Immune checkpoint inhibitors (ICPI) have improved progression-free survival in several solid tumors. Side effects are related to overstimulation of the immune system. Thyroid dysfunction (TD) is the most common endocrine immune-related adverse event of ICPI.
ObjectiveTo describe the clinical presentation and the course of TD in cancer patients treated with ICPI referred to an endocrinology outpatient clinic.
Material and methodsThis was a descriptive, retrospective and multicenter study of patients with TD associated with ICPI in six Spanish hospitals.
Results120 patients (50.8% women), mean age 60 ± 12 years were included. The initial TD was hypothyroidism in 49% of patients and hyperthyroidism in 51%, with an average of 76 (41–140) and 43 (26–82) days respectively between the onset of ICPI and the analytical alteration. Significantly, the earlier the first analytical determination was, the greater the prevalence of hyperthyroidism. A turnover was observed in 80% of subjects during follow-up, mostly from hyperthyroidism to hypothyroidism. Twenty-one percent received double ICPI therapy. The most frequent form of presentation in monotherapy was hypothyroidism (57%), and in double therapy it was hyperthyroidism (77%) (p = 0.002). Patients under double therapy showed thyroid alterations earlier than those in the monotherapy group (p = 0.001). After a follow-up of 205 (112–360) days, half of the patients continued under levothyroxine treatment.
ConclusionsHypothyroidism and hyperthyroidism present in a similar proportion in cancer patients undergoing ICPI therapy. Our results suggest that transitory hyperthyroidism may not be detected in a relevant number of cases. In addition, TD in double therapy presents earlier. This should be taken into account in the follow-up protocols of these patients.
Los inhibidores de los puntos de control inmunitario (IPCI) han conseguido elevados porcentajes de respuesta en diversas neoplasias. Los efectos secundarios se relacionan con la hiperestimulación del sistema inmune y los que afectan a la glándula tiroides se encuentran entre los más frecuentes.
ObjetivoDescribir la presentación clínica y evolución de la disfunción tiroidea (DT) en pacientes oncológicos en tratamiento con IPCI remitidos a las consultas de endocrinología.
Material y métodosEstudio descriptivo, retrospectivo y multicéntrico de pacientes con DT asociada a IPCI en seis centros hospitalarios españoles.
ResultadosSe incluyeron 120 pacientes (50,8% mujeres), edad 60 ± 12 años. La DT inicial fue el hipotiroidismo en el 49% de los pacientes y el hipertiroidismo en el 51%, con una media de 76 (41–140) y 43 (26–82) días, respectivamente entre el inicio de IPCI y la alteración hormonal. El diagnóstico de hipertiroidismo estaba significativamente asociado a una evaluación analítica más temprana. En un 80% se observó un viraje durante el seguimiento, en la mayoría de hipertiroidismo a hipotiroidismo. El 21,7% recibió doble terapia con IPCI. La forma de presentación más frecuente en monoterapia fue el hipotiroidismo (57%), y en doble terapia fue el hipertiroidismo (77%) (p = 0,002). Los pacientes en doble terapia presentaron alteraciones tiroideas significativamente más tempranas que los del grupo en monoterapia. Tras un seguimiento de 205 (112–360) días, el 50% de los pacientes continuaba en tratamiento con levotiroxina.
ConclusionesEl hipotiroidismo y el hipertiroidismo se presentan en una proporción similar en la DT asociada a IPCI, aunque es posible que el hipertiroidismo transitorio no sea detectado en un gran número de casos. La DT en la doble terapia es más precoz, hecho que debiera tenerse en cuenta en los protocolos de seguimiento de estos pacientes.
Immune checkpoint inhibitors (ICIs) have revolutionised the treatment of several malignant neoplasms.1 At present, the Food and Drug Administration (FDA) has approved seven ICIs for the treatment of advanced solid tumours, which are classified according to their mechanism of action as CTLA-4 (cytotoxic T lymphocyte antigen-4) inhibitors: ipilimumab and tremelimumab; PD-1 (programmed death-1) inhibitors: nivolumab and pembrolizumab; and PD-L1 (programmed death-ligand 1) inhibitors: atezolizumab, avelumab and durvalumab. In addition, there are new drugs being developed from these groups, as well as clinical trials with new therapeutic targets such as LAG-3 (lymphocyte-activation gene 3) inhibitors, TIM-3 (T-cell immunoglobulin and mucin-domain containing-3) inhibitors and IDO (indoleamine-2,3-dioxygenase) inhibitors,2 so it is expected that the use and development of this therapeutic group will increase in the coming years.
