To analyse the main characteristics of patients and the health outcomes obtained and to evaluate the impact of peripheral artery disease (PAD) in patients treated in our multidisciplinary Diabetic Foot Unit.
Research design and methodsObservational prospective study. 273 patients from two different populations (with and without PAD – classified according to the presence of distal pulses) treated over a 14-month period in the multidisciplinary Diabetic Foot Unit were included. The data on patient characteristics and outcomes were analysed for the purpose of comparison. For the inference study, a comparison of medians with the non-parametric test for independent samples for the quantitative variables and a χ2 test for the comparison of proportions in qualitative variables were performed.
ResultsPatients with PAD ulcers were older (60 (54–67) vs. 64 (75–81), p=0.000) and had a higher macrovascular burden (8.1% vs. 29% for ischaemic heart disease history, p=0.000; 6.7% vs. 18.1% for cerebrovascular disease history, p=0.004). Their Texas Score was higher (p=0.000) and their major amputation rate was higher (1.4% vs. 12.3%, p=0.001). They had less background of previous ulcers (52.6% vs. 26.8%, p=0.000), their episode duration was shorter (4 (0–10) vs. 0 (0–3) weeks, p=0.000), and their proportional need for antibiotic therapy was lower (64.4% vs. 51.4%, p=0.03).
ConclusionsThe differences found between ulcers with and without vascular involvement support the need for a different approach and for the inclusion of vascular surgeons on the team. The multidisciplinary care model for diabetic foot patients could be effective and improve health outcomes.
Analizar las características principales de los pacientes y de los resultados obtenidos, así como evaluar el impacto de la presencia de enfermedad arterial periférica (EAP) en pacientes atendidos en nuestra unidad de pie diabético multidisciplinar.
MétodosEstudio observacional prospectivo. Se incluyeron 273 pacientes de 2 poblaciones diferentes (con y sin EAP —clasificados según la presencia de pulsos distales—) atendidos en un periodo de 14 meses en la unidad de pie diabético multidisciplinar. Las características y los resultados de los pacientes fueron analizados para su comparación. Para el estudio inferencial se realizó un test no paramétrico de comparación de medianas para las variables cuantitativas; para las variables cualitativas se realizó un test χ2.
ResultadosLos pacientes con úlceras con EAP eran mayores (60 [54-67] vs. 64 [75-81]; p=0,000) y presentaban una mayor carga macrovascular (8,1 vs. 29% para cardiopatía isquémica, p=0,000; 6,7 vs. 18,1% para enfermedad cerebrovascular, p=0,004). Su Texas Score era superior (p=0,000) y su tasa de amputaciones mayores fue superior (14 vs. 12,3%; p=0,001). Presentaban menos antecedentes de úlceras previas (52,6 vs. 26,8%; p=0,000), la duración de sus episodios fue inferior (4 [0-10] vs. 0 [0-3] semanas; p=0,000), y la proporción de necesidad de antibioterapia fue inferior (64,4 vs. 51,4%; p=0,03).
ConclusionesLas diferencias encontradas entre las úlceras con y sin afectación vascular apoya la necesidad de un abordaje diferente y la presencia de cirujanos vasculares en el equipo. El modelo de atención multidisciplinar al pie diabético puede ser efectivo y mejorar los resultados en salud.
