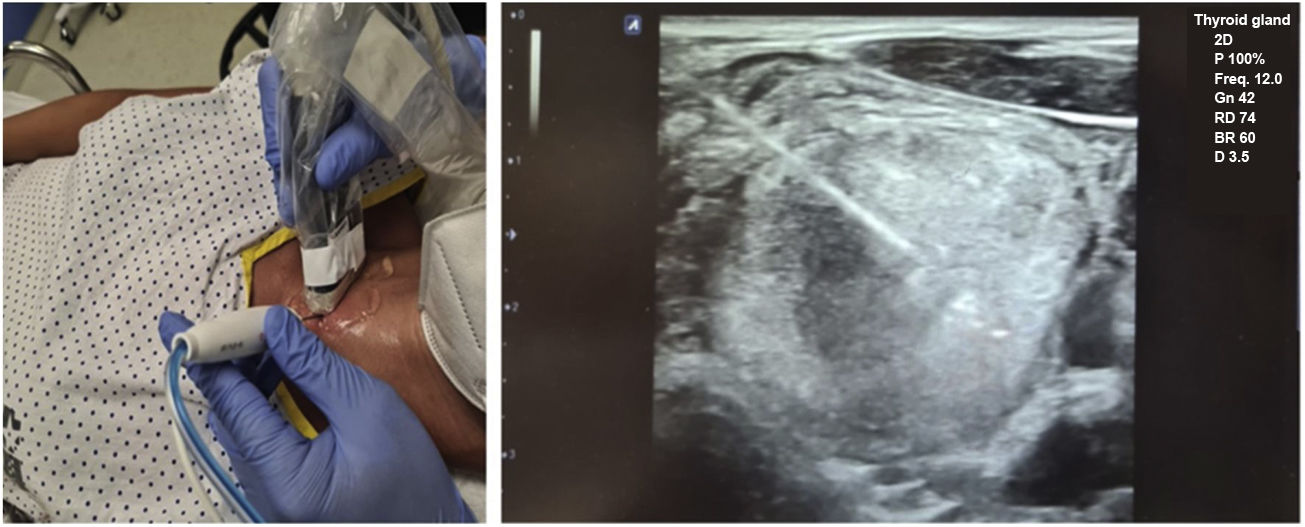
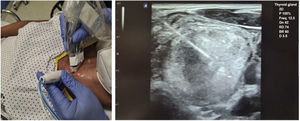
Before ablation, each patient was placed in the supine position with their neck hyperextended, and venous access was placed in their arm. The most favourable access point was determined and ultrasound-guided anaesthesia (lidocaine 2%) was introduced pericapsularly and into the subcutaneous cellular tissue of the puncture site (at which point the procedure is considered to have started). Then, a 19 G, 7-cm long and with a 1-cm active straight tip, self-cooling monopolar electrode (STAR TIR RF 19-07s10f.), connected to a radiofrequency generator producing an alternating electric current (Cool-tip VIVA RFSystem, StardMed), was inserted (being able to use 0–200 W of power, with a maximum output load of 50 Ω), and simultaneously to a refrigerated peristaltic pump. Once the electrode had been inserted via a transisthmic approach, it was positioned inside the TN to be treated using the 'moving shot' technique26 until ablation was complete (Fig. 1).
In large TNs, when the procedure duration was long or manipulation was exhaustive/laborious, a single 20-mg dose of intravenous methylprednisolone was administered during RFA to reduce post-treatment perithyroidal oedema.
Post-procedural care, assessment and follow-upOnce the procedure had been completed, patients remained under observation in bed for approximately 1−2 h and ice was applied to the site. A follow-up neck ultrasound was performed to assess the complete or partial ablation of the TN and the absence of immediate complications. After confirming a positive outcome and oral tolerance, patients were discharged and prescribed on-demand analgesia.
During follow-up, a clinical assessment [(disappearance of compressive symptoms/signs shown before treatment: yes/no, symptoms/signs suggestive of chromic complications: yes (specify)/no)], and ultrasound (volume reduction rate, achievement of treatment success: yes/no) were performed at months 1, 3, 6 and 12 following the procedure and every 6–12 months after that at the doctor’s discretion. In addition, thyroid function was assessed at months 1, 3 and 6 (or 12), and it was decided whether or not to initiate or adjust medication for its control.
