Patients with primary hyperparathyroidism (PHP), even asymptomatic, have an increased cardiovascular risk. However, data on reversibility or improvement of cardiovascular disorders with surgery are controversial. Our aims were to assess the prevalence of classic cardiovascular risk factors in patients with asymptomatic PHP, to explore their relationship with calcium and PTH levels, and analyze the effect of parathyroidectomy on those cardiovascular risk factors.
Patients and methodsA retrospective, observational study of two groups of patients with asymptomatic PHP: 40 patients on observation and 33 patients who underwent surgery. Clinical and biochemical data related to PHP and various cardiovascular risk factors were collected from all patients at baseline and one year after surgery in the operated patients.
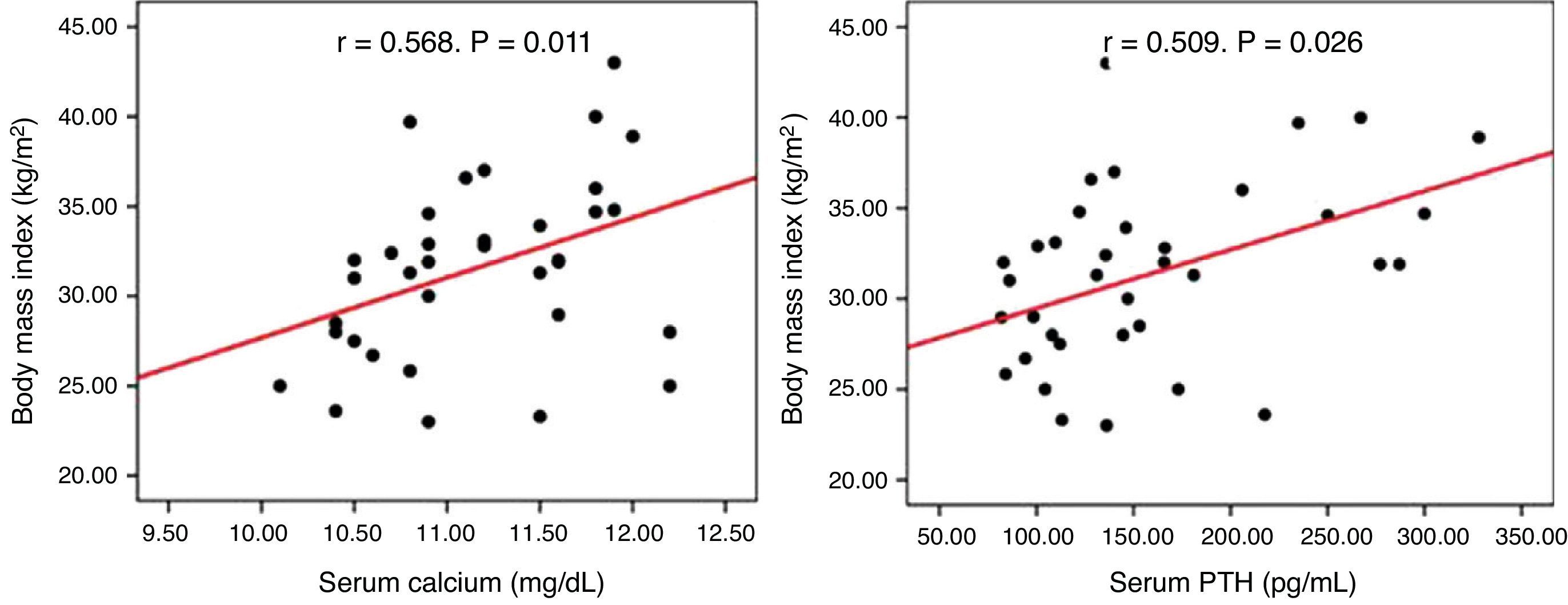
ResultsA high prevalence of obesity (59.9%), type 2 diabetes mellitus (25%), high blood pressure (47.2%), and dyslipidemia (44.4%) was found in the total sample, with no difference between the study groups. Serum calcium and PTH levels positively correlated with BMI (r=0.568, p=0.011, and r=0.509, p=0.026 respectively) in non-operated patients. One year after parathyroidectomy, no improvement occurred in the cardiovascular risk factors considered.