Diabetic foot is an important complication and its prevalence varies from 4% to 15%.1 A diabetic patient's risk of contracting this disease in the course of their life has been estimated at 25%, and the condition can be present at diagnosis of the disease. Among diabetic complications, diabetic foot is one of the main causes of hospitalisation, together with cardiovascular and cerebrovascular disease,2,3 which signifies, in addition to high economic costs, huge morbidity and mortality in those who have it, since most of them will undergo amputation within the first four years after the diagnosis of diabetic foot, and since the post-amputation mortality rate has been estimated at 39%–68%. In the EURODIALE study (a one-year proactive follow-up study performed in 10 European countries)4 the minor amputation rate was 18% and the major amputation rate was 4%. Estimates of the risk of amputation of up 30–40 times higher between diabetics and non-diabetics have been reported.5,6
The most important risk factors for ulceration are age, a history of previous ulcers and the presence of sensorimotor polyneuropathy and/or peripheral arterial disease (PAD). It has been estimated that 50% of patients with diabetic foot present sensorimotor polyneuropathy, 15% PAD and the remaining 35% both.1 However, a higher prevalence of PAD has been described in developed countries,7–9 with a prevalence of up to 66% of PAD in diabetic foot units,10 which could be important for the organisation of health resources, since vascular involvement in these patients is also known to be associated with an increased risk of ulcer, infection, major amputation, morbidity and mortality.11–13
With regard to treatment, the role that multidisciplinary units can play in improving diabetic foot care and health outcomes should be emphasised. The scientific evidence in support of such clinical management is currently being studied, with few scientific articles published so far, and with great variability among them, particularly in terms of these units’ operating methods.14,15 In this context, a recent systematic review carried out by the International Working Group on the Diabetic Foot (IWGDF), in which the role of various interventions in diabetic foot ulcer prevention was evaluated, analysed precisely the establishment of multidisciplinary units was for these patients. Although no evidence was presented for the prevention of first ulcers, evidence was found in favour of the prevention of recurrent ulcers.16
In our Autonomous Community, both the Andalusian Health Plan and the Andalusian Comprehensive Diabetes Plan17 promote the existence of at least one multidisciplinary unit for diabetic foot care in the Diabetes Day Hospital in all provinces, which must be the gateway to hospital care for these patients. For this reason, in 2013 a multidisciplinary Diabetic Foot Unit was established in our hospital, coordinated by the Endocrinology and Nutrition Unit, in which endocrinologists, general surgeons, nurses and nursing assistants participated. For this purpose, patients with diabetic foot ulcers with peripheral pulse were treated at the Diabetes Day Hospital one day a week. In January 2019, vascular surgeons joined this unit, and patients with diabetic foot ulcers without peripheral pulse were treated on a different day of the week.
The objectives of this study are: to analyse the main characteristics of patients and the health outcomes obtained and to evaluate the impact of PAD in patients treated in our multidisciplinary Diabetic Foot Unit.
Research design and methodsAn observational prospective study was carried out between January 2019 and February 2020, with a 6-month follow-up. Two different populations of patients presenting active diabetic foot ulcers treated over a 14-month period by a multidisciplinary team in the Diabetic Foot Unit, operating in the Diabetes Day Hospital of the Virgen del Rocío University Hospital were included:
- -
Population 1: Patients with peripheral pulse (both dorsalis pedis and posterior tibial pulses) treated jointly by General Surgery and Endocrinology in the diabetic foot unit (on Wednesdays).
- -
Population 2: Patients without peripheral pulse treated jointly by Vascular Surgery and Endocrinology in the diabetic foot unit (on Thursdays).
Non-diabetic patients, those with at-risk diabetic foot without active ulcer and those with ulcers in places other than the foot were excluded.
This study was approved by the Hospital's ethics committee, which waived the need for consent. All data were fully anonymised before they were accessed.
Two types of variables were collected: variables to describe the type of patient treated in our unit and others to describe our health outcomes. The following information was collected for the first type: medical history number, sex, age, type of diabetes, HbA1c at the first visit, Texas scale score at the first visit, referring medical unit, relevant background such as the presence of arterial hypertension, dyslipidaemia, history of smoking, diabetic nephropathy, diabetic retinopathy, ischaemic heart disease and/or cerebrovascular disease and a history of previous ulcers. The following variables were collected for health outcomes: duration of the episode (in weeks), number of visits to the Diabetes Day Hospital during the episode, need for antibiotic therapy, need for intravenous antibiotic therapy, antibiotic prescribed, need for hospital admission, amputation, type of amputation (major – those including ankle amputation – or minor –those distal to the ankle –), revascularisation (in population 2) and referral to Rehabilitation.
The members of the unit were endocrinologists, nurses, general surgeons and vascular surgeons. We were also assisted by specialists in Infectious Diseases, Radiology, Nuclear Medicine and Orthopaedics. The screening of patients to assess in which office they were to be treated was carried out by our specialised nurses and in the event of doubt they were given an appointment with the vascular surgeon first in order to perform other tests such as the ankle-brachial index (ABI). Mixed cases were also treated in the vascular surgeon's office.