A combined endpoint of comprehensive clinical-morphological-functional control was defined, including achievement of TS, normal thyroid function (maintenance or improvement depending on the case) without medical treatment, and absence of compressive symptoms or signs following RFA, which was assessed at all post-six month visits.
Statistical analysisThe statistical analysis was performed using IBM-SPSS Statistics version 21. The Kolmogorov-Smirnov test was used to evaluate the distribution of quantitative variables. The quantitative variables are expressed as mean/median and standard deviation (SD)/range depending on the case, and the qualitative variables as absolute and relative frequencies (percentage). Student's t-test-test and the Mann–Whitney U test were used to compare the mean values of two groups with normal and non-normal distribution, respectively. Changes during follow-up versus the baseline parameters were analysed using the Wilcoxon signed-rank test in variables with non-normal distribution. To test the statistical significance of the VRR and TS between TN subtypes, the analysis of variance (ANOVA) of one factor for related samples was applied. The coefficient of variation (relative SD) was used to compare the degree of variation between subgroups. A p-value <0.05 was considered statistically significant.
ResultsDescriptiveA total of 702 new TN patients were assessed during the study period. Of these, 100 RFA procedures were performed (on 83 patients with 85 TNs, with a BV of 21.48 ± 15.89 ml) and a mean of 1.17 RFA sessions per TN (range 1−2). In total, 70 TNs (82.36%) received a single treatment, while the other 15 TNs (17.64%) required a second RFA session during follow-up (after a mean of 17.3 ± 8.19 months of initial treatment; range: 2.5–34.3 months). The general characteristics of the patients, TNs and procedures are shown in Table 2.
General characteristics of the patients/thyroid nodules treated and procedures performed.
| Patients treated | 83 |
|---|---|
| Gender M/F | 17/66 |
| Age in years (mean ± SD, range) | 46.5 ± 13.27, 20.6−88.4 |
| TSHa pre-treatment (mean ± SD; median, range) in mIU/l | 1.62 ± 0.98; 1.47; 0.01−4.54 |
| Number of patients with autoimmune thyroid disease | 11 |
| 2 hypothyroid patients (receiving treatment with LT4) | |
| Number of patients with hyperthyroidism | 8 (3 with clinical hyperthyroidism and 5 with subclinical hyperthyroidism)* |
| Thyroid nodules (TNs) treatedg | 85 |
|---|---|
| Single TNs/in the context of MNG | 54/31 |
| Location of TNs treated (RTL, LTL, isthmus) | 57/27/1 |
| Composition (100% solid, >80%, between 50% and 80%) | 76/5/4 |
| Maximum initial diameter in mm (mean ± SD; median and range) of the TNs | 44.23 ± 11.24; 42, 24.9–73.9 |
| 94.11% of the TNs had a maximum initial diameter >30 mm | |
| Baseline volume (BV) in ml (mean ± SD; median and range) of the TNs | 21.48 ± 15.89; 17.62 (4.37−72.24) |
| 96.47% of the TNs had a BV > 5 ml; 42.35% >20 ml and 12.94% >40 ml | |
| -No. of medium TNs or with a BV < 12 ml (mean BV) | 30 (7.67 ml) |
| -No. of large TNs or with a BV of 12−30 ml (mean BV) | 39 (19.69 ml) |
| -No. de very large TNs or with a BV > 30 ml (mean BV) | 16 (48.85 ml) |
| RFA procedures/sessions performed | 100 |
|---|---|
| 1.17 per TN (range 1−2) | |
| 1st session: | 85 |
| Duration (mean ± SD; median and range) in min | 56.8 ± 22.4; 55 (15−135) |
| Maximum power (mean ± SD; median and range) in W | 36.9 ± 8.86; 35 (25−60) |
| Number of procedures that required intravenous (IV) analgesia in the procedureb | 22 |
| Number of procedures in which IV corticosteroids were administeredc | 32 |
| Number of incomplete RFAsd | 21 (24.7%) |
| 2nd session (re-treatment): | |
| Number of TNs that required a second RFA sessione | 15/85 (17.64%) |
| Duration (mean ± SD; median and range) in minf | 58.7 ± 23.9; 60 (25−105) |
| Maximum power (mean ± SD; median and range) in W | 36.9 ± 8.48; 35 (25−55) |
| Number of procedures that required intravenous (IV) analgesia in the procedureb | 5 |
| Number of procedures in which IV corticosteroids were administeredc | 3 |
| Treatment indications | |
| - Number of symptomatic TNs** | 58 |
| - Number of asymptomatic TNs with compressive signs | 18 |
| - Number of asymptomatic TNs >30 mm that had shown significant growth*** | 3 |
| - Number of hyperfunctioning TNs not eligible for other treatment options*** | 8 |