Due to their mechanism of action, the adverse effects produced by these drugs are related to the state of immune activation that they trigger, which is why they are referred to as immune-related adverse events (irAEs).3 Although irAEs may affect any organ, their most commonly observed manifestations are in the skin, the digestive system and the endocrine glands, and they are generally mild in intensity. While they are common in all types of cancer, they show certain differences in relation to the family to which they belong.4 The most common endocrine disorders are hypophysitis and thyroid dysfunction, although cases of adrenalitis, diabetes mellitus type 1 and hypoparathyroidism have been reported.5,6 The frequency and severity of endocrine disorders are not yet well defined due to variability in data collection. However, screening for altered hormone levels in therapeutic protocols is increasingly common, which translates into earlier detection.7 The published incidence of thyroid disorders is variable and depends on the pattern of the disorder, as well as the use of ICIs in monotherapy or dual therapy.8 A review by Byun et al. found that the incidence of hypothyroidism and thyroiditis associated with ipilimumab was estimated at 5.6% and 3.2%, respectively, similar to that reported for anti-PD-1, with a hypothyroidism incidence of 5.9% and a hyperthyroidism incidence of 3.3%. The incidence of hypothyroidism for anti-PD-L1s was 4.3%.9 Other groups have reported their experience at the national level with regard to the endocrine adverse effects induced by immunotherapy.10,11 In Spain, the evidence is based on case reports and small series.12 In light of the above, we propose this descriptive, retrospective and multicentre study, with the objective of describing the clinical presentation and course of the thyroid disorders reported in cancer patients treated with ICIs in six Spanish hospitals.
Material and methodsStudy designWe conducted a retrospective review of the medical records of cancer patients referred to the Endocrinology Department. The data collected comes from six national public referral hospitals for cancer treatment in Spain: Hospital Universitario Vall d’Hebron [Vall d’Hebron University Hospital] (Catalonia), Complejo Hospitalario de Navarra [Navarra Hospital Complex] (Navarre), Hospital Universitario de Burgos [Burgos University Hospital] (Castile and Leon), Hospital 12 de Octubre [12 de Octubre Hospital] (Madrid), Hospital Ramón y Cajal [Ramón y Cajal Hospital] (Madrid) and Hospital Virgen del Rocío [Virgen del Rocío Hospital] (Andalusia).
Patients120 cancer patients undergoing treatment with ICIs referred to the Endocrinology Department for thyroid dysfunction from December 2011 to July 2018 were included. The anonymity of the patients has been preserved at all times through the encoding of the records and cumulative analysis of the data, with both procedures being carried out by different research teams. Patients who, prior to immunotherapy had some type of thyroid dysfunction were also included when the introduction of ICIs produced a change in their hormonal status. Treatments that included the anti-CTLA4, anti-PD-1 and anti-PD-L1 families approved by the summary of product characteristics, as well as treatments from new therapeutic families (anti-LAG-3) in a phase I–II clinical trial regime, were analysed, both in monotherapy and in combination, regardless of the type of neoplasm. Variables related to the drug (type, start and discontinuation dates, duration of treatment, reason for discontinuation) were analysed in relation to other treatments received that could alter thyroid function (glucocorticoid therapy, previous treatment with chemotherapy or tyrosine-kinase inhibitors, [TKIs]) and history of cervical radiotherapy, prior use of radioiodine or iodinated contrast agents in the last three months.
The thyroid profile was documented before the start of immunotherapy, it being considered valid if the hormone test had been carried out within the six months prior to initiation of the ICIs. Data were collected on the frequency with which thyroid function tests were performed. At least four follow-up tests were recorded in each case. For the evaluation of thyroid function, the reference ranges of the laboratory of each hospital for thyroid-stimulating hormone (TSH) and free thyroxine (FT4) were considered. Data were also collected on the presence of thyroid autoantibodies: anti-thyroglobulin (anti-Tg), anti-thyroid peroxidase (anti-TPO) and anti-TSH receptor (TSI).
Assessment of thyroid functionThe variables of interest for thyroid function were defined according to the criteria of the European Thyroid Association (ETA)13,14: a) Subclinical hypothyroidism: TSH elevated above the reference limit and FT4 within the reference range; b) Clinical hypothyroidism: TSH elevated above the reference limit with FT4 below the lower reference limit; c) Subclinical hyperthyroidism: TSH below the lower reference limit with FT4 and FT3 normal; d) Clinical hyperthyroidism: TSH below the lower reference limit and elevation of free thyroid hormones above the upper reference limit.