Every week, patients with peripheral pulse were treated on Wednesdays, whereas those patients without peripheral pulse were treated on Thursdays. Visit frequency depended on ulcer severity. When patients only needed treatment with cures by the nursing staff they were referred back to Primary Care offices (and if necessary to the outpatient general surgeon or vascular surgeon) to guarantee a steady flow of patients in the Diabetic Foot Unit. At every visit, Endocrinologists evaluated their glycaemic control and changed their treatment if necessary. Patients who could be discharged from the diabetic foot unit but continued to present poor glycaemic control would continue to be evaluated at the Diabetes Day Hospital.
Two measurements were used for HbA1c: either a recent value (<1 month) in venous blood in a routine analysis or the measurement performed at the same visit with the capillary HbA1c measurement system 501 Analyzer (HemoCue AB, Angelholm, Sweden) if no recent tests were available.
The PRIOAM18 guideline was followed for the prescription of antibiotic therapy: treatment was initiated empirically and titrated according to cultures obtained by wound aspiration and transported in portagerm® medium results.
All patients who required hospitalisation were subsequently reviewed in our multidisciplinary unit.
Post-amputation patients and those who presented chronic stable Charcot foot or acute Charcot foot after 4–6 months were referred for outpatient rehabilitation using discharge prescribed by the nurse and immobilisation.
Data collection and analysis were performed with the Statistical Package for Social Science (SPSS®) 22 version for Windows (IBM Corporation, New York, USA). The descriptive analysis was carried out by obtaining median and quartiles of the quantitative variables (expressed as P50 (P25–P75)) and the frequency of the qualitative variables (expressed as n (%)) for each population. For the inference study, a comparison of medians with the non-parametric test for independent samples for the quantitative variables and a χ2 test for the comparison of proportions in qualitative variables were performed. A p-value of less than 0.05 was considered statistically significant.