ConclusionsOur results confirm the high prevalence of obesity, type 2 diabetes mellitus, high blood pressure, and dyslipidemia in patients with asymptomatic PHP. However, parathyroidectomy did not improve these cardiovascular risk factors.
Los pacientes con hiperparatiroidismo primario (HPP), incluso asintomático, presentan un mayor riesgo cardiovascular. Sin embargo, los datos sobre la reversibilidad o mejoría de las alteraciones cardiovasculares con la cirugía son controvertidos. Los objetivos de nuestro estudio fueron evaluar la prevalencia de factores de riesgo cardiovascular clásicos en pacientes con HPP asintomático, examinar su relación con los niveles de calcio y PTH y analizar el efecto de la paratiroidectomía sobre los mismos.
Pacientes y métodosEstudio retrospectivo observacional de 2 grupos de pacientes con HPP asintomático: 40 pacientes en observación y 33 pacientes intervenidos. Se recogieron datos clínicos y bioquímicos relacionados con el HPP y de diversos factores de riesgo cardiovascular en todos los pacientes de forma basal, y al año de la cirugía en el grupo de pacientes intervenidos.
ResultadosEncontramos una elevada prevalencia de obesidad (59,9%), diabetes mellitus tipo 2 (25%), hipertensión arterial (47,2%) y dislipidemia (44,4%) en la muestra total, sin diferencias entre los grupos de estudio. En el grupo que se mantuvo en observación las concentraciones séricas de calcio y PTH se relacionaron positivamente con el IMC (r=0,568, p=0,011 y r=0,509, p=0,026 respectivamente). En los pacientes intervenidos, al año de la cirugía no hubo mejoría de los factores de riesgo cardiovascular considerados.
ConclusionesNuestros resultados confirman la elevada prevalencia de obesidad, diabetes mellitus tipo 2, hipertensión arterial y dislipidemia en pacientes con HPP asintomático. Sin embargo, el tratamiento quirúrgico no supuso una mejoría en estos factores de riesgo cardiovascular.
Primary hyperparathyroidism (PHP) is a common endocrine disease which is now usually diagnosed at an asymptomatic stage. Its classical renal and bone manifestations are therefore increasingly uncommon.
The clinical implications of mild or asymptomatic hyperparathyroidism are controversial. In recent years, different authors have assessed mortality and cardiovascular changes in this disease, showing an increase in mortality1,2 similar to moderate and severe forms of the condition, and a greater incidence of high blood pressure (HBP),3 left ventricular hypertrophy,4 vascular calcification,5 and type 2 diabetes mellitus6 in this population.
The relationship between hyperparathyroidism and cardiovascular risk is complex, and various hypotheses have been postulated: the action of intracellular calcium mediated by parathyroid hormone (PTH) on insulin sensitivity,7 the direct effect of PTH on vascular and cardiac muscle proliferation,8 or the dysfunction of the renin–angiotensin–aldosterone system.9
However, the potential cardiovascular effects of the disease are not taken into consideration in treatment decisions. It should be noted that although patients with PHP, even mild, have a greater incidence of cardiovascular changes, the reversibility or improvement of these changes with surgery has not been consistently shown.
Our study objectives were to assess the prevalence of cardiovascular risk factors in both patients with asymptomatic PHP under observation and patients who had undergone surgery, to examine its potential relationship to calcium and PTH levels and, finally, to analyze the effect of parathyroidectomy on such levels.
Patients and methodsDesign and patientsThis was an observational, retrospective study of two groups of patients with asymptomatic PHP diagnosed and evaluated at the department of endocrinology and nutrition of Hospital Universitario San Cecilio in Granada between 2000 and 2007. Those patients for whom all clinical and laboratory data were available were selected (73 of 89 patients). A first group consisted of 40 patients (out of a total of 48) under observation due to the absence of surgical criteria or surgery refusal or contraindication, and a second group consisted of 33 patients (out of 41) who underwent surgery between 2007 and 2008 and were followed up one year after surgery.