Definition of time of onset of thyroid disordersIn most centres, thyroid function was assessed before the start of each ICI treatment cycle, and every two to three weeks thereafter. The time of onset of thyroid dysfunction was defined as the number of days between the administration of the first dose of immunotherapy and the date of the first documentation of biochemical alteration. Change time was defined as the time in days from the onset of the first verified thyroid alteration to the time of diagnosis of the change in this dysfunction.
Statistical analysisFor the statistical analysis, SPSS® (version 24) was used. Continuous variables were expressed as means ± standard deviation for normal variables and as medians ± interquartile range (IQR) for non-normal variables. The categorical variables were expressed as percentages. For the differences between groups in continuous variables, the Student's t test or the Mann–Whitney U test was used, while for the categorical variables χ2 was used. In the analysis of the possible predictors of thyroid disorders, the multivariate logistic regression model was used to assess the association between six possible predictors (age, gender, time to diagnosis of thyroid disorder [days], type of therapy, previous therapy received and previous corticosteroid therapy) and the occurrence of thyroid disorder as a result, with hypothyroidism or hyperthyroidism in the reference category. Statistical significance was considered to be p < 0.05.
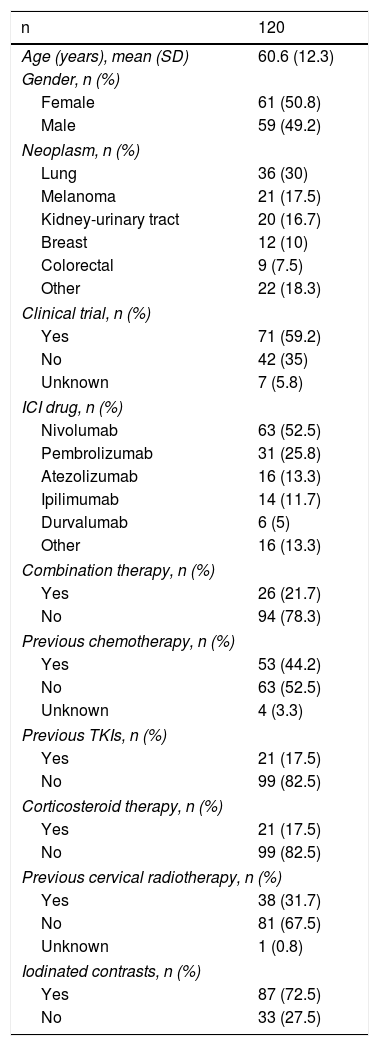
ResultsPatients studiedThe main clinical characteristics of the 120 patients who were included are shown in Table 1. In total, 18% had received prior treatment with TKIs and in nine patients (7.5%) the use of other drugs with known effect on thyroid function was recorded (amiodarone, beta-blockers, etc.). As expected, due to the oncological context, a large percentage of the cohort (72.5%) had been exposed to iodinated contrast in the last three months.
Main clinical characteristics of the patients studied.
| n | 120 |
|---|---|
| Age (years), mean (SD) | 60.6 (12.3) |
| Gender, n (%) | |
| Female | 61 (50.8) |
| Male | 59 (49.2) |
| Neoplasm, n (%) | |
| Lung | 36 (30) |
| Melanoma | 21 (17.5) |
| Kidney-urinary tract | 20 (16.7) |
| Breast | 12 (10) |
| Colorectal | 9 (7.5) |
| Other | 22 (18.3) |
| Clinical trial, n (%) | |
| Yes | 71 (59.2) |
| No | 42 (35) |
| Unknown | 7 (5.8) |
| ICI drug, n (%) | |
| Nivolumab | 63 (52.5) |
| Pembrolizumab | 31 (25.8) |
| Atezolizumab | 16 (13.3) |
| Ipilimumab | 14 (11.7) |
| Durvalumab | 6 (5) |
| Other | 16 (13.3) |
| Combination therapy, n (%) | |
| Yes | 26 (21.7) |
| No | 94 (78.3) |
| Previous chemotherapy, n (%) | |
| Yes | 53 (44.2) |
| No | 63 (52.5) |
| Unknown | 4 (3.3) |
| Previous TKIs, n (%) | |
| Yes | 21 (17.5) |
| No | 99 (82.5) |
| Corticosteroid therapy, n (%) | |
| Yes | 21 (17.5) |
| No | 99 (82.5) |
| Previous cervical radiotherapy, n (%) | |
| Yes | 38 (31.7) |
| No | 81 (67.5) |
| Unknown | 1 (0.8) |
| Iodinated contrasts, n (%) | |
| Yes | 87 (72.5) |
| No | 33 (27.5) |
ICIs: immune checkpoint inhibitors; SD: standard deviation; TKIs: tyrosine-kinase inhibitors.