ResultsDescription of the patients treatedA sample of 135 patients was obtained for population 1 (with peripheral pulse) and 138 patients for population 2 (without peripheral pulse), making a total of 273 patients included in the study. In both groups the male sex predominated: 108 (80%) and 95 (68.8%) patients were male, respectively.
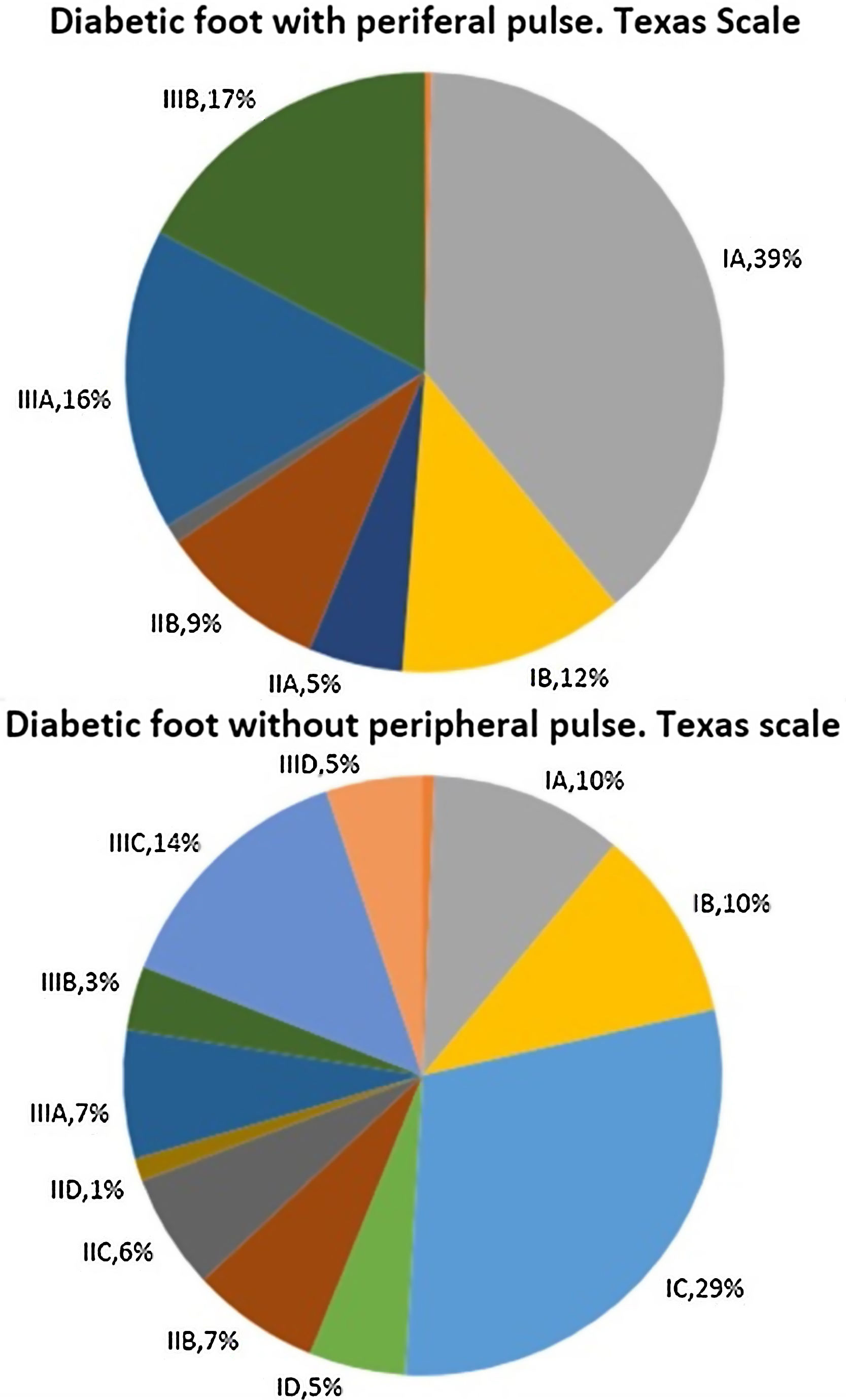
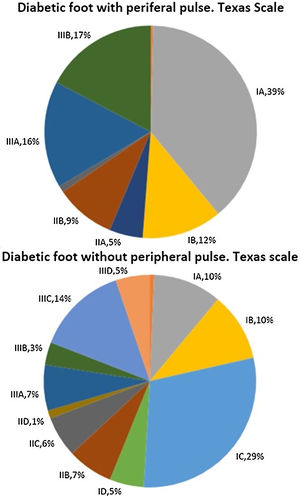
Table 1 presents the variables describing our cohort in each population sample and in the total number of patients included. In addition, the p-value obtained when both samples’ proportions and medians are compared is shown. As can be seen, patients without peripheral pulse are older, more often hypertensive and dyslipidaemic, have significantly less history of diabetic retinopathy and previous ulcers and have a significantly greater history of macrovascular disease (both ischaemic heart disease and cerebrovascular disease).
Description of the patients treated in our Diabetic Foot Unit.
| Variable | Total | Patients with peripheral pulse | Patients without peripheral pulse | p-Value |
|---|---|---|---|---|
| Age (years) | 66 (57–78) | 60 (54–67) | 64 (75.7–81) | 0.000 |
| Type 2 diabetes | 246 (83.1%) | 118 (87.4%) | 128 (92.8%) | 0.091 |
| HbA1c (%) | 7.5 (6.5–9.07) | 7.6 (6.7–9.52) | 6.9 (5.6–8.05) | 0.226 |
| Arterial hypertension | 202 (68.2%) | 88 (65.2%) | 114 (82.6%) | 0.001 |
| Dyslipaemia | 185 (62.5%) | 78 (57.8%) | 107 (77.5%) | 0.000 |
| Smoking history | 146 (53.4%) | 83 (61.4%) | 63 (45.6%) | 0.117 |
| Diabetic nephropathy | 95 (34.8%) | 47 (34.8%) | 48 (34.8%) | 0.996 |
| Diabetic retinopathy | 108 (36.5%) | 64 (47.4%) | 44 (31.9%) | 0.009 |
| History of previous ulcers | 108 (36.5%) | 71 (52.6%) | 37 (26.8%) | 0.000 |
| Ischaemic heart disease | 51 (17.2%) | 11 (8.1%) | 40 (29%) | 0.000 |
| Cerebrovascular disease | 34 (12.4%) | 9 (6.7%) | 25 (18.1%) | 0.004 |
The variables in which significant differences were observed between the group of patients with and without peripheral pulse are bolded.
Fig. 1 describes patient distribution according to their score on the Texas scale at their first visit to our Diabetic Foot Unit. When the difference in their distributions were analysed, statistically significant differences were found with a p-value=0.000. Patients without peripheral pulse obtained scores that reflected a greater initial severity according to this scale.
