Pituitary neuroendocrine tumours (PitNETs) constitute a heterogeneous group of tumours with a gradually increasing incidence, partly accounted for by more sensitive imaging techniques and more extensive experience in neuroradiology in this regard. Although most PitNETs are indolent, some exhibit aggressive behaviour, and recurrence may be seen after surgical removal. The changes introduced in the WHO classification in 2017 and terminological debates in relation to neuroendocrine tumours warrant an update of the guidelines for the diagnosis, preoperative and postoperative management, and follow-up of response to treatment of PitNETs. This multidisciplinary document, an initiative of the Neuroendocrinology area of the Sociedad Española de Endocrinología y Nutrición [Spanish Society of Endocrinology and Nutrition] (SEEN), focuses on neuroimaging studies for the diagnosis, prognosis and follow-up of PitNETs. The basic requirements and elements that should be covered by magnetic resonance imaging are described, and a minimum radiology report to aid clinicians in treatment decision-making is proposed. This work supplements the consensus between the Neuroendocrinology area of the SEEN and the Sociedad Española de Anatomía Patológica [Spanish Society of Pathology] (SEAP) for the pathological study of PitNETs.
Los tumores neuroendocrinos hipofisarios (TNEH) constituyen un grupo heterogéneo de tumores cuya incidencia ha experimentado un aumento progresivo a la que han contribuido técnicas de imagen más sensibles y mayor experiencia en neurorradiología en este ámbito. Aunque la mayoría de los TNEH son indolentes, algunos presentan un comportamiento agresivo, y puede aparecer recurrencia tras la extirpación quirúrgica. Los cambios introducidos por la clasificación de la OMS en 2017 y las controversias terminológicas con relación a los tumores neuroendocrinos hacen necesario actualizar las recomendaciones para el diagnóstico, manejo pre- y postoperatorio y seguimiento de respuesta al tratamiento de los TNEH. Este documento multidisciplinar, iniciativa del área de Neuroendocrinología de la Sociedad Española de Endocrinología y Nutrición (SEEN), se centra en los estudios neurorradiológicos de imagen médica para el diagnóstico, pronóstico y seguimiento de los TNEH. Se describen los requisitos básicos y aspectos que deben cubrir los estudios con resonancia magnética nuclear, y se propone un informe mínimo de radiología que ayude al clínico en sus decisiones terapéuticas. Este trabajo complementa así el consenso entre el Área de Neuroendocrinología de la SEEN y de la Sociedad Española de Anatomía Patológica (SEAP) para el estudio anatomopatológico de los TNEH.
Pituitary neuroendocrine tumours (PitNETs) constitute a heterogeneous group of tumours in radiological, histological and clinical terms1. In recent years, there has been a progressive increase in their incidence, justified by an increase in experience in expert neuroradiological examination and by the development of more sensitive imaging techniques2,3. PitNETs constitute 15–25% of intracranial neoplasms1,4–7; the most common are adenohypophyseal (85–90% of cases). However, in the sellar region, it is also possible to find other lesions, such as metastases of multiple origins, mesenchymal, meningeal or neural neoplasms, cysts, and inflammatory processes4–7. It is estimated that 15% of the population would present a tumour found by neuroimaging or pathological anatomy. PitNETs with clinical repercussions represent one out of every 10008–10.
Although most PitNETs are indolent, with slow growth and an optimal response to treatment, some present aggressive behaviour (between 5% and 15% depending on the series11), characterised by local invasion, resistance to treatment and, very rarely, the appearance of metastases12. In addition, in some patients, despite surgical removal, recurrence occurs13. For all these reasons, the terminology used to define this group of tumours has been the subject of debate. Since 2016, the term PitNET has replaced the traditional concept of pituitary adenoma1. Regarding their classification, the most recent, carried out by the WHO in 2017, groups PitNETs according to the profile of pituitary hormones and transcription factors they express6. This classification has facilitated a different approach to their pathological diagnosis14,15. It has enabled a more precise classification of tumours, drastically reducing the percentage of null ones16–18.
The recent changes introduced in the WHO classification in 20176 and the terminological debate in relation to neuroendocrine tumours make it necessary to review the guidelines for the diagnosis, preoperative and postoperative management, and follow-up of response to treatment of PitNETs. Recently, a consensus has been drawn up between the Neuroendocrinology Knowledge Area of the Sociedad Española de Endocrinología y Nutrición (SEEN) [Spanish Society of Endocrinology and Nutrition] and the Sociedad Española de Anatomía Patológica (SEAP) [Spanish Society of Pathological Anatomy] for the anatomopathological study of PitNETs15. In a complementary manner, this multidisciplinary document, an initiative of the Neuroendocrinology area of the SEEN, focuses on neuroradiological imaging studies for the diagnosis, prognosis and follow-up of PitNETs.
Magnetic resonance imaging (MRI) as the imaging test of choice in the study and follow-up of PitNETsCurrently, MRI is considered the reference test in the imaging diagnosis of diseases of the hypothalamic-pituitary region8,19–21. With its higher contrast resolution and the possibility of obtaining multiplanar images, it enables the identification of normal structures, diagnosis of lesions, planning of surgical treatment, monitoring of pituitary disease and evaluation of response to treatment8.
Indications for MRI in the diagnosis of PitNETsMRI is indicated for diagnosing various conditions where an anatomical or functional alteration of the pituitary gland is suspected. These include hyper- or hypofunction of the adenohypophysis, dysfunction of the neurohypophysis, suspected compression of adjacent structures in the sellar region, the appearance of temporary visual field defects, or paralysis associated with cranial nerves III, IV, and VI.
Indications for other medical imaging techniquesComputed axial tomography (CT)CT was the technique used to study the pituitary region before the development of MRI, but the latter has displaced it due to its greater capacity for contrast resolution, avoiding the presence of beam hardening artifacts in the region of the sella turcica and exposure to ionising radiation from CT19. Therefore, CT is currently used for primary diagnosis only when MRI is contraindicated (e.g. some patients with pacemakers, ocular metallic foreign bodies, cochlear implants or obesity that technically precludes the use of MRI) and as a complement to MRI for the differential diagnosis of meningiomas, craniopharyngiomas and germinomas, since it allows the identification of calcifications or ossifications characteristic of these tumours. CT is also used to assess the bony integrity of the skull base and for surgical planning.