****In all these TNs, hyperfunction was the primary reason for RFA indication.
We measured serum thyrotropin (TSH) together with serum-free T4 (and serum-free T3 in hyperfunctioning nodules), by direct chemiluminescence immunoassay (Advia Centaur, Bayer normal range 0.55–4.78 mIU/l, 0.8–1.8 ng/dl and 2.3–4.2 pg/ml, respectively); serum anti-Tg antibodies (normal range 0–115 IU/ml) and serum anti-thyroid peroxidase antibodies (normal range 0–10 IU/ml) by competitive electrochemiluminescence signal (Cobas e411; Roche).
5 of whom were on prior antithyroid treatment (methimazole/thiamazole, mean dose 8 ± 6.70, range 5−20 mg/day).
All in correlation with some compressive sign (clinical, on radiography, spirometry or OGD study).
Paracetamol 1 g or dexketoprofen 25 mg single dose due to pain (mild/moderate) and/or locoregional/cervical postural discomfort.
<100% of the parenchyma of the nodule treated with RFA (according to the operator). The causes for this were: 10 (47.6%) due to the location of all or part of the nodule (partially endothoracic); 2 (9.52%) due to the characteristics of the nodule (macrocalcifications or areas with echogenicity that made it difficult to visualise structures of risk/ the tip of the electrode); 3 (14.2%) due to lack of patient cooperation; and 1 case (4.67%) in which ablation was stopped due to an acute complication (perthyroidal haematoma, treated on an outpatient basis with cryotherapy and compression and admitted for one day for observation).
The reasons for re-treatment were: in 8 TNs (53.33% of all re-treated nodules), a volume reduction <50% of the baseline volume after at least six months of follow-up, with a vascularised nodular remnant that did not decrease in size in at least two consecutive check-ups; in 6 TNs (40%) because, despite the fact that volume reduction was >50% after at least six months, there was a vascularised and clinically-significant nodular remnant (in the opinion of the patient or clinician); and 1 TN (6.66%) underwent early re-treatment because, after incomplete ablation, a viable remnant >50% of the baseline volume and stable versus the one-month check-up persisted at three months.
TN volume decreased progressively and significantly (p < 0.01 for all times versus the baseline value), showing a VRR versus BV of 54.43 ± 19.56% at one month of follow-up, which corresponded to a TN mean residual volume (MRV) on that date of 10.19 ± 11.21 ml; 67.69 ± 17% at three months (MRV of 8.12 ± 9.36 ml); 70.38 ± 15.46% at six months (MRV of 7.76 ± 9.30 ml); 70.67 ± 17.27% at 12 months (MRV 7.66 ± 8.27 ml); and 70.24 ± 17.7% (MRV of 6.5 ± 7.01 ml) at the end of follow-up (15.84 ± 12.18 months) (Fig. 2).
Analysing only those TNs followed up for at least six months after RFA (63 TNs, with a BV of 21.81 ± 16.04 ml), the VRR at the end of follow-up (mean 19.74 ± 9.61 months) was 76.99 ± 10.51%, which corresponded to an MRV of 7.08 ± 9.1 ml. Some 90.47% of these maintained TS at the end of follow-up (78.9% after a single session).