Variables collectedEpidemiological (age and sex) and clinical history data (time since PHP onset, prior diseases, and concomitant treatments) were collected, and a physical examination including the measurement of body mass index (BMI) and systolic (SBP) and diastolic (DBP) blood pressure was performed at study start in all patients (before surgery in those to be operated on) and at one year of follow-up in patients who had undergone surgery.
Laboratory tests were performed at baseline in all patients and at 12 months in those who had undergone surgery, including basic blood chemistry with kidney and liver function tests and basal blood glucose levels; glycosylated hemoglobin (HPLC, ADAMS A1c analyzer, HA-8160, Menarini); serum triglyceride levels (TG), total cholesterol (TC), HDL cholesterol (enzymatic procedures), and LDL cholesterol (Friedewald formula) Plasma levels of calcium and phosphorus, PTH (Intact PTH test, Roche Diagnostics SL Barcelona, Spain), and 25-OH-vitamin D (25-Hydroxyvitamin D 125I RIA, DiaSorin, Stillwater, Minnesota, USA) were also measured.
Based on data from the clinical history, physical examination and biochemical parameters tests, the presence or absence of the following cardiovascular risk factors was evaluated: obesity (BMI>30kg/m2), type 2 diabetes mellitus diagnosed based on the criteria of the American Diabetes Association (ADA)10 or glucose-lowering treatment, HBP defined as SBP≥140mmHg and/or DBP≥90mmHg or antihypertensive treatment, and dyslipidemia according to the standards of the Third Report of the National Cholesterol Education Program (NCEP)11 or lipid-lowering treatment.
Data analysisThe statistical software used was SPSS version 15.0. Quantitative variables were given as mean (standard deviation), and dichotomic variables as a percentage. The normality of variables was analyzed using a Kolmogorov–Smirnov test. A value of p<0.05 was considered statistically significant. A Chi-square test was used to compare qualitative variables, or a Fisher's exact test if the conditions for the former were not met. For quantitative variables, a Student's t test was used to compare means or a Mann–Whitney test was used for independent samples (between-group differences) and paired samples (within-group differences) depending on the distribution of the variable of interest. The relationship between quantitative variables was analyzed using a bivariate Pearson correlation test if the distribution was normal or a bivariate Spearman correlation test if the variables were not normally distributed.
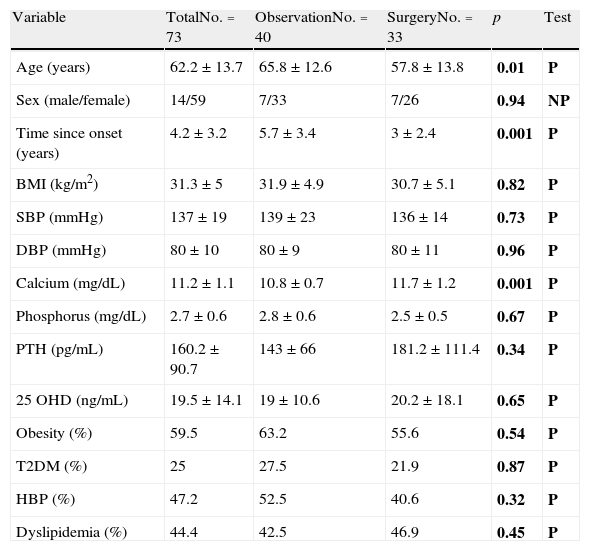
ResultsPrevalence and control of cardiovascular risk factorsTable 1 shows the general characteristics and prevalence of the different cardiovascular risk factors considered in the total sample and by study group. Patients referred for surgery were younger, had a shorter disease duration, and had higher calcium levels. However, no differences were found in physical examination data and the prevalence of cardiovascular risk factors.