Positron emission tomography (PET) with 11C-methionine11C-methionine PET/CT (MET-PET/CT) is a sensitive and complementary technique to MRI to detect pituitary adenomas or residual tissue after pituitary surgery. The main advantage of MET-PET/CT over 18F-fluorodeoxyglucose (FDG) lies in its better pituitary/brain uptake ratio22.
This modality has greater sensitivity, especially in recurrent microadenomas22. It can provide information when MRI or FDG are negative, especially in the case of microadenomas23,24. Its detection rate is higher than that of MRI in certain PitNETs25, and it is also useful for assessing the response to treatment with somatostatin analogues in these tumours23.
Petrosal sinus catheterisationPetrosal sinus catheterisation is used in the diagnosis of Cushing's disease when the MRI image is inconclusive or in adenomas with a diameter of less than 6 mm26,27. Although it reaches sensitivities of over 90% to establish the pituitary origin of the disease, it is an invasive technique. It is not as specific in demonstrating the lateralisation of the tumour in one half or the other of the adenohypophysis28.
MRI requirements for the diagnosis and follow-up of PitNETsConsiderations on the power of MRI equipmentThe pituitary gland does not usually measure more than 8 mm in length, and microadenomas can be very small, reaching no more than a millimetre in diameter. Therefore, it is essential that the images have good spatial resolution and optimal contrast resolution, that is, that they have an adequate signal-to-noise and contrast-to-signal ratio, respectively.
The first consideration when it comes to performing an MRI study of the pituitary on a 1.5 T or a 3 T scanner is the availability of each centre. Although there has been an increase in 3 T teams in recent years, they are still a minority, so indications for these scans must be carefully defined. Using equipment with a larger field may be useful if there is suspicion of a microadenoma, and the 1.5 T scanner does not exclude this possibility29. It is also useful for more accurately assessing subtle differences between normal and abnormal tissue, predicting invasion of adjacent structures and evaluating the pituitary stalk29,30. However, high-field equipment poses technical difficulties, as it is more sensitive to artifacts, especially susceptibility artifacts, so they must be appropriately adjusted, and they pose greater limitations in some patients.
Use of contrast in MRIGadolinium contrast is used for MRI, both in the diagnosis and follow-up of pituitary lesions. Gadolinium contrast is used for MRI in the diagnosis and follow-up of pituitary lesions. For this reason, official reports have been issued on its use and restrictions31.
The clinical practice guidelines for PitNETs recommend a neuroradiological follow-up ranging from 3 to 12 months for aggressive tumours8. Still, they have not taken a position concerning non-aggressive ones. Clinicians need to be aware of the need to minimise patient exposure to gadolinium contrast32, adapt the interval between studies to each clinical situation, and avoid using contrast when it is unnecessary20,33.
Basic MRI protocol for the exploration of the neurohypophysis and the study of PitNETsThe pituitary MRI protocol can be divided into three parts21,34, described in Table 1.
Basic MRI protocol for the examination of neurohypophysis and study of PitNETs.
| Study | Acquisition | Methodology | Objective |
|---|---|---|---|
| Morphological study | Routinely, sagittal and coronal plane sequences are performed, and very occasionally axial ones, enhanced on T1, without and with contrast, and on T2. | Fine slice sequence with high spatial resolution and a good signal-to-noise ratio.The cut should be fine, with a thickness between 2 and 3 mm. Can be reduced to 1 mm or 1.5 mm on 3 T equipment.Small field of view, between 140 and 160 mm. | Identify normal or pathological structures, their relationship with neighbouring structures, and determine whether they are solid, cystic, with a fatty, haemorrhagic or protein component.Distinguish areas with a high content of free water because they are hyperintense (ventricles, basal cisterns or cystic lesions).Delimit the intracranial vessels (carotids or the circle of Willis), hypointense due to the rapid flow of blood.Identify haemorrhagic content (e.g. hypointense signal on T2 in the pituitary).Very useful if the use of gadolinium contrast is contraindicated. |
| Dynamic study | Series of coronal T1-weighted images during the arterial phase of gadolinium administration. | A fast repetitive scan (low resolution) is performed, with coronal sequences in 10 min cycles.During the first sequence, a bolus of 10 ml at 2.5 ml/s + bolus of 20 ml of physiological serum is administered.The successive sequences make it possible to check how the signal intensity of the gland increases over time. | Identify a microadenoma by its slower enhancement than the rest of the pituitary tissue. The pituitary gland and pituitary stalk lack a blood-brain barrier, resulting in rapid and progressive enhancement that begins at the top of the gland and spreads to the rest. |
| Post-contrast study | Sagittal and coronal T1-weighted spin echo slices. | Observe the normal homogeneous enhancement that should appear in the late phase after administering gadolinium. |
An initial MRI scan report for suspected PitNETs should include all relevant information to define the lesion. Table 2 is a proposed basic report based on the following points.