Compressive clinical signs and symptoms disappeared in all patients (100%) with prior history of such symptoms within the first three months of follow-up. In total, 98.7% of patients with normal thyroid function retained this function during follow-up. All patients with either clinical or subclinical hyperthyroidism before RFA (n = 8) improved after treatment, and 80% (four out of five) of those using antithyroid drugs no longer required them within three months.
Some 88% of patients followed for at least six months achieved the combined endpoint of morphological, functional and clinical control, which was maintained until the end of follow-up in all cases.
SafetyAn acute complication arose in nine of the 100 RFA procedures performed (most of which were mild and all transient), and side effects during follow-up were only reported in seven patients (Table 3).
Safety results.
| Number of acute complications | 9 out of 100 procedures |
|---|---|
| 3 Severe | 2 Periprocedural transient dysphoniaa |
| 1 Moderate perithyroidal haematomab | |
| 6 Mild | 3 Symptomatic orthostatic hypotension |
| 2 Hypertensive crisesc | |
| 1 Mild perithyroidal haematomad |
| Number of medium-term side effects | 7 out of 100 procedures |
|---|---|
| 6 Subclinical hyperthyroidism (mild and transient asymptomatic)e | |
| 1 Subclinical hypothyroidismf |
Lasted minutes but subsided following treatment with IV corticosteroids, discharged the same day, asymptomatic and with subsequent confirmation of adequate vocal cord function.
Prevented the treatment from being completed and required admission for observation due to its size.
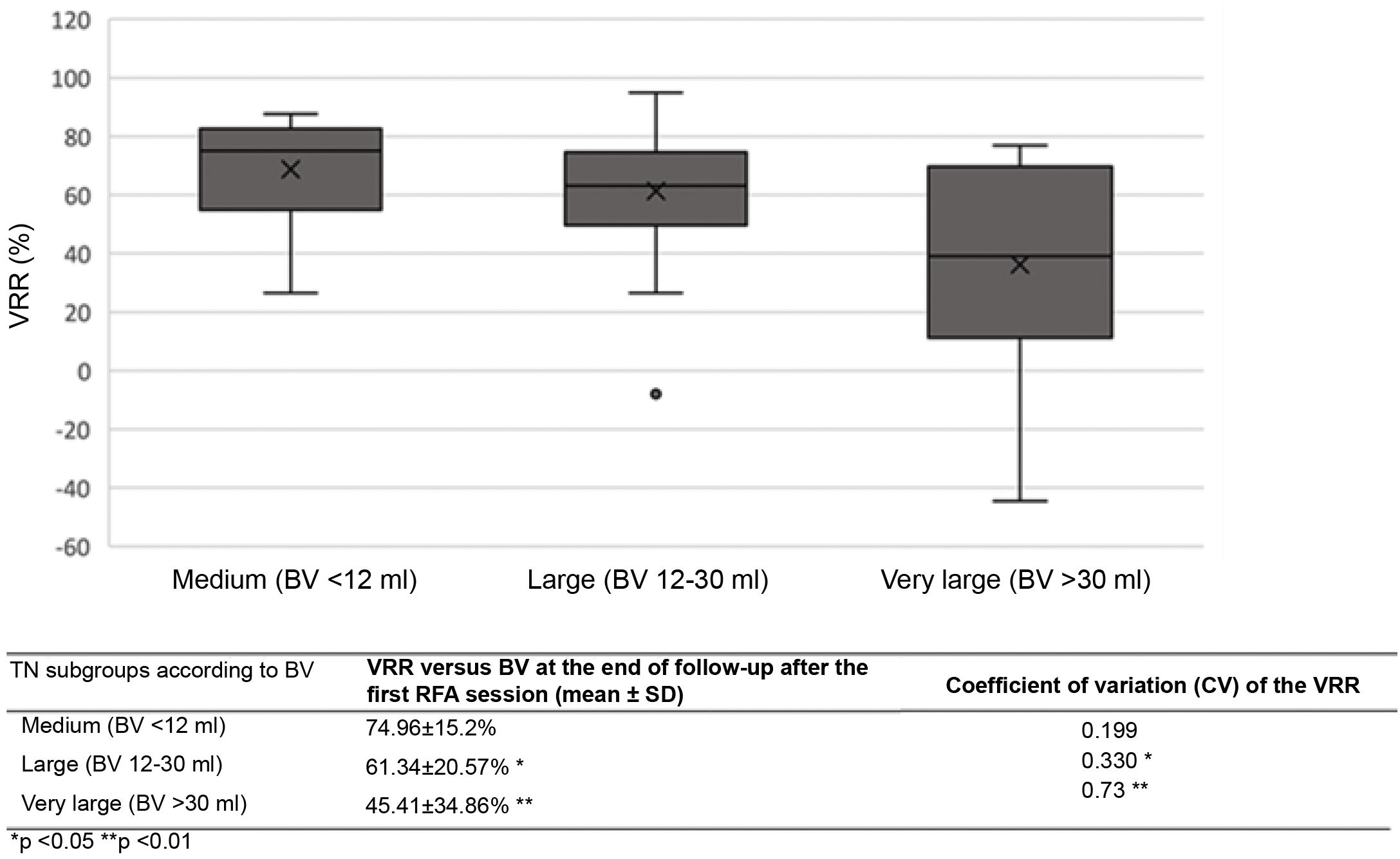
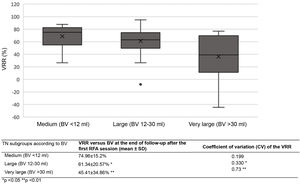
The VRR after the first treatment varied significantly according to TN size (medium, large or very large) (Table 5). Variability in the volume reduction percentage after initial RFA (measured by the coefficient of variation [CV]) was significantly greater for very large TNs than for the other two groups throughout all follow-up stages.