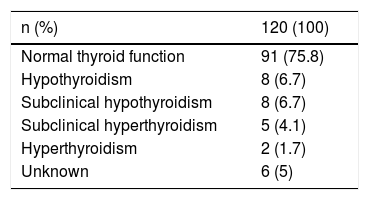
Before starting immunotherapy, 75% of patients were euthyroid and in six cases (5%) the baseline status was unknown (Table 2). Thyroid autoimmunity was not studied in most patients either before or after initiating ICIs, and a prior thyroid ultrasound was only available in two subjects with multinodular goitre.
Changes in thyroid functionThe first laboratory tests were obtained 31 days (20–60) after the start of treatment with ICIs. Globally and for the entire cohort, in the first laboratory tests a trend towards a decrease in TSH was observed (−0.33 mU/l [−1.5 to 1.8]) together with a slight increase in FT4 (0.07 ng/dl [−0.1 to 0.5]) compared to the baseline test.
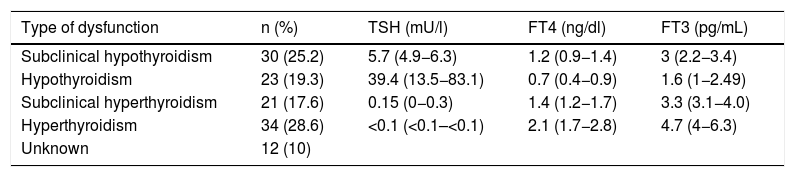
The first thyroid dysfunction was detected 55 days (27.5–105) after the start of immunotherapy. The type of initial thyroid dysfunction and the accompanying hormonal values are summarised in Table 3. Thyroid disorders presented asymptomatically in almost half of the cases (46.7%). The most common symptom was asthenia (10.8%) and, in a small proportion of cases, signs and symptoms of hyperthyroidism (3.3%). No clinical manifestations were recorded in 39.2%.
Initial thyroid dysfunction after initiation of ICIs. Note that, without taking into account the unknown cases, the proportion of patients who initially manifest any form of hypothyroidism (44.5%) is similar to those who manifest any form of hyperthyroidism (46.2%). TSH, FT4 and FT3 expressed as mean (range).
| Type of dysfunction | n (%) | TSH (mU/l) | FT4 (ng/dl) | FT3 (pg/mL) |
|---|---|---|---|---|
| Subclinical hypothyroidism | 30 (25.2) | 5.7 (4.9−6.3) | 1.2 (0.9−1.4) | 3 (2.2−3.4) |
| Hypothyroidism | 23 (19.3) | 39.4 (13.5−83.1) | 0.7 (0.4−0.9) | 1.6 (1−2.49) |
| Subclinical hyperthyroidism | 21 (17.6) | 0.15 (0−0.3) | 1.4 (1.2−1.7) | 3.3 (3.1−4.0) |
| Hyperthyroidism | 34 (28.6) | <0.1 (<0.1–<0.1) | 2.1 (1.7−2.8) | 4.7 (4−6.3) |
| Unknown | 12 (10) |
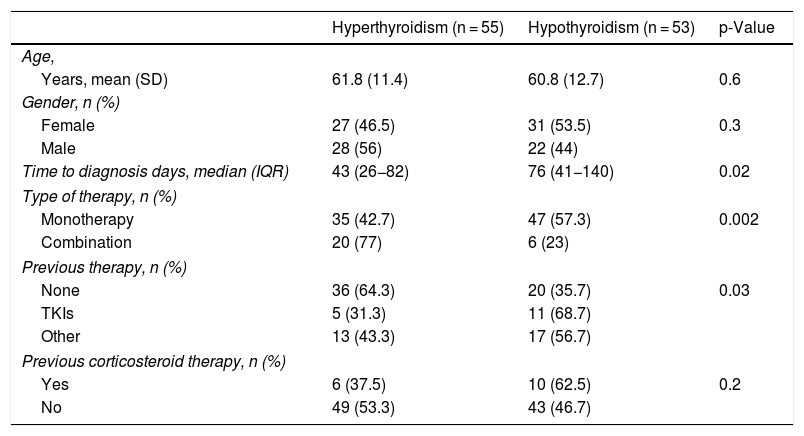
The patients were subdivided into two groups depending on whether the first manifestation was hypothyroidism (n = 53) or hyperthyroidism (n = 55). The 12 cases for which there were no laboratory test records in the nine weeks after the start of treatment were excluded. It should be noted that when the first laboratory tests were carried out within the first two months of treatment, 60% of the patients had hyperthyroidism as the first manifestation, whereas when the first laboratory tests were delayed beyond 60 days, in 70% of the cases hypothyroidism was recorded as the first disorder (p = 0.012). The differences in the characteristics of both forms of presentation are shown in Table 4, which shows that of the variables that demonstrated significant differences in the univariate study, only monotherapy/dual therapy remained significant in the multivariate regression analysis (p = 0.02).