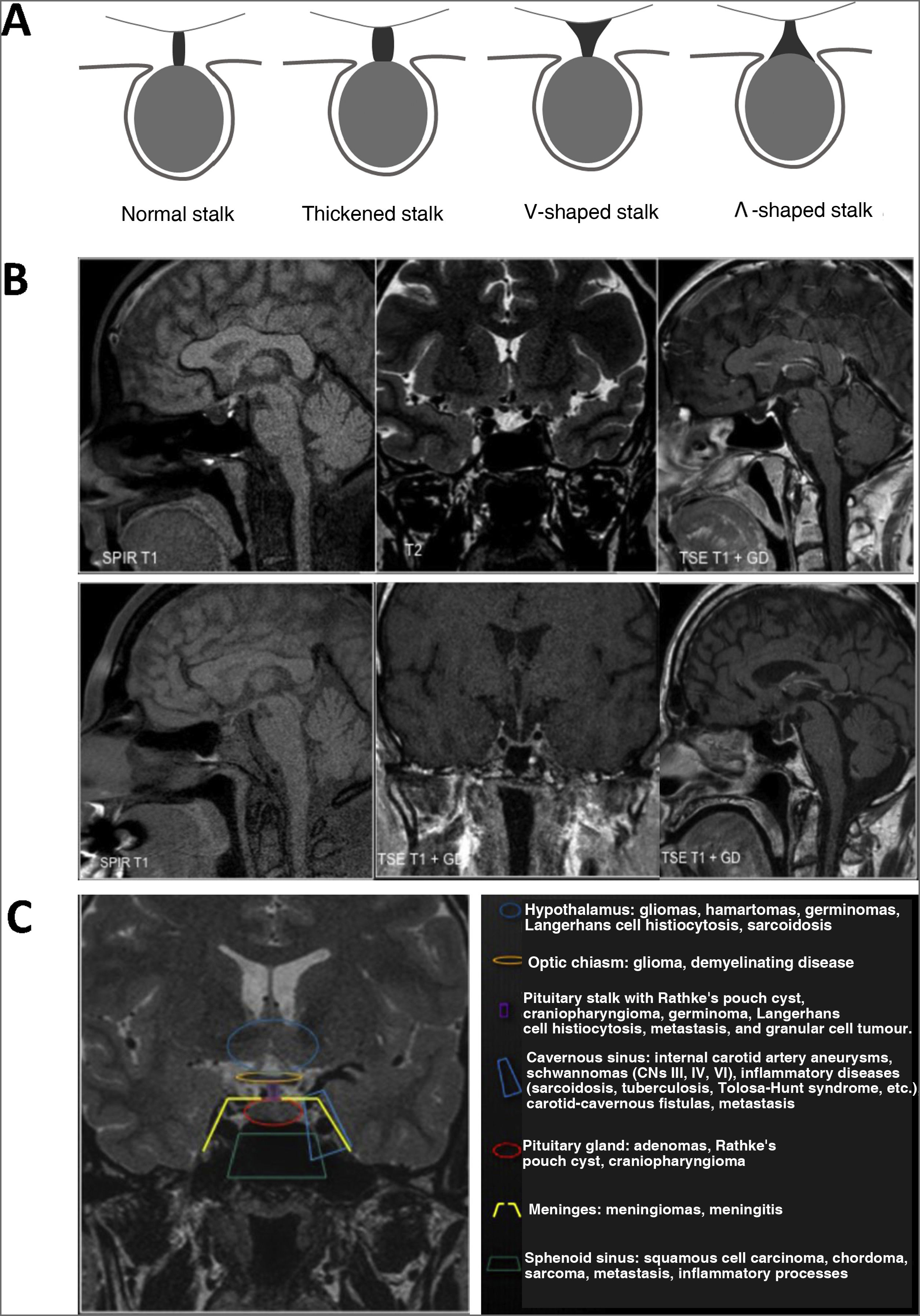
The first objective is to determine whether or not there is an alteration in the normal anatomy of the pituitary gland. In this way, the following should be taken into account: the volume and morphology of the sella, the size, signal and shape of the gland, the suprasellar content (stalk [Fig. 1A], chiasm and floor of the third ventricle) and the cavernous sinuses.
Morphological alterations. A) Stalk morphologies. B) Upper panels: ectopic neurohypophysis and hypoplasia of the stalk and adenohypophysis; lower left panel: diabetes insipidus (absence of normal hypersignal on T1 of the neurohypophysis). Lower centre and right panels: empty sella turcica (coronal and sagittal section). C) Classification of sellar and parasellar lesions according to their origin.
It should be remembered that the pituitary size is greater in neonates and women, especially in the pubertal period or during pregnancy and postpartum, where the shape of the pituitary with a convex superior border should not be confused with a pituitary adenoma. In some cases, primary glandular deficiencies can also be associated with physiological growth of the pituitary gland, for example, in the case of primary hypothyroidism35. Therefore, when faced with an anatomical alteration, it must be evaluated whether it is a normal variant or a lesion36 (Fig. 1B).
Origin of the pituitary lesionFor the differential diagnosis, it is necessary to determine the origin of the pituitary lesion, for which the guidelines shown in Fig. 1C can be followed.
Characterisation of the lesionTo characterise the lesions, at least the morphological sequences enhanced on T1 and T2 are required. The signal characteristics will be described in the different enhancements of the pituitary lesion compared with the healthy gland or the adjacent temporal lobe. These characteristics allow a certain approximation to the histological/tissue characterisation of the lesions to differentiate solid lesions from cystic and mixed lesions while also attempting to know the content of cystic lesions (protein, haemorrhagic, cholesterol, etc.). In this sense, contrast uptake is also used for characterisation.
Dynamic T1 imaging, or dynamic contrast-enhanced MR imaging (DCE-MRI), is used to assess vascularisation and the degree of tissue permeability. In pituitary disease, it has been used to differentiate normal pituitary tissue from pituitary adenomas. As pituitary tissue is more vascularised than adenomas, normal pituitary tissue uptake curves over time show a higher and faster enhancement peak with a steeper slope than adenomas37 (Table 1).
Including coronal sequences on T2 can help identify microprolactinomas (hyperintense on T2), avoiding the use of contrast. Also, silent corticotroph adenomas may have a microcystic pattern on T2 and their identification is important because they are more aggressive. Finally, in acromegaly, it offers information to predict the response to analogues33.
Size of the lesionThe size must be defined by measuring the three axes of the space (anteroposterior [A], craniocaudal [B] and transverse [C]), and this information at least must be included in the report. The geometric formula can be used⠀to calculate the volume Vol=A×B×C2, with the limitation that we assume the lesion to be spherical38. A 3D volumetric analysis can also be carried out with specific software, and although it is a more laborious option, it allows a better definition of irregular or lobulated adenomas. However, although this last approach is more accurate and would be of special interest in post-surgical follow-up, it is not yet routinely applied in clinical practice and is used more in research studies. Both methods have been compared38 and are limited by the intra- and interobserver variability in the delimitation of the lesions, especially when the difference in intensity of the adenomas concerning the normal parenchyma is scarce.
In addition, it is important always to use the same volume calculation method to estimate evolutionary changes correctly.
