Different epidemiological studies show the relationship between osteoporosis, cardiovascular disease, and cardiovascular-related mortality. Previously considered as age-related but independent diseases, this review reports emerging data showing the common mechanism of both conditions. The relationship between bone-related proteins such as osteocalcin and osteoprotegerin and vascular disease is of special interest. In addition, recent data suggest the influence of bone metabolism on energy balance which may be relevant for cardiovascular disease.
Diferentes estudios epidemiológicos muestran la relación que existe entre osteoporosis, enfermedad cardiovascular y la mortalidad asociada a ésta. Aunque tradicionalmente estas enfermedades se han considerado como procesos independientes relacionados con la edad, como se muestra en este trabajo de revisión, cada vez existe una evidencia más sólida que confirma que ambas entidades comparten mecanismos fisiopatológicos. Entre ellos destacan la relación entre proteínas de origen óseo como la osteocalcina y la osteoprotegerina con la patología vascular. Además, recientemente se han producido diversos avances en el conocimiento de la relación entre metabolismo óseo y metabolismo energético, lo que pudiera tener implicaciones en la patología cardiovascular.
The relationship between osteoporosis, cardiovascular disease, and mortality associated to the latter has previously been reported.1–3 Although these diseases have traditionally been considered as age-related separate conditions, there is increasingly solid evidence to confirm that both conditions share pathophysiological mechanisms. A short revision will be made of the epidemiological aspects and the factors common to bone disease and vascular pathology that may explain this relationship, paying special attention to most recent advances in his field.
EpidemiologyThe relationship between low bone mass, fractures, and cardiovascular mortality is well documented. Females with a low bone mass have increased cardiovascular mortality ranging from 22% to 40% per each decrease by one standard deviation (SD) in bone mineral density (BMD),4,5 and cardiovascular mortality decreases 24% in males per each one SD decrease in BMD in the hip.6 In addition, deaths from cardiovascular disease are 30% more common in females with vertebral fractures.7