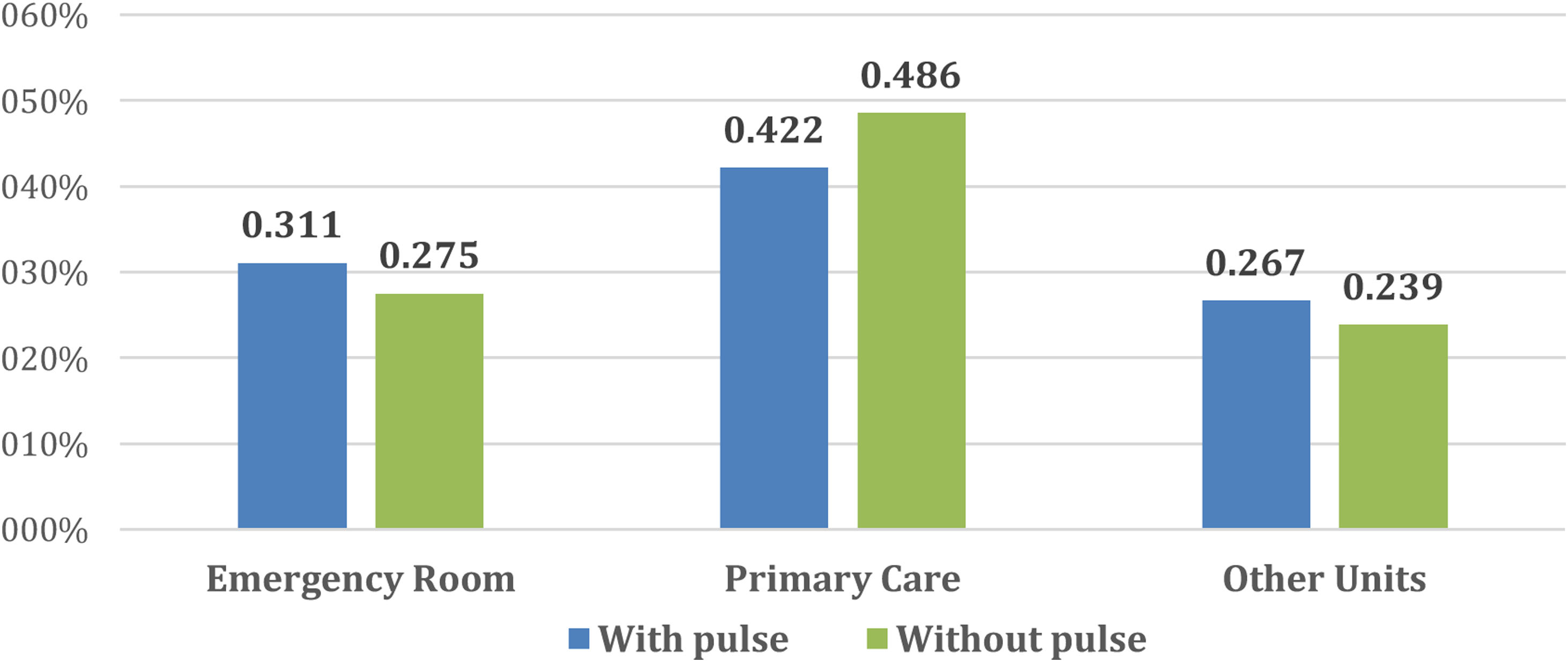
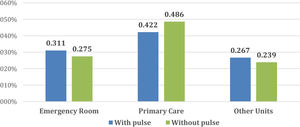
Fig. 2 describes the medical units from which patients were referred to our Diabetic Foot Unit. In both cases, the units that referred most patients were the Emergency Rooms and Primary Care. The rest were referred by Endocrinology, General Surgery and Vascular Surgery and to a lesser extent other services such as Infectious Diseases, Orthopaedics, Internal Medicine, Dermatology and Nephrology.
Description of health outcomesFor health outcomes, Table 2 shows the results of some of the variables used for the description of the total patients included in the study and in each population, in addition to the p-value obtained when the results for both populations were statistically compared. The results show that patients with peripheral pulse have significantly longer episodes and visit our Day Hospital more often. Significant differences were also found in the proportion of referrals to the Rehabilitation unit, which was higher in patients with peripheral pulse. No differences were found in the need for hospital admission.