No significant differences were detected in the VRR or in the proportion of patients who achieved TS when analysed by other demographic parameters (age, gender), clinical parameters (manifestation or absence of symptoms, reason for indication), context (MNG or single TN), location (right, left) or TN characteristics (solid component proportion, echogenicity), or by procedure characteristics (maximum power used or procedure duration).
To analyse the impact of the learning curve on our results, they were classified into three subgroups: A) the first 10 TNs treated, B) TNs 11–40, and C) TNs 41–85 (Table 4). The proportion of TNs that achieved >50% volume decrease, and therefore TS (both after one RFA session and at the end of follow-up), increased progressively as the number of procedures performed increased, to the point that from TN number 49, all patients treated achieved TS (and with no evidence of complications).
Efficacy results by treatment order in nodules followed up for at least six months.
| Baseline volume (BV) in ml | Volume reduction rate (VRR) versus BV (%) at the end of follow-up after 1 RFA session (mean ± SD) | Proportion (%) of TNs that achieved treatment success (TS) at the end of follow-up after 1 RFA session | Proportion (%) of TNs that achieved TS at the end of follow-up (after 1 or 2 RFA sessions) | |
|---|---|---|---|---|
| Subgroups according to order number | ||||
| Group A: first 10 TNs treated | 29.87 ± 22.81 | 51.37 ± 31.66 | 50 | 70 |
| Group B: TNs #11 to 40 | 20.61 ± 16.27* | 65.74 ± 29.02 | 83.3** | 90** |
| Group C: TNs # 41–85 | 18.57 ± 9.66*,a | 71.17 ± 14.82**,a | 90.9** | 100** |
Comparison of studies of the treatment of predominantly solid, normal-functioning TNs with a BV of ≥20 ml by RFA.