Similarly, bone mass is decreased in patients with cardiovascular disease regardless of age,8 and presence of peripheral artery disease and/or ischemic heart disease is associated to an increased risk of hip fracture.9 A significant association has also been reported between the presence of myocardial infarction and low BMD10 and between the presence of osteoporosis/osteopenia and an increased risk of obstructive coronary disease in both sexes.11,12 Finally, a relationship has been reported of presence of cerebrovascular disease13 and peripheral artery disease14 with low bone mass and fragility fractures.
There is also a relationship between atherosclerosis markers and bone disease. Thus, most cross-sectional studies have reported an inverse association between the presence, severity, and progression of calcification at abdominal aorta and bone mass at lumbar and femoral level in both post-menopausal females15 and males.16 Moreover, aortic calcification is associated to an increased risk of hip fracture in post-menopausal women.17 Carotid atheromasis, another marker of cardiovascular disease, has been associated to a lower bone mass in post-menopausal women18 and to an increased risk of non-vertebral fracture.19 Similarly, presence of vertebral fracture is associated to a greater risk of occurrence of carotid plaque in post-menopausal women with BMD,13 and bone mass loss measured in metacarpus is associated to progression of atherosclerosis in women.15
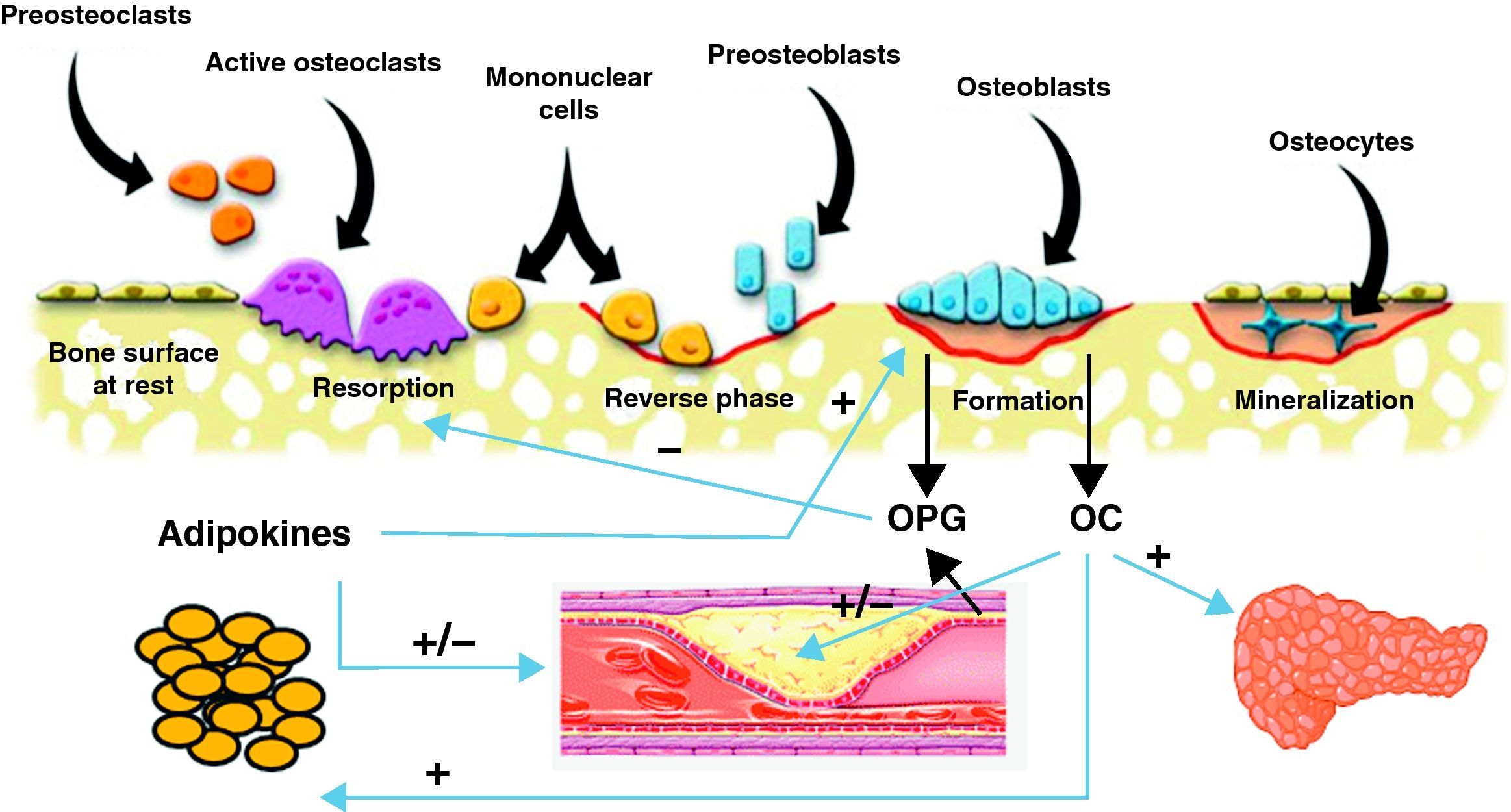
Common pathophysiological mechanismsIt has been known for some time that these two conditions share risk factors that could account for the association between them. Such factors include age, estrogen depletion, a sedentary lifestyle, alcohol consumption, smoking, and dietary factors such as calcium intake, consumption of saturated fatty acids, and deficient vitamin C and K levels. There has been in recent years a great interest in research of the common factors and mechanisms involved in the pathophysiology of both conditions (Fig. 1).
Scheme of the interrelationship between bone cells, vascular system, adipose tissue, and energy metabolism. Osteoblasts are the source of osteoprotegerin (OPG) and osteocalcin (OC). OC has been shown to have effects of energy metabolism through which it may also influence cardiovascular disease. OPG not only inhibits bone resorption, but is also involved in the incidence and severity of cardiovascular disease. Finally, adipose tissue secretes adipokines that influence bone cells and vascular tree.