Differences in the presentation of thyroid dysfunction.
| Hyperthyroidism (n = 55) | Hypothyroidism (n = 53) | p-Value | |
|---|---|---|---|
| Age, | |||
| Years, mean (SD) | 61.8 (11.4) | 60.8 (12.7) | 0.6 |
| Gender, n (%) | |||
| Female | 27 (46.5) | 31 (53.5) | 0.3 |
| Male | 28 (56) | 22 (44) | |
| Time to diagnosis days, median (IQR) | 43 (26−82) | 76 (41−140) | 0.02 |
| Type of therapy, n (%) | |||
| Monotherapy | 35 (42.7) | 47 (57.3) | 0.002 |
| Combination | 20 (77) | 6 (23) | |
| Previous therapy, n (%) | |||
| None | 36 (64.3) | 20 (35.7) | 0.03 |
| TKIs | 5 (31.3) | 11 (68.7) | |
| Other | 13 (43.3) | 17 (56.7) | |
| Previous corticosteroid therapy, n (%) | |||
| Yes | 6 (37.5) | 10 (62.5) | 0.2 |
| No | 49 (53.3) | 43 (46.7) | |
IQR: interquartile range; SD: standard deviation; TKIs: tyrosine-kinase inhibitors.
Pharmacotherapy for thyroid dysfunction was started in 57 patients. Of the subjects who developed clinical hypothyroidism, 52 were treated with levothyroxine. Before starting replacement therapy with levothyroxine, the mean TSH value was 23.1 ug/dl (10.3–59.6). The mean starting dose of levothyroxine was 75 mcg/day (50–100). Of the patients who developed clinical hyperthyroidism, five subjects were treated with synthetic antithyroid drugs. In all cases, antithyroid treatment began with an undetectable TSH value. This treatment was started 91 (56–154) days after the introduction of the ICIs.
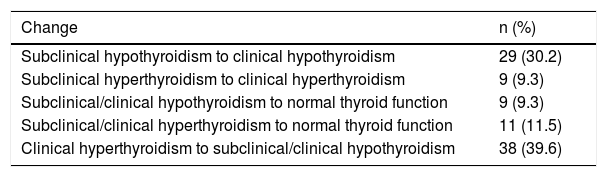
Changing thyroid functional statusIn 58 patients, a change in thyroid status was verified during follow-up (changing), while the rest progressed from subclinical to clinical status, 38 with hypothyroidism and nine with hyperthyroidism, as shown in Table 5. The time from diagnosis of dysfunction to changing or progression was 42 (28–84) days.
Type of change by initial thyroid dysfunction. It should be noted that 20.8% of patients changed from a clinical or subclinical form of thyroid disorder to normal thyroid function.
| Change | n (%) |
|---|---|
| Subclinical hypothyroidism to clinical hypothyroidism | 29 (30.2) |
| Subclinical hyperthyroidism to clinical hyperthyroidism | 9 (9.3) |
| Subclinical/clinical hypothyroidism to normal thyroid function | 9 (9.3) |
| Subclinical/clinical hyperthyroidism to normal thyroid function | 11 (11.5) |
| Clinical hyperthyroidism to subclinical/clinical hypothyroidism | 38 (39.6) |
During the follow-up, antithyroid antibodies were requested in 25% of cases. In this subgroup, anti-Tg antibodies were positive in 27.8%, anti-TPO in 31.8% and TSI in 14.3% of cases.