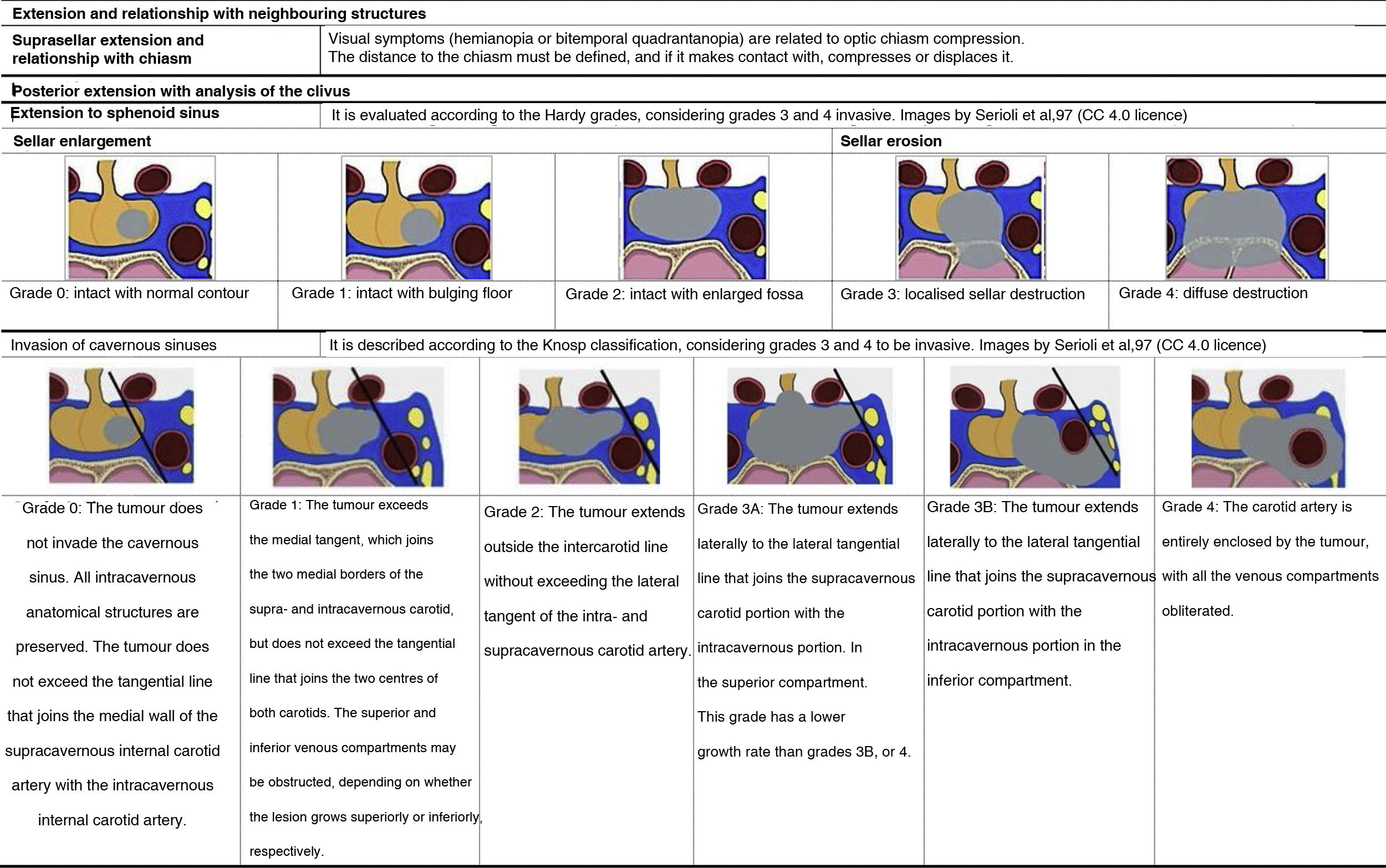
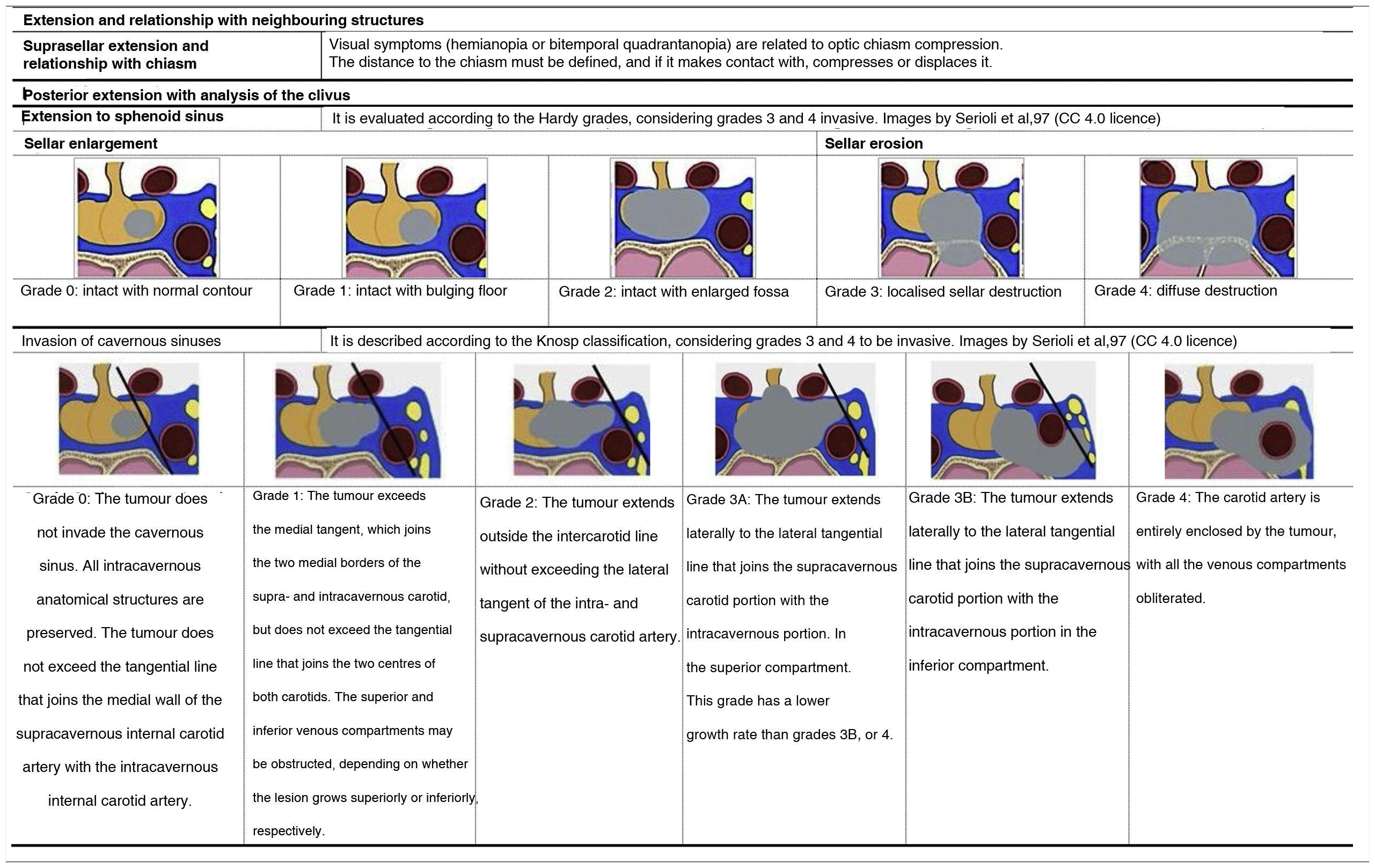
Extension and relationship with neighbouring structuresThe report must describe the extent of the injury and its relationship with neighbouring structures, considering the established classifications summarised in Table 3.