| Type of ablation and electrode used | N(#TNs) | Proportion of non-functioning TNs | Solid component proportion | BV (ml) Mean ± SD and (range) or [IQR] | Mean RFA sessions; proportion of patients with 1 session (range) | Overall VRR versus BV at 6 months. Mean ± SD or (IQR) | Overall VRR versus BV at 12 months. Mean ± SD or (IQR) | Overall VRR versus BV at end of follow-up. Mean ± SD or [IQR] | Proportion of TNs with TS at end of follow-up | Regrowth rate | Differences in VRR in nodule subgroups versus BV | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Spiezia et al. (2009)18 | Fixed with self-expanding multi-tip monopolar electrode | 94 | 70.21% | ≥70% | 24.5 ± 2.1; (4.5–103) | 1.47; 66% one session (range 1–3)**** | 57.8 ± 9% | 78.6 ± 2% | 79.4 ± 2.5% (at 24 months) | NS | 34%. | NS. However, an inverse correlation was found between VRR and BV (<0.01) |
| Valcavi et al. (2015)27 | "moving shot" with self-cooling rigid single-tip monopolar electrode | 40* | 100% | ≥80% | 30.0 ± 18.2; (6.5−90) | 1 | 55.1 ± 18.5% | 67.7 ± 17.3% | 80.1 ± 16.1% (at 24 months) | NS | 0% | NS |
| Cesareo et al. (2015)44 | "moving shot" with self-cooling rigid single-tip electrode | 42 | 100% | ≥70% | 24.5 ± 19.6 (3.4–89) | 1 | 68.6 ± 13.5% | NS | N/A | NS | NS | Yes in TNs with BV >30 ml (and with higher coefficient of variation) |
| Cesareo et al. (2017)45 | "moving shot" with self-cooling rigid single-tip monopolar electrode | 48 | 100% | ≥70% | 23.5 ± 18.6 (NS) | 1 | 68.6 ± 13.65% | 71.1 ± 14.3% | N/A | NS | 4.2% | Yes in TNs with BV <12 ml, 12−30 ml and >30 ml |
| Pacella et al. (2017)29 | "moving shot" with self-cooling rigid single-tip monopolar electrode | 138** | 100% | ≥70% | 24.5 ± 17.9 (NS) | 1 | 57 ± 21% | 62 ± 22% | N/A | 69% | NS | Yes in TNs with BV <13.1 ml, 13.1–30 ml and >30 ml |
| Deandrea et al. (2019)43 | "moving shot" with self-cooling rigid single-tip monopolar electrode | 215*** | NS | ≥70% | 20.9 [15–33] | (range 1−2) | 56.90% | 64.11% | 66.9% after a mean of 35 months | NS | 4.1% | Yes in TNs with BV <10 ml, 10−20 ml and >20 ml |
| Deandrea et al. (2019)41 | "moving shot" with self-cooling rigid single-tip monopolar electrode | 337 | 100% | ≥70% | 20.7 [13.7–33.1] | 1 | 63.50% | 70% | N/A | NS | NS | Yes in TNs with BV <15 ml, 15−30 ml and >30 ml |
| Guang et al. (2019)32 | "moving shot" with self-cooling rigid single-tip bipolar electrode | 194+ | 100% | ≥70% | 21.2 ± 19.7 (1.1–91.1) | 1.5 ± 0.6; 50.5% one session (range 1−3) | NS | NS | NS++ | NS | 0% | Yes in TNs with BV <5.1 ml, 5.1−13.1 ml, 13.1−30 ml and >30 ml |
| Hamidi et al. (2018)37 | "moving shot" with self-cooling rigid single-tip monopolar electrode | 14 | 92.30% | ≥50% | 24.2 [17.7–42.5] | 1 | 44.2% (40.7–59) | 54.3% (29.2–57.9) | 44.6% [42.1–59.3] after a mean of 8.6 months | NS | NS | NS |
| Familiar et al. (2020)38 | "moving shot" with self-cooling rigid single-tip monopolar electrode | 24 | 83.30% | ≥50% | 25.4 ± 15.5 (8.2–66.1) | 1 | 57.5 ± 24% | 65.4 ± 20.7% | N/A | 65% at 6 months and 81.2% at 12 months | NS | Yes in TNs with BV >20 ml as well as the percentage of TS |
| Cesareo et al. (2021)35 | "moving shot" with self-cooling rigid single-tip monopolar electrode | 30+++ | 100% | ≥80% | 26.5 ± 21.2 (NS) | 1 | 66 ± 16% | 70.9 ± 16.9% | N/A | 89.7% at 12 months | 10% | NS |
| Our series | "moving shot" with self-cooling rigid single-tip monopolar electrode | 85 | 94.11% | ≥50% (but 95.2% of TNs ≥80%) | 21.48 ± 15.89 (4.37–72.24) | 1.17; 82.3% one session, (range 1−3) | 70.38 ± 15.46% | 70.67 ± 17.27% | 70.24 ± 17.7% after a mean of 15.84 months | 90.47% | 8.23% | Yes after 1 session in TNs with BV <12 ml, 12−30 ml and >30 ml (and with higher coefficient of variation) |
TN: thyroid nodule; VRR: volume reduction rate; BV: baseline volume; TS: treatment success; NS: not specified; N/A: not applicable.