Clinical course of thyroid dysfunctionAfter a follow-up of 205 (112–360) days from the start of immunotherapy to the last laboratory tests, 60 patients (50%) were undergoing treatment with levothyroxine (94 [56–112] mcg/day), 47 patients (39%) were not receiving any thyroid-related therapy and the rest were unknown. Of the 60 patients who continued with levothyroxine, 38 belonged to the group of patients who initially presented with clinical hypothyroidism or progressed from the subclinical to the clinical form, while the remaining 21 cases had changed from hyperthyroidism to clinical hypothyroidism. At the end of this period of time, only 39 patients (33.3%) continued with ICIs, while immunotherapy had been suspended in the rest; in 38 cases (32.5%) due to progression of the neoplasm, in 17 (14.6%) due to adverse effects (three of them due to thyroid disorder), in 9 (7.7%) due to death and in another 14 (12%) due to other causes. At the end of the follow-up, six patients had complete remission of their neoplasm, 45 stable disease, 45 progressed, and there were a total of 13 deaths. No differences were found between this final clinical status and the type of initial dysfunction, or with the time elapsed until the onset of the dysfunction.
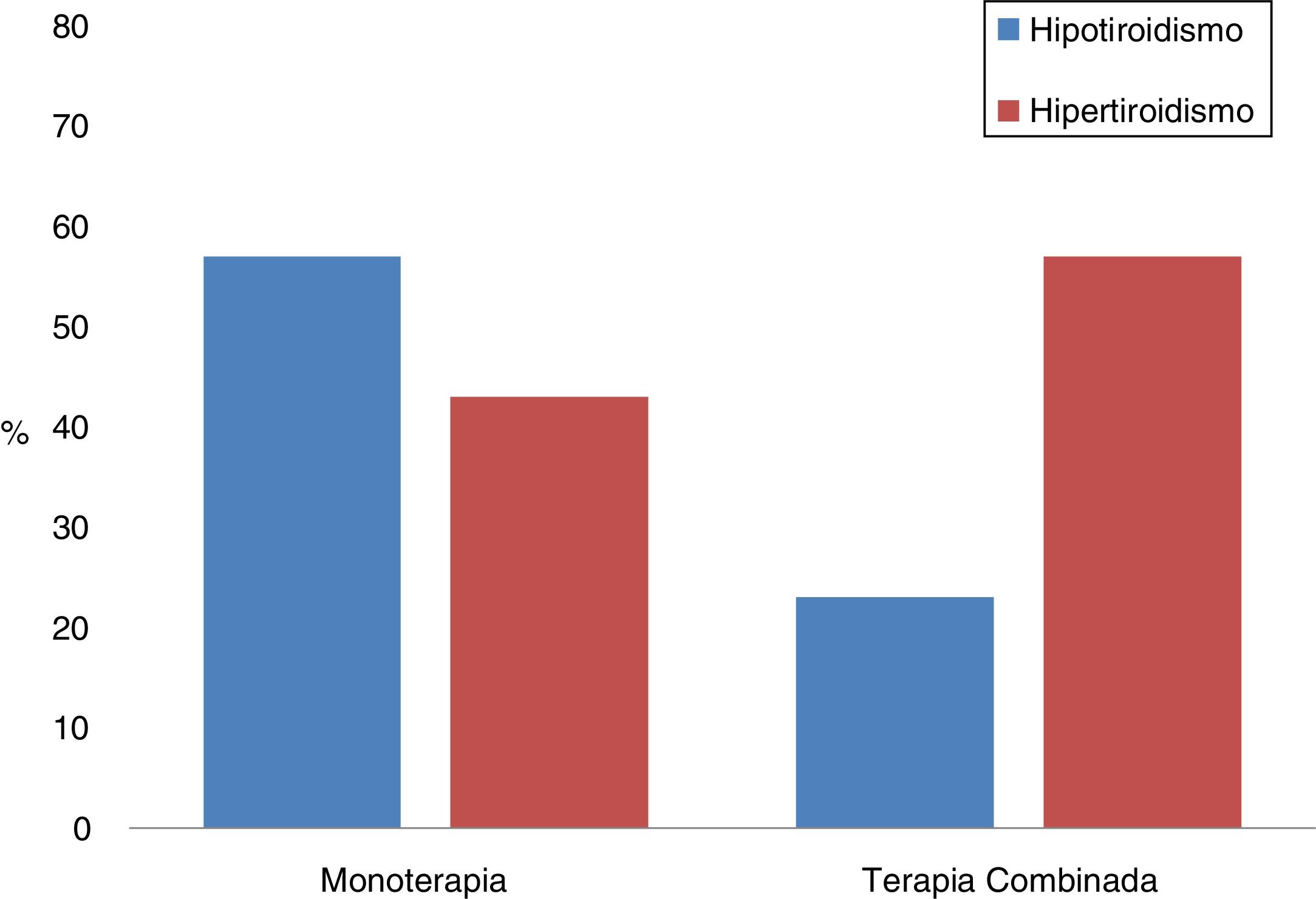
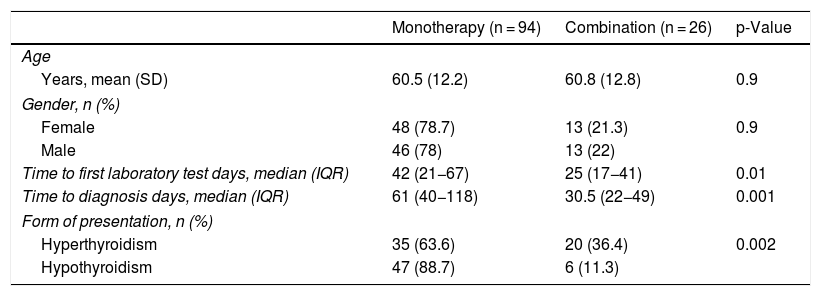
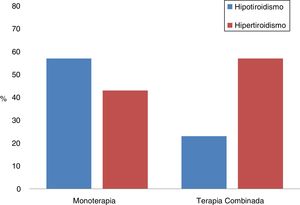
Combination therapyThe differences between the patients who had been treated with monotherapy or in combination with two ICIs were analysed; a total of 26 (21.7%) received dual ICI therapy (Table 6, Fig. 1). It should be noted that the most common form of presentation in monotherapy was hypothyroidism (57%), while in combination therapy it was hyperthyroidism (77%). Furthermore, in combination therapy, the first thyroid disorder was observed 30 days after starting treatment, and this period was exactly double in monotherapy (p = 0.001). However, there were also differences between both groups in relation to the time elapsed between the start of immunotherapy and the first laboratory tests, being 25 (17–41) days in those who received two drugs and 42 (21–67) in those who were treated with monotherapy (p = 0.01).
Characteristics of the patients according to whether they received monotherapy or combination therapy.
| Monotherapy (n = 94) | Combination (n = 26) | p-Value | |
|---|---|---|---|
| Age | |||
| Years, mean (SD) | 60.5 (12.2) | 60.8 (12.8) | 0.9 |
| Gender, n (%) | |||
| Female | 48 (78.7) | 13 (21.3) | 0.9 |
| Male | 46 (78) | 13 (22) | |
| Time to first laboratory test days, median (IQR) | 42 (21−67) | 25 (17−41) | 0.01 |
| Time to diagnosis days, median (IQR) | 61 (40−118) | 30.5 (22−49) | 0.001 |
| Form of presentation, n (%) | |||
| Hyperthyroidism | 35 (63.6) | 20 (36.4) | 0.002 |
| Hypothyroidism | 47 (88.7) | 6 (11.3) | |
IQR: interquartile range; SD: standard deviation.
The global incidence of thyroid disorders associated with ICI treatment is as high as 35% depending on the series and the type of drug used.15 When compared with placebo, the relative risk of developing hypothyroidism is 8.3 (95% CI 4.7–14.6), while for hyperthyroidism it is 5.5 (95% CI 1.3–22.5).16 Prevalence increases with combination therapy.17 The mechanism by which thyroid dysfunction develops with ICIs is unknown. However, the role they play in immune tolerance suggests an imbalance in favour of an exaggerated immune response as the most accepted theory.18