Comparison with previous MRI studiesIf it is not the first diagnostic study, the study should be obtained using the same protocol and configuration as the original or the previous ones19. Ideally, it will be performed on the same MRI equipment, with the images stored in the centre's picture archiving and communication system (PACS). The report should compare previous studies and put the current study in context19,21,39.
- –
Clinical context of the case: initial diagnosis and treatment performed.
- –
Comparison with previous radiological studies, both with the diagnostic and follow-up studies.
- –
If surgery has been carried out, consider the changes inherent to the intervention, both in the immediate postoperative period and in subsequent monitoring.
- –
Identify possible tumour remnants or recurrence (tissue that behaves like the initial lesion in the diagnostic study).
- –
Describe possible complications: cerebrospinal fluid leaks, haemorrhages, etc.
If an aggressive PitNET is suspected, a correct staging of the disease should be performed. It is necessary to investigate the possible presence of metastases when faced with data such as accelerated growth, invasive tumour, and growth despite adequate treatment and lack of consistency between symptoms, biochemical data and radiological findings40. The main locations of metastases are the spinal cord, cervical lymph node chains and, less frequently, the liver, bone and lung. For this study, the imaging options are full-body PET and CT. FDG-PET has greater sensitivity in staging various oncological processes compared to full-body CT. Aggressive PitNETs have high avidity for FDG, so they should be considered an imaging technique for remote disease screening. In the case of confirmed metastases, PET with 68Ga-DOTATATE can be useful to assess the expressivity of somatostatin receptors41 and to evaluate alternative treatment with analogues labelled with 177Lu42.
Imaging tests in the surgical planning for PitNETsPresurgical evaluationIn this context, the purposes of the neuroradiological study of pituitary adenomas are those already described: to identify the lesion and define the tumour's spatial relationships with the surrounding structures. Among these, the following stand out: the effect on the optic nerves/chiasm, the extension in the cavernous sinuses and its relationship with the adjacent internal carotid artery, the parasellar extension and the invasion of the base of the skull/foramina ovale, clinoid process, planum sphenoidale, clivus and sphenoid sinuses. In addition to the basic MRI study described above (Table 2), it is useful to have complementary sequences such as MR angiography43,44, especially when suspecting a possible vascular nature of the sellar lesion or stenosis of the adjacent internal carotid artery.
MRI data for surgical planning for microadenomasPituitary microadenomas are the most common intrasellar neoplasms, and their diagnosis is based on the presence of indirect and direct findings. The indirect findings are: a) lateral displacement of the pituitary stalk; b) morphological alteration of the upper contour (sellar diaphragm), and c) irregularity or focal convexity of the sellar floor. The direct findings identify round or oval lesions, with a different signal from the rest of the glandular parenchyma in baseline sequences or with less contrast uptake, compared to the rest of the adenohypophysis. Microadenomas may show hyperintensity on T1-weighted images due to increased protein content or haemorrhage from part or all of the lesion.
In the case of microadenomas, it is important to determine the lateralisation, that is, the precise location in one of the two halves of the anterior lobe, if the aim is to preserve the healthy pituitary half to maintain functionality.
MRI data for surgical planning for macroadenomasPituitary macroadenomas usually extend outside the sella turcica. For planning, it is necessary to clarify the structure of the lesion, its consistency (firm, cystic, necrotic or haemorrhagic) and its relation with the surrounding anatomical structures. The tumour usually extends cranially, tending to compress the optic chiasm; it may remain subdiaphragmatic or rupture the sellar diaphragm. The position of the adenoma should be described when it extends into the suprasellar region. The adenoma may extend downward into the sphenoid sinus or laterally into the cavernous sinus45. It is of utmost importance to assess whether the cavernous sinus is compressed or invaded (Table 3) or if there is an invasion of the third ventricle and to plan the approach (either transsphenoidal or transcranial). Likewise, the neurosurgeon needs to know a priori if there is an invasion of the adventitia of the adjacent internal carotid artery because, in this case, if the resection is aggressive, it can tear the carotid artery and cause massive arterial bleeding.
One common way to approach it surgically is by nasal endoscopy. Therefore, it is convenient to describe the position according to four regions established by two virtual planes: one passes through the inferior surface of the chiasm and the mammillary bodies and the other through the posterior margin of the chiasm and the dorsum sellae. The resulting regions are the suprachiasmatic, the subchiasmatic, the retrosellar and the ventricular.
Usefulness of CT in surgical planningIn selected cases, CT will provide more detail about the presence of calcified components of the lesion and achieve a precise definition of the bone boundaries. If the surgical approach chosen is the transsphenoidal one, the CT will offer relevant information on the degree of pneumatisation of the sphenoid sinus; if it is transcranial (e.g. supraorbital), we will obtain information on the degree of pneumatisation of the frontal sinus. In addition, CT allows a detailed overview of the bone map of the nasal passages, paranasal sinuses and skull base. It allows the study of the anatomical landmarks referenced in nasosinusal endoscopic surgery (nasal turbinates, uncinate processes, etc.) and the degree of pneumatisation and integrity and positioning of the bony walls of the sphenoid and frontal sinuses.
Prediction of post-surgical remissionThe key factor for predicting postoperative remission is the degree of invasion of the cavernous sinus46,47. The importance of the differentiation between grades 3A and 3B within Knosp grade 3 (Table 3) in predicting the success of surgery has recently been described, being significantly less in grade 3B47–49. With this objective of predicting post-surgical remission and recurrence, diffusion-weighted imaging (DWI) sequences and the apparent diffusion coefficient (ADC) could be useful50.
Intraoperative assessment by MRIThe use of intraoperative MRI (iMRI) has been described since 199451. However, its implementation in clinical practice is still very low, and the literature on the matter is controversial. In a recent review52 on iMRI modalities (low field, high field and neuronavigation), advantages are cited, such as better visualisation of the cavernous sinus, real-time information for the surgeon, and therefore the possibility of increasing the rate of complete tumour resection and preservation of healthy gland, among other things. On the other hand, the disadvantages are the duration of the surgery and the increase in costs.