General characteristics and cardiovascular risk factors in the overall sample and by study group.
| Variable | TotalNo.=73 | ObservationNo.=40 | SurgeryNo.=33 | p | Test |
| Age (years) | 62.2±13.7 | 65.8±12.6 | 57.8±13.8 | 0.01 | P |
| Sex (male/female) | 14/59 | 7/33 | 7/26 | 0.94 | NP |
| Time since onset (years) | 4.2±3.2 | 5.7±3.4 | 3±2.4 | 0.001 | P |
| BMI (kg/m2) | 31.3±5 | 31.9±4.9 | 30.7±5.1 | 0.82 | P |
| SBP (mmHg) | 137±19 | 139±23 | 136±14 | 0.73 | P |
| DBP (mmHg) | 80±10 | 80±9 | 80±11 | 0.96 | P |
| Calcium (mg/dL) | 11.2±1.1 | 10.8±0.7 | 11.7±1.2 | 0.001 | P |
| Phosphorus (mg/dL) | 2.7±0.6 | 2.8±0.6 | 2.5±0.5 | 0.67 | P |
| PTH (pg/mL) | 160.2±90.7 | 143±66 | 181.2±111.4 | 0.34 | P |
| 25 OHD (ng/mL) | 19.5±14.1 | 19±10.6 | 20.2±18.1 | 0.65 | P |
| Obesity (%) | 59.5 | 63.2 | 55.6 | 0.54 | P |
| T2DM (%) | 25 | 27.5 | 21.9 | 0.87 | P |
| HBP (%) | 47.2 | 52.5 | 40.6 | 0.32 | P |
| Dyslipidemia (%) | 44.4 | 42.5 | 46.9 | 0.45 | P |
25 OHD: 25-hydroxyvitamin D; T2DM: type 2 diabetes mellitus; HBP: high blood pressure; BMI: body mass index; NP: nonparametric test; P: parametric test; DBP: diastolic blood pressure; SBP: systolic blood pressure; PTH: parathyroid hormone.
In bold, p values and the statistical test used.
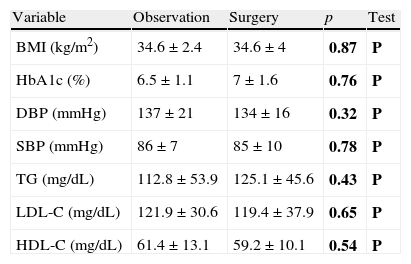
No significant differences were seen either in the BMI in obese patients, blood glucose control in diabetic patients, blood pressure control in hypertensive patients, or the lipid profile in dyslipidemic patients depending on the treatment prescribed (Table 2).
Differences in glycosylated hemoglobin, blood pressure, and lipid profile by study group.
| Variable | Observation | Surgery | p | Test |
| BMI (kg/m2) | 34.6±2.4 | 34.6±4 | 0.87 | P |
| HbA1c (%) | 6.5±1.1 | 7±1.6 | 0.76 | P |
| DBP (mmHg) | 137±21 | 134±16 | 0.32 | P |
| SBP (mmHg) | 86±7 | 85±10 | 0.78 | P |
| TG (mg/dL) | 112.8±53.9 | 125.1±45.6 | 0.43 | P |
| LDL-C (mg/dL) | 121.9±30.6 | 119.4±37.9 | 0.65 | P |
| HDL-C (mg/dL) | 61.4±13.1 | 59.2±10.1 | 0.54 | P |
HbA1c: glycosylated hemoglobin; HDL-C: HDL cholesterol; LDL-C: LDL cholesterol; NP: nonparametric test; P: parametric test; DBP: diastolic blood pressure; SBP: systolic blood pressure; TG: triglycerides.
In bold, p values and the statistical test used.