Obesity is a clear risk factor for development of cardiovascular disease. Adipose tissue is currently considered as a metabolically active tissue, and study of the effect of various cytokines secreted by adipose tissue on bone and vascular system has become relevant in recent years. Leptin, an adipokine that is increased in patients with obesity, has a dual effect because it slows bone formation at hypothalamic level by inhibiting osteoblast formation, and also stimulates formation by a direct effect upon bone cells.20 Recent studies show the influence of leptin on the cardiovascular system, including effects upon cardiac remodeling.21 However, clinical studies evaluating the association between leptin and cardiovascular disease have not been conclusive.22,23 Adiponectin, another adipokine that is decreased in obesity, has been shown to promote bone formation,24 and in vascular pathology reduces atherogenesis by inhibiting adhesion capacity of monocytes and accumulation of modified lipoproteins in the vascular wall.25 Adiponectin also decreases endothelial damage and stimulates nitric oxide production by endothelial cells.26 In this setting, low adiponectin levels could partially contribute to the atherogenetic process.
Vascular calcification and bone remodelingThis is undoubtedly the field where the greatest advances have recently been made in understanding of the common pathophysiological mechanisms shared by vascular pathology and bone remodeling. Different proteins produced by bone cells, such as osteocalcin (OC), osteopontin, osteoprotegerin (OPG), receptor activator of nuclear factor kappa-β ligand (RANKL), and bone morphogenetic proteins (BMPs) are found in atherosclerotic lesions.27 Presence of osteoblastic cells in the vascular wall has also been shown.28
Influence of these factors in the pathogenesis of cardiovascular disease has been demonstrated through studies in animal models. Knockout mice for bone matrix GIa protein (MGP), a protein widely distributed in tissue including bone and vascular wall, experience severe vascular calcification. This protein has therefore been attributed an inhibitory effect of arterial calcification.29 OC is a protein synthesized by osteoblasts or bone-forming cells which is released into the bloodstream after undergoing a carboxylation process. Although OC has traditionally been considered as a bone formation marker, its role as hormone is increasingly being recognized. In animal models of peripheral artery disease, severity of the condition is related to the number of mononuclear cells immunoreactive to OC,30 which may therefore be related to vascular involvement.
In humans, the known relationship between low levels of bone remodeling markers and severity of atherosclerosis has recently been extended. Thus, OC secretion is parallel to MGP secretion in both normal vessels and atherosclerotic plaques,27 and serum OC levels have shown a negative correlation to carotid intima-media thickness in Asian patients with type 2 diabetes mellitus (T2DM).31 In addition, patients with carotid atheromasis and aortic calcification have lower OC levels as compared to healthy controls.32 In Asian patients also, low serum OC levels were associated to a lower risk of ischemic heart disease.33 Other authors have reported different results, including a higher prevalence of carotid atheromasis in healthy post-menopausal women with elevated OC.34 The conflicting results of these studies may partly be explained by differences in baseline characteristics of patients and racial differences in the degree of adiposity, and probably in adipokine levels also. Although it is currently unknown whether or not OC produced by osteoblasts or osteoblast-like cells in the arterial wall has a modulatory role on atherosclerosis, future studies should confirm the value of OC measurement for diagnosis and monitoring of vascular disease, because initial data are promising.