None of the 40 TNs with a BV ≤ 17 ml required more than one treatment session, yielding a mean VRR versus BV of 70.43 ± 21.27% (15.43 ± 13.29 months, range 0.9–53.1 months). This was significantly higher than the 56.36 ± 25.06% of nodules >17 ml after the first treatment (not so after >1 RFA session, which yielded a VRR in these nodules of 66.82 ± 22.29%).
Similarly, although 93.33% (14/15) of TNs treated with >1 RFA session manifested compressive symptoms before the first session, all such symptoms abated within one month of the first procedure, and patients remained asymptomatic throughout follow-up.
In contrast, in four (26%) of the re-treated TNs, the first RFA session was considered incomplete, and one (6.6%) was a hyperfunctioning TN that caused clinical hyperthyroidism. Moreover, the mean BV of those TNs that required a second treatment session was 38.1 ± 17.37 ml (range 17.27–72.24 ml), while the mean BV of those only requiring one RFA session was significantly lower (17.9 ± 13.16 ml, range 4.37–68.21 ml, p < 0.00001).
The mean duration of follow-up after the second treatment of the 15 TNs treated with more than one session was 6.95 ± 5.63 months. Despite this, the VRR at the end of follow-up was 73.74 ± 14.14%, with 86.66% of patients achieving TS (and 73.33% >70% reduction of BV).
There were no differences between the final VRR achieved for TNs treated with one session and those treated with two (69.50 ± 18.37% vs 65.86 ± 33.44%, p = 0.68).
RecurrenceIn total, seven TNs (8.23%), with a BV of 21 ± 7.86 ml, showed significant regrowth during follow-up after the first treatment (clinically significant remnant of the TN and >50% of the BV despite having previously achieved TS). This occurred after a mean of 21.75 months (range 13–34) of follow-up.
DiscussionThe morphological efficacy of RFA has traditionally been evaluated as the VRR or the proportion of TNs that achieve TS and clinical significance by the partial or complete abatement of compressive symptoms.8,17,22–24,35 However, the VRR decreases significantly in predominantly solid and/or large TNs, during response variability and rate of recurrence increase.18,23,27–29 The overall results of our series in these three parameters are in line with the literature. Although the largest series or those conducted in centres with extensive experience (predominantly Korean and Italian) have found similar or slightly superior results to ours in terms of VRR and TS, these studies were generally performed on patients either: 1) with significantly smaller TNs on average than in our series; or 2) with predominantly cystic mixed TNs or in which the proportion of this component was not analysed.8,17,19,22–24,26,28,33,36
Following an exhaustive review of the literature published by the completion date of this manuscript, we only found 11 studies in addition to ours that analysed the outcomes of treatment with RFA in predominantly solid TNs and with a mean BV of ≥20 ml. These show variable VRRs of between 44.6% and 80.1% versus baseline at six to 24 months of follow-up (Table 5). Of these, the only ones conducted outside of Italy or Asia were two small series, with medium-term efficacy results inferior and more heterogeneous in principle than ours. The authors themselves highlight that this could be explained by the need for a greater LC to optimise efficacy.37,38
In our study, the first cases were grouped separately to analyse the LC, which was consistent with the approach adopted by Familiar et al. and Aysan et al.38,39, who also found lower and more heterogeneous VRRs in the first 10 cases compared to subsequent patients. However, it isn't easy to extrapolate conclusions regarding our results since, as mentioned above, in our series, the TNs treated at the beginning had a greater BV. Nevertheless, the proportion of TNs with BV ≥ 20 ml was similar in both the first patients treated, and the TNs treated later. Despite this, the VRRs versus BV were still statistically significant for the first 10 cases, and there were no differences in the rate of complications. To date, there has only been one other study40 to assess the LC for TN RFA operators prospectively. The authors propose a cut-off point of experience similar to that found by our study (>60 cases) to reach an optimal and reproducible morphological efficacy rate of the procedure (both in terms of VRR and TS). This is consistent with the range of >50–60 cases that some authors propose (empirically) to ensure efficacy and minimise the likelihood of side effects.30,31,41 However, like our study, Aysan et al.39 failed to identify any differences in the rate of complications between the first ten patients and the remainder in their cohort of 100 treated cases.