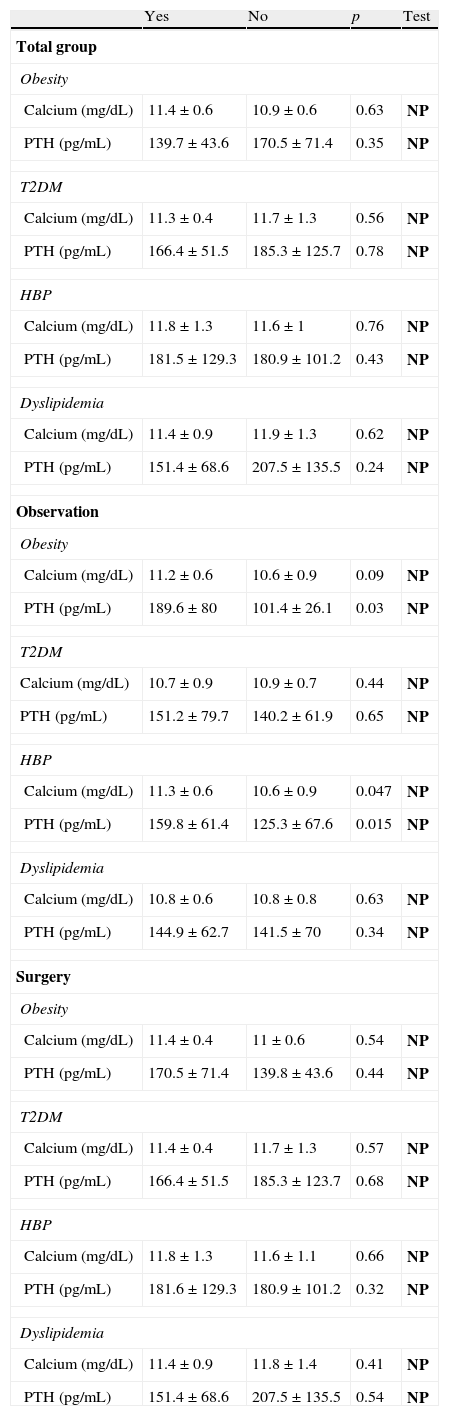
Table 3 shows calcium and PTH levels by diagnosis of obesity, type 2 diabetes mellitus, HBP, and dyslipidemia in the overall sample and by group. There were no statistically significant differences between mean calcium and PTH levels in the overall sample or in patients who had been operated on. In the observation group, however, significantly higher calcium and PTH levels were found in obese and hypertensive patients.
Differences in calcium and PTH depending on the diagnosis of obesity, type 2 diabetes mellitus, high blood pressure, and dyslipidemia in the overall sample and by group.
| Yes | No | p | Test | |
| Total group | ||||
| Obesity | ||||
| Calcium (mg/dL) | 11.4±0.6 | 10.9±0.6 | 0.63 | NP |
| PTH (pg/mL) | 139.7±43.6 | 170.5±71.4 | 0.35 | NP |
| T2DM | ||||
| Calcium (mg/dL) | 11.3±0.4 | 11.7±1.3 | 0.56 | NP |
| PTH (pg/mL) | 166.4±51.5 | 185.3±125.7 | 0.78 | NP |
| HBP | ||||
| Calcium (mg/dL) | 11.8±1.3 | 11.6±1 | 0.76 | NP |
| PTH (pg/mL) | 181.5±129.3 | 180.9±101.2 | 0.43 | NP |
| Dyslipidemia | ||||
| Calcium (mg/dL) | 11.4±0.9 | 11.9±1.3 | 0.62 | NP |
| PTH (pg/mL) | 151.4±68.6 | 207.5±135.5 | 0.24 | NP |
| Observation | ||||
| Obesity | ||||
| Calcium (mg/dL) | 11.2±0.6 | 10.6±0.9 | 0.09 | NP |
| PTH (pg/mL) | 189.6±80 | 101.4±26.1 | 0.03 | NP |
| T2DM | ||||
| Calcium (mg/dL) | 10.7±0.9 | 10.9±0.7 | 0.44 | NP |
| PTH (pg/mL) | 151.2±79.7 | 140.2±61.9 | 0.65 | NP |
| HBP | ||||
| Calcium (mg/dL) | 11.3±0.6 | 10.6±0.9 | 0.047 | NP |
| PTH (pg/mL) | 159.8±61.4 | 125.3±67.6 | 0.015 | NP |
| Dyslipidemia | ||||
| Calcium (mg/dL) | 10.8±0.6 | 10.8±0.8 | 0.63 | NP |
| PTH (pg/mL) | 144.9±62.7 | 141.5±70 | 0.34 | NP |
| Surgery | ||||
| Obesity | ||||
| Calcium (mg/dL) | 11.4±0.4 | 11±0.6 | 0.54 | NP |
| PTH (pg/mL) | 170.5±71.4 | 139.8±43.6 | 0.44 | NP |
| T2DM | ||||
| Calcium (mg/dL) | 11.4±0.4 | 11.7±1.3 | 0.57 | NP |
| PTH (pg/mL) | 166.4±51.5 | 185.3±123.7 | 0.68 | NP |
| HBP | ||||