Recent studies show the influence exerted by bone cells upon energy metabolism and glucose homeostasis, factors which have both potential impact on the cardiovascular system. In experimental animals, OC increases insulin release and sensitivity, decreases visceral fat, and increases energy expenditure.35In vivo studies support an association of serum OC levels to the degree of insulin resistance and to insulin release, and also a positive effect of weight loss on levels of this hormone.36 Finally, in T2DM, serum OC levels are negatively related to glucose levels and degree of metabolic control, as measured through HbA1c values,31 and hypoglycemic treatment induces increased OC levels.37
OsteoprotegerinOsteoprotegerin (OPG) is a protein from the TNF (tumor necrosis factor) family that regulates the osteoclastogenesis process through inhibition of RANKL, receptor activator of nuclear factor kappa β. The OPG/RANKL system is a critical factor that determines the degree of osteoblast activation and, thus, the degree of bone destruction. OPG is secreted by osteoblasts or bone-forming cells, and also by vascular cells, including endothelial cells and muscle cells in coronary arteries (Fig. 1). Recent data suggest that OPG may be a significant factor regulating arterial calcification and may represent an indicator of vascular damage. In addition, the relationship between higher OPG levels and an increased incidence of and mortality from cardiovascular disease has been confirmed in different study populations.
In coronary artery disease, elevated plasma OPG levels are associated to disease presence and severity.38 OPG levels in turn correlate to severity of peripheral artery disease39 and OPG is expressed in high amounts in carotid atherosclerotic lesions.40 These two findings combined support the role of OPG as a cardiovascular event marker.
OPG has also been related to markers of subclinical atherosclerosis such as carotid IMT (intima-media thickness). In post-menopausal women with no cardiovascular disease, high OPG levels are positively related to endothelial dysfunction, arterial rigidity, and IMT.41 By contrast, other studies found no association between OPG and IMT after adjusting for age and sex42 or reported an inverse relationship.43
OPG is increased in T2DM patients of both sexes as compared to controls with no diabetes mellitus, and its value as a mortality predictor appears to be even more important in this patient group than in the non-diabetic population.44 Increased OPG levels occur in diabetic patients from the early disease stages, and although regulation of OPG secretion at vascular level is not fully known, we know that blood glucose does not appear to have a significant role and that no relation exists to leptin or adiponectin, which suggests that obesity is not the reason for OPG increase in this patient group.45
As regards vascular complications in diabetic patients, high OPG levels have been related to an increased risk of silent myocardial ischemia46 and microangiopathic complications.47 As in the general population, serum OPG levels are related to the presence and severity of coronary calcification in patients with T2DM.
While in vitro studies clearly show the influence of the OPG/RANKL system on vascular physiology and pathology, results of clinical studies have been conflicting in some cases. Better understanding of the factors involved in its regulation and advances in procedures to measure proteins of this system have led to achievement of consistent results in the most recent studies. However, it has not been elucidated yet whether elevation of serum OPG levels in different cardiovascular conditions represents a compensatory mechanism to prevent vascular damage or is responsible for such damage.
The Wnt pathwayThis pathway consists of 19 soluble glycoproteins having a critical role in regulation of osteoblast development and, thus, of the bone formation process. These proteins bind to two receptors, LRP-5 and LRP-6, starting signaling of nuclear factors involved in bone formation. This signaling pathway is also involved in regulation of vascular calcification and in differentiation of smooth muscle cells into osteoblasts.48 Recent data suggest an inhibition of the Wnt pathway by the oxidative stress characteristic of aging,49 which would represent a common mechanism for development of osteoporosis and atherosclerosis.
Cbfa1 and Runx2Cbfa1 (core-binding factor a1) and Runx2 (Runt-related transcriptional factor-2) are two factors that induce differentiation of bone-forming cells, osteoblasts, and participate in the mineralization process.50 Runx2 expression has been shown in atherosclerotic lesions in humans, but not in healthy vessels, thus suggesting a potential role of these factors in vascular calcification. They also appear to have a role in the vascular calcification process induced by oxidative stress.51
Fibroblast growth factor 23Fibroblast growth factor 23 (FGF 23) is a 251-amino acid protein secreted by osteocytes (cells immersed in bone matrix) which has a significant role in mineral metabolism. FGF 23 has a particularly significant role in renal physiology. Its levels already increase from the early stages of renal failure, and has therefore been implicated in both normal physiology and changes of phosphorus and calcium metabolism characteristic of renal failure.52 It has also been recently suggested that it may be a pathogenetic mechanism in cardiovascular disease, based on the association of the latter to renal failure and on the fact that other growth factors from this family are related to lipid and glucose metabolism Thus, in Caucasian populations, a negative correlation between serum levels of FGF 23 and HDL and a positive correlation between FGF23 and triglycerides, body mass index, waist/hip ratio and visceral fat, and also with the risk of metabolic syndrome have been shown.53 The recent association found between this growth factor synthesized by bone cells, fat mass, and lipid changes may represent a new common mechanism explaining the relationship between bone metabolism and cardiovascular risk.