Variables analysing health outcomes obtained in the overall sample and in both populations.
| Variable | Total | Diabetic foot with peripheral pulse | Diabetic foot without peripheral pulse | p-Value |
|---|---|---|---|---|
| Episode duration (weeks) | 0 (0–3) | 4 (0–10) | 0 (0–3) | 0.000 |
| Visits during episode | 2 (1–4) | 3 (1–6) | 1 (1–2) | 0.000 |
| Need for antibiotic therapy | 158 (53.4%) | 87 (64.4%) | 71 (51.4%) | 0.03 |
| Need for iv antibiotic therapy | 82 (27.7%) | 38 (28.1%) | 44 (31.9%) | 0.501 |
| Need for hospital admission | 87 (29.4%) | 38 (28.1%) | 49 (35.5%) | 0.192 |
| Length of hospital stay (days) | 5 (3–8) | 4.5 (2–7) | 5 (3–9) | 0.329 |
| Need for amputation | 74 (27%) | 31 (23%) | 43 (31.2%) | 0.065 |
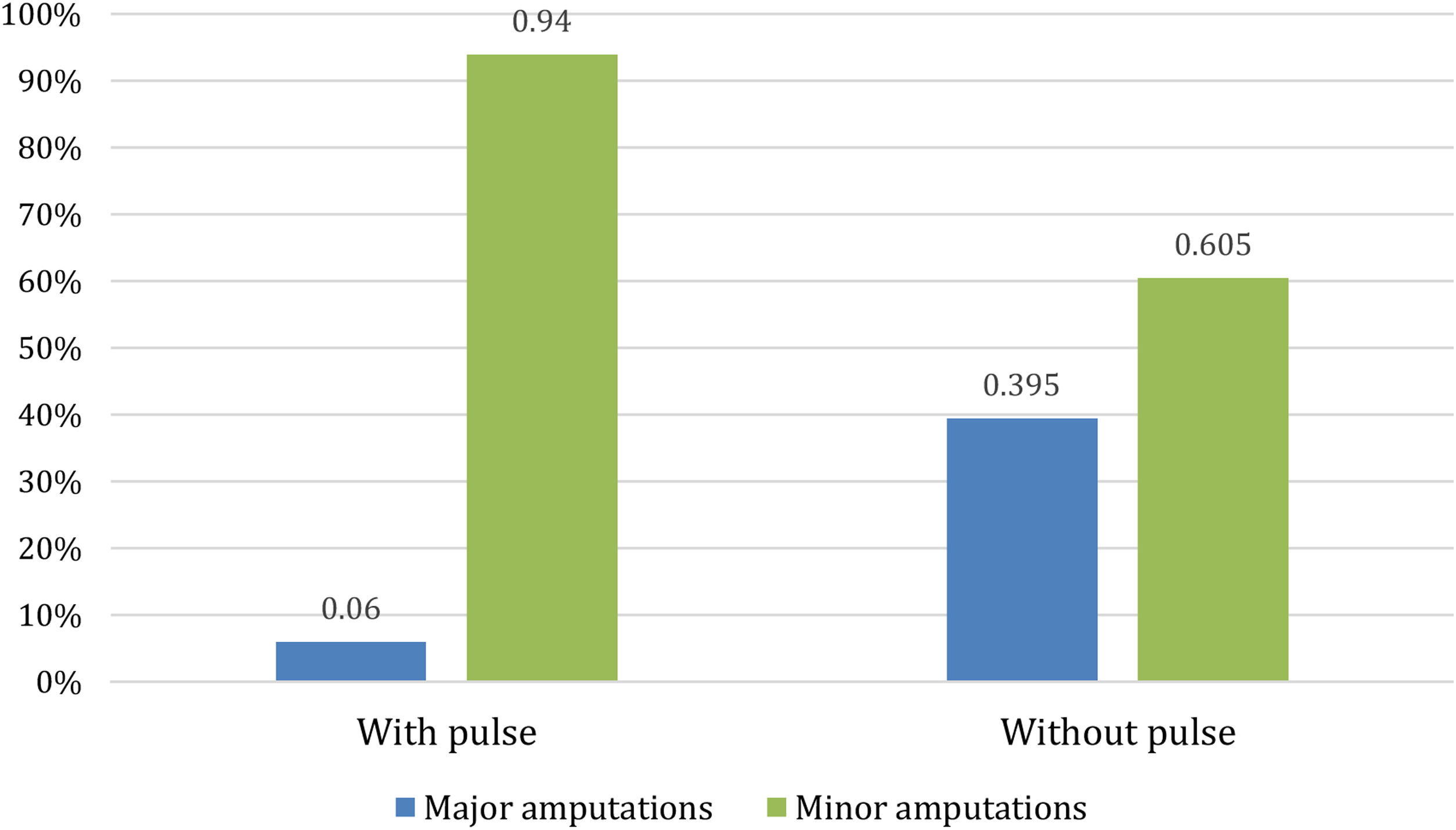
| Major amputation | 19 (6.9%) | 2 (1.4%) | 17 (12.3%) | 0.001 |
| Minor amputation | 55 (20.1%) | 29 (21.4%) | 26 (18.8%) | |
| Need for revascularization | 28 (20.3%) | |||
| Referral to Rehabilitation | 70 (25.6%) | 47 (34.8%) | 23 (16.7%) | 0.001 |
The variables in which significant differences were observed between patients with and without peripheral pulse are bolded.
As can be seen in Table 2, a significantly greater proportion of patients with presence of peripheral pulse required antibiotic therapy than those without peripheral pulse, although this difference was not observed when the use of intravenous antibiotic therapy was analysed. Among the total number of patients who required antibiotic therapy (158), the most prescribed antibiotic in both populations was amoxicillin-clavulanate, followed by ciprofloxacin. Other antibiotics prescribed were clindamycin, cefadroxil, cotrimoxazole, cloxacillin, levofloxacin, linezolid, vancomycin, ceftriaxone, metronidazole and piperacillin-tazobactam.
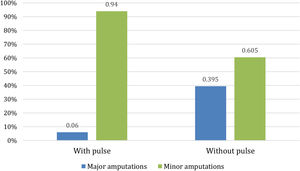
With regard to amputations, Table 2 shows that there were no significant differences in the percentage of patients who eventually had an amputation between the two populations, although the p-value of 0.065 approached significance. However, there were differences when an analysis of whether patients underwent minor or major amputations was performed. As Fig. 3 shows, for patients with peripheral pulse, 31 (23%) required amputation, of whom only 2 (6%) required a major amputation. For patients without peripheral pulse, 43 (31.2%) required amputation, 17 (39.5%) of them being major amputations.