Post-surgical evaluation by MRIThere is no consensus regarding the optimal time to perform the post-surgical MRI study53,54. Immediate post-surgical MRI (within the first week after surgery) detect tumour remnants during evolutionary follow-up, serving as the basis for subsequent follow-up MRI scans55–57. It is useful to diagnose possible complications and to assess the normal gland and the degree of resection of the lesion21,58 (Fig. 2, upper panel). The following can be taken into account for reference:
- –
If the signal intensity and contrast uptake are similar to those of the lesion in the pre-surgical study, said tissue is suspected of being a tumour remnant.
- –
When contrast enhancement is linear and peripheral, it is more likely to correspond to post-surgical changes59. Knowledge of the radiological characteristics of the materials implanted in the sellar region is also very important. Some materials, such as fat, can be identified up to years after surgery, whereas others, such as haemostatic materials, only be for a few weeks after surgery. On the other hand, it is important to bear in mind that the disposition and characteristics of the fat used to cover the surgical defect of transsphenoidal surgery vary between the study immediately after surgery and the subsequent follow-up studies.
- –
In the dynamic study, identifying the suspected tumour remnant with an uptake thickness >3.9 mm is related to tumour remnants with a sensitivity of 89% and specificity of 97%60.
Upper: evolution of pituitary adenoma four °months after surgery. Lower: radiological follow-up with MRI for non-functioning PitNETs.
Translated and adapted from Cortet-Rudelli et al.55, 2015.
In late postoperative follow-up, and especially in non-functioning PitNETs, MRI is usually performed between the third and sixth month after the intervention21,55. Although in the immediate post-surgical follow-up scan, it is possible to get an indication of whether partial or complete resection has been achieved, it is not until 4–6 months after surgery that the materials of the surgical site are reabsorbed and that the degree of tumour resection can be clearly assessed. In functioning PitNETs, routine radiological follow-up is unnecessary if hormonal remission is confirmed and complications or recurrence are not suspected. Fig. 2 (lower panel) outlines a post-surgical follow-up protocol for non-functioning PitNETs. The existence of tumour remnants after surgery, especially invading the cavernous sinuses, is the factor that most defines the risk of recurrence, and, after analysing the different series, it is estimated that this recurrence appears in 47% of cases if there are remnants vs 15% if they are none55. The variables related to a lower recurrence rate are older age and a greater extent of tumour resection61.
There are no specific recurrence markers in non-functioning PitNETs. Some 85% of recurrences usually occur in the first five years. Among patients not treated with radiotherapy, 20% present recurrence before five years and more than 50% after 10 years62. Late recurrences are also common, so long-term follow-up with MR imaging should be performed at least until other reliable methods that predict tumour recurrence have been identified62.
Imaging tests in planning radiotherapy treatmentVolumetric T1-weighted sequences with contrast provide precision when outlining the adenomas and the risk organs surrounding them, mainly the chiasm and the optic nerves. In radiotherapy and after surgery that requires abdominal fat grafting, fat suppression sequences can be useful for defining residual adenoma56,63. Reconstructions must be obtained in the axial plane since most radiotherapy planners only use this plane to be able to make a rigid or deformable registration between the planning MRI and the planning CT. The latter will be required in most cases (except Gamma Knife or MR-Linac units) for dose calculation. In patients who cannot undergo an MRI, a CT with slice reconstruction every 1−2 mm and adding iodinated contrast is the test of choice56.
Currently, the use of metabolic imaging in planning radiotherapy for treating pituitary adenoma is experimental, with various techniques under study57,64–66.
Usefulness of MRI in the prognosis of PitNETs and monitoring of response to treatmentMRI in the prognosisThe depth of invasion is a marker of aggressiveness of a PitNET. However, to be considered an aggressive adenoma, it must also present an early recurrence (6–12 months after surgery), rapid tumour growth, and resistance to conventional treatment40,67. In any case, the definition of an aggressive PitNET is achieved in combination with the histopathological diagnosis using proliferation indicators67 and theragnostics68.
Most studies that relate the radiological aspect and the histological and molecular characteristics of aggressiveness have been done concerning acromegaly69–74. According to these relationships, different subtypes of GH-producing PitNETs have been described70. In this pathology, signal hypointensity on T2 has been related to other tumour characteristics, histological (densely granulated pattern) and clinical (such as better response to somatostatin analogues [SSA]) and, therefore, with its prognosis69,71–73. Expression of somatostatin receptor 3 (SSTR3) and dopamine D5 receptor (DRD5) are associated with extrasellar or suprasellar extension. In addition, the expression of DRD5 is greater in hyperintense adenomas on T2, and its expression is directly related to Knosp grades and tumour diameter69. On the other hand, the absence of the normal hypersignal on T1 of the neurohypophysis in the pre-surgical MRI is a predictor of post-surgical diabetes insipidus74.
In a recent meta-analysis that included tumours of various strains, of which 9.5% were neuroendocrine and 2% pituitary, ADC was related to the degree of cell proliferation and histopathological characteristics such as the expression of different receptors, nuclear polymorphism and proliferation potential (especially MIB-1, Ki67), which would help plan the surgical approach and predict the tumour behaviour75. A strong correlation between low ADC values and MIB-1 (Ki67) has been described, demonstrating the potential of diffusion imaging as a biomarker of what was previously known as atypical and proliferative adenomas76.
Morphological criteria to determine the response to radiotherapy treatmentThe parameter that has traditionally provided information on the response to treatment is the size of the lesion77. Since these are slow-growing tumours, a reduction and even stability in size have been considered favourable responses. For this reason, it is important that, in addition to the tumour characteristics, the reduction, stability or increase in volume is also registered. When the patient has received radiotherapy treatment, the increase in signal on T2-weighted imaging is considered a criterion of favourable response to treatment. On the other hand, the loss of signal on T2 (hyposignal) in the follow-up of adenomas should be evaluated with caution, since it may reflect a transformation to a more fibrous or more cellular tumour (malignant degeneration)77.
In adenomas larger than 3 cm, the measurements' differences before and after radiotherapy have been evaluated by calculating the volume using the three diameters. The results support performing 3D volumetric analysis, instead of 2D, for the measurement of large or giant adenomas78. 3D volumetry is more sensitive to changes in size, but obtaining it is more complex79.
Prediction of response to SSA treatment in acromegalyThe hypointensity of PitNETs on T2-weighted sequences has been associated with the presence of a densely granulated pattern80, as well as with a significantly better response to SSA after surgery73. Furthermore, the reduction in GH with treatment is also significantly greater81–83, with a greater reduction in GH production and tumour size the greater the hypointensity detected84. Hypointensity on T2 also correlates with a greater reduction in GH after an octreotide test and a smaller size and invasiveness at diagnosis82,84. However, the reason for the better response to SSA in hypointense adenomas is unknown33.