Vitamin DThere is ample evidence for the role of vitamin D in pathogenesis of cardiovascular disease. Low vitamin D levels have been shown to be an independent risk factor for development of arterial hypertension, diabetes mellitus, heart failure, stroke, peripheral artery disease, ischemic heart disease, and mortality associated to these conditions.54 However, interventional studies assessing the effect of vitamin D treatment on different cardiovascular outcomes have not shown consistent results. Thus, there is currently inadequate evidence to recommend its use for the treatment and/or prevention of cardiovascular disease, although more research is warranted in this area.
Therapeutic implicationsStatinsSeveral authors have examined the association of plasma lipid levels to BMD and presence of fracture with conflicting results. More consistent results have been found with regard to statin influence on bone metabolism. Statins stimulated bone formation in in vitro studies,55 and epidemiological studies appear to show that women treated with statins, both menopausal56 and with DM,57 have higher BMD values. By contrast, the effect on fracture risk is controversial, as cross-sectional studies appeared to show a protective effect,58 but this not confirmed by prospective studies,59 although benefits were seen on fracture union.60
Antihypertensive drugsAlthough is has not been fully elucidated whether arterial hypertension is a risk factor for low bone mass and/or fractures, the influence of different antihypertensive drugs on bone metabolism has widely been studied. Thus, thiazides decrease urinary calcium excretion and have been shown to prevent bone mass loss in post-menopausal women.61 A direct effect on osteoblast differentiation and bone mineralization has also been shown.62
Angiotensin II stimulates bone loss by inducing increased RANKL levels, and treatment with ARBs (olmesartan) inhibits this effect.63 Evidence from clinical studies is conflicting, with some data showing an increased bone mass64 and others no effect. Such differences are probably explained by ACE polymorphisms.
Finally, beta-blockers exert an effect upon bone remodeling through the sympathetic nervous system. A large, prospective population study showed these drugs to decrease risk of fracture,65 although their use for fracture prevention is currently not recommended.
BisphosphonatesSeveral studies suggest that bisphosphonates have an antiatherogenic action by a direct mechanism on the vascular wall, and also by their indirect effects upon other cardiovascular risk factors. In vitro, they inhibit calcification outside bone, reduce lipid accumulation and fibrosis in atherosclerotic lesions, and decrease calcium deposits.66 Oral etidronate decreased carotid IMT in patients with T2DM and osteopenia,67 and in post-menopausal women long-term treatment with oral bisphosphonates decreased LDL-C and increased HDL-C levels,68 improving arterial compliance and decreasing arterial resistance irrespective of changes in lipid levels.
ConclusionsSignificant advances have been made in recent years in the already known epidemiological relationship between osteoporosis, fractures, and cardiovascular disease. Thus, the relationship existing between bone cells and vascular system is increasingly recognized. Advances have also been made in understanding of the influence of proven cardiovascular risk factors, such as obesity and dyslipidemia, on bone metabolism. Finally, different studies have shown the effect of drugs widely used for bone conditions, such as bisphosphonates, on the vascular system, as well as the influence of statins and antihypertensive drugs on bone mass and risk of fractures. An improved understanding of the pathophysiological mechanisms common to both conditions may contribute to future development of drugs active upon bone metabolism and cardiovascular disease.
Conflicts of interestThe authors state that they have no conflicts of interest.
Please cite this article as: Reyes-García R, et al. Enfermedad cardiovascular y metabolismo óseo. Endocrinol Nutr. 2011;38:353–9.