The pattern of thyroid disorders published in the literature includes: subclinical or clinical hypothyroidism, silent thyroiditis with transient thyrotoxicosis, and subclinical or clinical hyperthyroidism.3 Other rare thyroid clinical manifestations have been reported: Graves' disease due to tremelimumab19; and thyroid storm in a patient with Hashimoto's disease during treatment with nivolumab and ipilimumab.20 Anecdotally, the development of thyroid-associated orbitopathy, a condition associated with Graves' disease, has been reported in a euthyroid patient during the course of treatment with nivolumab.21 In some cases, thyroid dysfunction occurs concomitantly with other endocrine irAEs, such as hypophysitis, diabetes mellitus type 1 with ketoacidosis and adrenal insufficiency, which can make diagnosis and management challenging.22,23 In our series, the initial clinical presentation occurred either as hyperthyroidism (clinical/subclinical) or hypothyroidism (clinical/subclinical), with no differences regarding gender or age for the presentation of thyroid disorder in monotherapy or combination therapy and in line with that reported by Al Mushref et al.24 The patients who presented with hypothyroidism as the first abnormal laboratory test did so within a median of 76 days after starting ICI therapy, similar to the mean of 12 (7–36) weeks reported by Tan et al. in a review that included 457 endocrine irAE cases, of which 152 were thyroid disorders.25 In the 55 patients for whom hyperthyroidism was the first thyroid disorder that presented, it occurred within a median of 27 days (19–50 days) after starting ICIs, also in line with the findings of other studies.26
From a clinical point of view, the behaviour in many cases is similar to that of subacute thyroiditis with an initial phase of hyperthyroidism followed by the development of hypothyroidism. While the patients in our series who initially presented with hyperthyroidism commonly changed to hypothyroidism, no patient with hypothyroidism developed thyrotoxicosis. In total, 96 patients (80%) from our cohort followed a similar clinical course to this pattern of thyroiditis. The true incidence of thyroiditis is possibly underestimated because previously no protocolised monitoring of thyroid function was conducted, hyperthyroidism and hypothyroidism were recorded as separate entities, and the often asymptomatic phase of thyrotoxicosis could have gone unnoticed. Growing evidence and methodical screening for thyroid disorders, increasingly common in follow-up, have increased the prevalence of this entity. Accordingly, and taking these limitations into account, the prevalence reported in the literature ranges from 6.5 to 54%.26 This series reinforces the idea that the initial phase of hyperthyroidism goes unnoticed in a high percentage of patients, given that the earlier the first laboratory tests are conducted, the higher the percentage of diagnosed cases of hyperthyroidism. It should be noted that in our cohort, only three patients required discontinuation of ICI treatment due to the severity of thyroid dysfunction. This result concurs with the study published by Garon-Czmil et al., in which 110 patients presented with thyroiditis associated with ICI treatment, the authors concluding that with optimisation of its management, in almost no case was it necessary to suspend ICI therapy.11 These results suggest that although thyroid dysfunction is a common irAE, when properly managed it allows ICI treatment to be maintained.