It is therefore important to establish how to define the intensity on T2 and it can be done in two ways.
- –
Comparing it with the healthy pituitary gland, provided that it can be well discriminated84.
- –
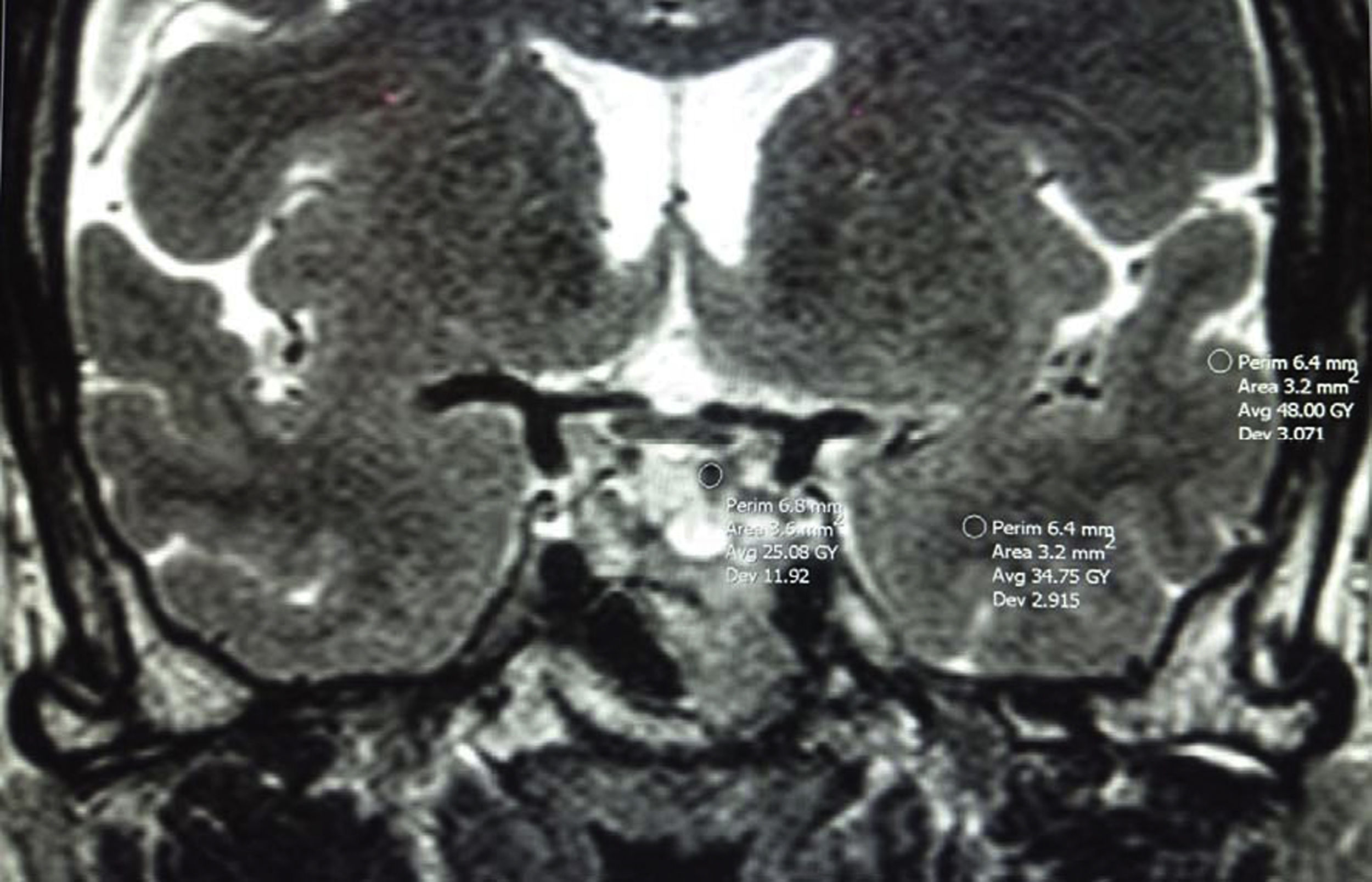
Or comparing it with the adjacent temporal lobe (cerebral white and grey matter [Fig. 3])78,80,84. It will be hypointense when the MRI signal is equal to or less than that of the white matter and less than that of the grey matter, hyperintense when the signal is equal to or greater than that of the grey matter, and isointense when the signal is between those of the white and grey matter. Potorac et al.84 propose that since the characteristics of iso- and hyperintense are similar, they could be grouped into two categories: hypointense and non-hypointense (iso- and hyperintense).
- –
It could be a qualitative or quantitative comparison (using the measurements of the region of interest (ROI) of the PitNET, white and grey matter [Fig. 3]). Methodologically, a good correlation between the use of ROI and direct visual assessment has been described33,84.
Another radiological parameter is the homogeneity/heterogeneity of the tumour. More homogeneous adenomas have higher GH levels at diagnosis, but this does not correlate with reduced GH or the volume or invasiveness of the adenoma82,83.
Prediction of response to treatment with dopamine agonists (DAs) in acromegalyAlthough DAs are indicated in cases with slightly elevated IGF-185, there is no evidence in the literature that the intensity on T2 can predict the response to DAs. However, it is suggested that DAs would be the treatment to consider in the case of poorly granulated tumours, with little expression of SSTR2 and hyperintense on T2 with slightly elevated IGF-185.
Prediction of response to DA treatment in prolactinomasProlactinomas are usually hypointense and occasionally isointense on T1 but rarely hyperintense (haemorrhage or protein content). On T2, 80% of prolactinomas are hyperintense, although with differences according to sex. Heterogeneity on T2 is more common in men with areas of focal necrosis or old haemorrhage, and heterogeneity is associated with poorer response to DAs. In young men, prolactinomas are usually larger and more invasive, have higher PRL levels and have a worse response to DAs than homogeneous adenomas86.
Adenomas hypointense on T2 are also more common in men, possibly due to the deposition of amyloid material in these tumours87–89. It has been suggested that hypointensity on T2 would be related to resistance to DAs, although the evidence is controversial86–88,90.
Regarding the response to treatment with cabergoline, it is essential to standardise the report, stating whether there is a volumetric reduction of the prolactinoma at three months as a criterion of good long-term response91.