DiscussionIn summary, the objective of our study was to analyse the main characteristics of patients and the health outcomes and to evaluate the impact of PAD in patients with active ulcer treated in our multidisciplinary Diabetic Foot Unit. In this last aspect, in our sample, the patients with PAD ulcers were older and had a higher macrovascular burden. Their Texas Score was higher and their major amputations rate was higher. Finally, they had less history of previous ulcers, their episode duration was lower and their proportional need for antibiotic therapy was also lower.
In terms of the characteristics of the patients treated in our unit, the majority were men. This concurs with the scientific literature, in which male sex is described as a risk factor for diabetic foot and also for a greater recurrence of ulcers.19,20 For patients with type 2 diabetes, in roughly 90% of the patients in both of the populations in this study, the median patient age was 66 and the median HbA1c was 7.5%. These results are similar to those reported by Jiménez et al.21 from a multidisciplinary diabetic foot care unit in another hospital in Spain, in which 92.1% of patients had type 2 diabetes with a median age of 69.5 and a median HbA1c of 7.9%.
Jiménez et al.21 also obtained similar data from patients with a history of smoking and cerebrovascular disease, although the proportion of patients with arterial hypertension, microvascular diabetic complications and ischaemic heart disease was higher in that study. It should be noted that while the number of patients included in the study by Jiménez et al. was similar, patients were recruited for 6 years with a maximum follow-up of 8.1 years, which might explain the higher number of complications, as the follow-up time is longer. By contrast, our unit recruited the same number of patients in 14 months. Finally, in this previous study, 45% of the patients had ulcer recurrence, a higher proportion than the percentage of patients with previous diabetic foot ulcers in our cohort (36.5%), which is also plausible given the longer follow-up time. In any event, these data highlight the critical need for greater monitoring and prevention of recurrence in this pathology.