In our series, in patients who received ICIs in combination therapy, it was observed that thyroid dysfunction occurred earlier than in the monotherapy group (30 days vs 60 days, p = 0.001), a finding similar to other reported series.27 Hypothyroidism as the first manifestation of thyroid dysfunction (TD) occurred in a higher proportion in the monotherapy group compared to the combination therapy group (88.9% vs 11.3%). However, it is interesting to note that the time that elapsed between the start of immunotherapy and the laboratory tests was also significantly shorter in the combination group, possibly reflecting the greater degree of laboratory test control in this group of patients.
Our study looks at thyroid dysfunction during immunotherapy in a significant cohort of patients in several Spanish centres. However, it has several limitations given the retrospective nature of the analysis and the lack of some data on clinical, biochemical and imaging endocrine evaluations. It has therefore not been possible to establish a relationship between the development of thyroid disorder and the type of tumour response to immunotherapy, a relationship that, although established for other irAEs,7 is not clear in relation to the thyroid. However, several studies seem to indicate that anti-TPO and anti-Tg antibody titres may be useful as a predictor of hypothyroidism, given that the prevalence of thyroid autoimmunity is higher among those who develop thyroid disorders than among those who do not.28 In other studies, the development of thyroid autoimmunity was not associated with the development of thyroid dysfunction.29 In this study, we only had available thyroid autoimmunity laboratory tests prior to ICI therapy in a small proportion of patients, so a comparative analysis could not be performed. Finally, we cannot draw conclusions about the incidence of thyroid dysfunction associated with therapy with these drugs, since only those cases referred to endocrinology with the disorder already verified were included.
ConclusionsIn summary, the incidence of both hypothyroidism and hyperthyroidism is similar as a form of presentation of thyroid dysfunction associated with ICIs, although it is possible that transient hyperthyroidism is not detected in a large number of cases. Thyroid dysfunction in combination therapy occurs earlier, a fact that should be taken into account in the follow-up protocols for these patients. Understanding the clinical presentation and management of these irAEs allows for better patient treatment and facilitates the continuity of cancer treatment, which has been shown to increase survival. The implications of this include a possible increase in the prevalence of thyroiditis, an increase in the number of patients on long-term thyroid treatment, and as a result an increase in follow-up by endocrinology departments.
This study is part of the research programme of the "Área de Conocimiento en Tiroides" [Thyroid Knowledge Area] of the Sociedad Española de Endocrinología y Nutrición (SEEN) [Spanish Society of Endocrinology and Nutrition].
FundingThis research has not received specific funding from public sector agencies, the commercial sector or non-profit organisations.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Fidilio E, Navarro-González E, Romero-Lluch AR, Iglesias P, Diez Gómez JJ, Anda Apiñániz E, et al. Alteraciones tiroideas asociadas con los inhibidores de los puntos de control inmunitario. Endocrinol Diabetes Nutr. 2021;68:408–415.