It has been described that early changes in intensity on T2 after treatment with cabergoline can predict a favourable response regarding regression and the almost total disappearance of the tumour92.
Cystic prolactinomas, more common in women, secrete less PRL86 and have traditionally been considered to be resistant. However, it has been shown that they can be treated effectively with DAs, normalising PRL in 18/22 cases and a mean volume reduction of 83.5% in 20/22 cases93.
Once controlled PRL levels have been achieved, routine follow-up with MRI is unnecessary94,95.
Future perspectives in pituitary imaging testsNew MRI techniquesIn addition to MR angiography, a sequence already implemented in practice for surgical planning, other sequences with potential utility in studying PitMETs have been investigated. Table 4 summarises the experimental and potential use of said sequences.
MRI sequences that have been applied in the investigation of pituitary disorders.
| MRI sequence | Characteristics | Possible applications |
|---|---|---|
| Magnetic resonance angiography | Provides detailed images of the arterial system | Detection of intrasellar aneurysms43Delimitation of cranial nerves in their cavernous sinus segment44 |
| 3D-GRE/3D-MPGRE | Higher contrast resolution and they are an alternative or an added value to the dynamic study. Volumetric acquisition with 1 mm voxel | Detection of the smallest microadenomas not visible on conventional imaging98–100 |
| FLAIR | An inversion recovery sequence with a long inversion time that removes the signal from the CSF which is, therefore, darker rather than brighter as would usually be seen on T2 sequences | In Cushing's disease, contrast withdrawal delay of a corticotropic microadenoma can be detected as FLAIR hyperintensity101 |
| DWI | Measures the diffusion of water molecules in biological tissues; most commonly used to detect cytotoxic oedema in the setting of acute cerebral ischaemia/infarction | Detection of pituitary apoplexy/acute pituitary infarction102To distinguish intrasellar/suprasellar cystic lesions103To distinguish between adenoma and craniopharyngioma104To obtain consistency information for surgical planning105,106 |
| Diffusion tensor | An extension of DWI that analyses the directional diffusion of water molecules in biological tissue | Tractography of the optic nerve for surgical planning and predicting the probability of visual recovery after transsphenoidal surgery107–109 |
| Apparent diffusion coefficient | A subtype of DWI | Its usefulness in predicting tumour consistency for surgical planning is still debatable110–117Assessment of aggressiveness by determining tumour heterogeneity (recurrence and proliferative potential)110To differentiate persistence vs inflammatory tissue/granulation in adenomas treated with surgery earlier than the morphological image118 |
| Weighted perfusion | Evaluates tissue perfusion at the capillary level | Evaluation of vascularisation of the adenoma before surgery119Identification of other vascular lesions (e.g. meningioma) that may be confused120,121 |
| Magnetic resonance spectroscopy | Provides insight into metabolic function by measuring different metabolites (e.g. N-acetyl aspartate, choline compounds, creatine/phosphocreatine) | Differentiation of adenoma from a normal gland122Differential diagnosis of suprasellar lesions123–126Prediction of the response of somatotropic tumours to therapy with somatostatin analogues127 |
| Magnetic resonance elastography | Measures shear wave propagation through the tissue of interest to estimate stiffness | Surgical planning estimating whether an adenoma is likely to be soft (and suctionable), intermediate or firm (requiring curettage for resection)128,129 |
| Steady-state balanced free precession (e.g. CISS; FIESTA-C) | Image contrast determined by the T2/T1 ratio of the tissue; in practice, very watery (good contrast between CSF and other structures) but also sensitive to post-contrast enhancement | Improved detection of adenoma in Cushing's disease130Better assessment of cavernous sinus invasion130Prediction of adenoma consistency131Improvements in the delineation of the optic nerves and chiasm in large pituitary tumours132 |
3D-GRE/3D-MPGRE: three-dimensional gradient echo sequences/three-dimensional multiplanar gradient echo sequences; DWI: diffusion-weighted imaging; FLAIR: fluid attenuation inversion recovery; CSF: cerebrospinal fluid.
Adapted from Bashari et al.20.
One of the advantages of the metabolic study of the pituitary gland is the variable affinity for the different radiotracers. This allows different studies to be combined to evaluate the pituitary gland and guide the clinician in therapeutic decisions. The merging of PET with MRI makes it possible to combine the sensitivity of metabolic tests with the anatomical resolution of MRI in a single study. Still, this equipment is hardly available in normal clinics due to its novelty and price, so the most common is co-registration software.
The combination of FDG and 68Ga-DOTATE benefits patients with important structural changes after previous interventions66. Under normal conditions, the pituitary gland does not show significant avidity for FDG, but pituitary adenomas do, even if they are benign. In the case of DOTATE, both healthy and pathological pituitary tissue show avidity. The combination of the two studies can make it possible to differentiate pituitary adenoma (FDG+/DOTATE+) and normal pituitary tissue (FDG–/DOTATE+)66. Although semi-quantitative methods (FDG/DOTATE ratio) can differentiate between healthy and pathological tissue, there are currently no standardised values.
Another useful tracer in the assessment of PitNETs is co-registration with methionine (MET). It makes it possible to differentiate active tumours from fibrosis, bleeding, cysts, etc. In addition, its better tumour-to-brain uptake ratio allows a better delineation of lesions and resolution. It has better sensitivity than FDG for detecting residual tumours, especially in patients with recurrent microadenomas22. However, FDG is highly specific; if a study is FDG positive, this patient likely has residual tumour tissue96. In cases in which MRI cannot differentiate between tumour and scar tissue, co-registration with these metabolic techniques is very useful. The main limitation of MET is its short half-life, which requires a cyclotron at the centre itself. Tracers with 18F-methionine are currently being developed, with promising results.
FundingThe article was financed by the Fundación de la Sociedad Española de Endocrinología y Nutrición (FSEEN) [Foundation of the Spanish Society of Endocrinology and Nutrition] thanks to an unrestricted grant from Pfizer. Pfizer has not participated in the drafting or content of the article. This document has received the endorsement of the SEEN and the Sociedad Española de Neurorradiología (SENR) [Spanish Society of Neuroradiology].
Conflicts of interestNone of the authors have conflicts of interest with respect to the content of this article.
The authors appreciate the collaboration of Dr Blanca Piedrafita, from Medical Statistics Consulting S.L., in the preparation and editing of the manuscript. All the authors contributed to the writing, critical review of content and approval of the article's final version.