There were differences between the characteristics of the two populations in our study: the patients with PAD were older, which could account for their higher proportion of arterial hypertension and dyslipidaemia. In addition, they presented with a higher proportion of macrovascular diabetic complications, both ischaemic heart disease and cerebrovascular disease, which is consistent with the presence of arteriopathy, another macrovascular diabetic complication. On the other hand, the difference in favour of a smaller number of patients with previous ulcers among patients with arterial disease is noteworthy and is in line with the results of several studies in which PAD has not been correlated with a higher risk of reulceration.21,22 All these differences were significant when they were analysed using statistical methods.
With regard to the description of health outcomes, one question of note is the shorter duration of episodes in patients with vascular disease, as well as the lower number of visits by them. This suggests a different performance of neuropathic ulcers in our sample, which present more infectious complications and have a more torpid resolution (they require more complex antibiotic management and there is a frequent presence of chronic osteomyelitis, including neuropathy-associated bone and deformational lesions – including Charcot's foot –) although the vascular ulcer is more severe and requires a more frequent surgical resolution (either by revascularisation or amputation), albeit with a faster evolution and fewer infectious complications. This result differs from the reports of the EURODIALE23 study and may also be due to the greater number of very elderly patients in this population and for whom a conservative management plan was prescribed in their Primary Care clinic. The difference in neuropathic and vascular ulcer performance also explains why the percentage of patients who received antibiotic therapy (essentially orally, as there were no differences in the intravenous route used in patients with greater severity) is higher in the group of patients with neuropathic involvement, as well as the higher percentage of patients who were referred to the Rehabilitation unit in this same group. The results that can be explained by PAD are the greater severity of ulcers according to the Texas scale, the trend towards a greater number of amputations and the greater number of major amputations, since, as was already commented, the literature reports a greater risk of greater severity of ulcers and major amputations in patients with vascular involvement.11–13,24 It would also be interesting, after a longer-term follow-up, to evaluate mortality in both groups to establish whether there are significant differences. In this regard, the EURODIALE study produced mortality data for about 9% of patients with PAD versus 3% mortality in those without PAD.25
Despite the emergence of data in favour of the creation of such multidisciplinary teams in diabetic foot care, at this moment in time few centres have actually set them up, especially if we take into account the fact vascular surgeons also need to treat arteriopathic ulcers, with regard to which published data seem to indicate a higher risk of complications.11–13,26 For the latter, we consider that our study is relevant, since it compares the outcomes in patients with diabetic foot with and without PAD, an issue that may be important in the organisation and management of multidisciplinary units such as ours.
The main limitation of our study is the diagnosis of PAD based solely on the absence of distal pulses, since the sensitivity of the examination for the detection of PAD is low and lower than ABI, which was only performed in patients whose classification was doubtful. In addition, it would have been more recommendable to use the SINBAD classification and the IDSA/IWGDF infection classification.27 This is further supported by our study's outcomes: the Texas scale at the first visit classified, as grades A or B, several patients who were subsequently reclassified as patients with PAD when their peripheral pulses were assessed or ABI if doubts. This should lead us to rethink the use of this scale in a Diabetic Foot Unit like ours. Another possible limitation of our study when the results are interpreted is the difference in the operating time of both work teams (with general surgeons and with vascular surgeons), since the team that treats diabetic foot without arterial disease has been operating for 7 years, while the office for diabetic foot with PAD in the Diabetes Day Hospital was launched when the data collection process was initiated. Finally, including other variables, such as the presence of osteomyelitis, the degree of chronic kidney disease or mortality, would have been interesting.
ConclusionsIn our experience, patients with diabetic foot and peripheral artery disease are older and have a higher macrovascular burden than patients with diabetic foot without vascular involvement. In addition, this type of patient exhibits a greater tendency to need amputation, particularly major amputation, which implies a higher social health cost and underlines the need to place greater emphasis on the prevention of diabetic foot ulcers in such patients. The differences found in our study between ulcers with and without vascular involvement support the need for a different approach and for the inclusion of vascular surgeons in the team, as envisioned in the current clinical guidelines.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Conflict of interestsThe authors declare that they have no conflict of interest.