The number of RFA sessions required to obtain our results (mean of 1.17 per TN, range 1−2, 82.35% of patients with a single treatment) is comparable to or fewer than that reported in RFA of TNs of similar characteristics and generally using lower maximum power than those described to date.18,28,30,32,41
All TNs with a BV ≤ 17 ml and almost two-thirds (64.8%) of TNs ≥20 ml could be treated with a single RFA session, despite the fact that several authors report that a second session tends to be required for TNs >12 ml, as well as for most 20−30-ml TNs.22,23,29,31,32 In 2017, Cervelli et al.25 conducted a comparative study of TNs ≥20 ml and <20 ml in which 51 TNs with >75% solid component were treated. Of these, 20 had a volume >20 ml (the mean BV, ST and range are not specified). The mean volume reduction of these 20 after treatment was 62.9% at six months, significantly lower than the volume reduction of smaller TNs. Of these 20 TNs ≥20 ml, 30% required two RFA sessions and the volume reduction after 6–12 months of follow-up after the second ablation was 86.4% and 88.2% versus the original, respectively. This proportion and these results are similar to our study. It is undoubtedly true that the mean BV of our patients treated with more than one session (38.1 ml) was strikingly higher (55% greater) than the mean BV of those treated with a single session. Despite that, all patients clinically benefited from the first procedure, and a second session facilitated the attainment of comparable final morphological efficacy results.
The morphological efficacy in the small proportion of hyperfunctioning TNs in our series seems to be in line with the literature,8,16,17,22,24,29,42 with a 73.5% reduction versus BV after a mean 13.8 months of follow-up. Strikingly, there were no significant differences in the VRR compared with normal-functioning TNs, despite also being large TNs (BV 22.15 ± 15.82 ml).
Our study also confirms the safety and efficacy of RFA with regard to functional control. Our study found that 98.7% of patients with normal thyroid function maintained this function until the end of follow-up. All patients with prior hyperfunctioning TNs significantly improved after treatment, and 80% of those who used antithyroid drugs no longer required them within the first three months.
The proportion, characteristics and context of side effects, as well as complications and recurrences, are similar to findings published in the literature.16,20,22,25,26,29,30 However, they highlight the need for more studies that explore event-predicting pre-procedural markers to allow preventive measures to be taken.
Our study has several limitations: being a retrospective study without a comparator group, which assesses compressive symptoms using a nominal dichotomous scale, it does not consider the impact of the treatment on patient quality of life. It has a limited follow-up period with a considerable loss of long-term data. However, other studies that do not suffer from these limitations suggest that after short- and medium-term results similar to ours, RFA is effective in the long term in most cases.17,21,28,43
ConclusionOur publication is one of the first outside Italy or China to study RFA treatment on predominantly solid, large TNs, which shows the reproducibility of the morphological efficacy results reported in centres with extensive experience in the technique. The number of procedures, the LC and our efficacy and safety results means that we can categorise our team within this latter group. To date, a combined endpoint of clinical, morphological and functional control of clinically-meaningful thyroid nodular disease with RFA has not been specifically assessed. The fact that 88% of our patients followed up for more than six months achieved this composite objective highlights the usefulness of RFA as an alternative tool for patient management in our setting. However, in patients with a TN BV >30 ml, the morphological results (but not the clinical or functional results) are more heterogeneous and, therefore, may require a second procedure. There is a need for prospective, controlled and longer-term studies in our setting to provide further strength to these results and to allow their sustainability over time to be assessed.
Conflicts of interestThe authors declare that they have no conflicts of interest.