| Calcium (mg/dL) | 11.8±1.3 | 11.6±1.1 | 0.66 | NP |
| PTH (pg/mL) | 181.6±129.3 | 180.9±101.2 | 0.32 | NP |
| Dyslipidemia | ||||
| Calcium (mg/dL) | 11.4±0.9 | 11.8±1.4 | 0.41 | NP |
| PTH (pg/mL) | 151.4±68.6 | 207.5±135.5 | 0.54 | NP |
T2DM: type 2 diabetes mellitus; HBP: high blood pressure; NP: nonparametric test; P: parametric test.
In bold, the statistical test used.
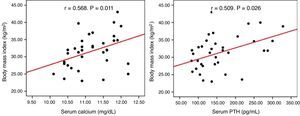
No correlation was seen between serum calcium and PTH levels and BMI, blood pressure, HbA1c, and lipids in the overall or surgical groups. In the group under observation, however, calcium (r=0.568, p=0.011) and PTH (r=0.509, p=0.026) levels positively correlated to BMI (Fig. 1).
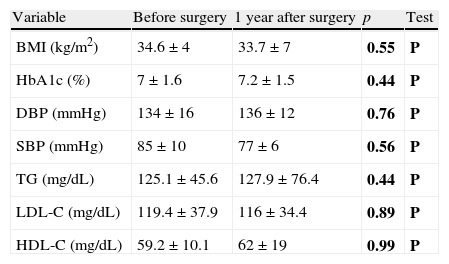
Changes over time in cardiovascular risk factors after parathyroidectomyIn surgical patients, no statistically significant differences were noted one year after surgery as compared to the preoperative study of the BMI in obese patients, HbA1c in diabetic patients, blood pressure control in hypertensive patients, or lipid profile in dyslipidemic patients (Table 4). There were no changes in the treatment of HBP, dyslipidemia and/or diabetes mellitus in patients at one year of follow-up as compared to the baseline evaluation.
Differences in the control parameters of type 2 diabetes mellitus, high blood pressure, and dyslipidemia in patients undergoing surgery before and one year after the surgical procedure.
| Variable | Before surgery | 1 year after surgery | p | Test |
| BMI (kg/m2) | 34.6±4 | 33.7±7 | 0.55 | P |
| HbA1c (%) | 7±1.6 | 7.2±1.5 | 0.44 | P |
| DBP (mmHg) | 134±16 | 136±12 | 0.76 | P |
| SBP (mmHg) | 85±10 | 77±6 | 0.56 | P |
| TG (mg/dL) | 125.1±45.6 | 127.9±76.4 | 0.44 | P |
| LDL-C (mg/dL) | 119.4±37.9 | 116±34.4 | 0.89 | P |
| HDL-C (mg/dL) | 59.2±10.1 | 62±19 | 0.99 | P |
HbA1c: glycosylated hemoglobin; HDL-C: HDL cholesterol; LDL-C: LDL cholesterol; NP: nonparametric test; DBP: diastolic blood pressure; SBP: systolic blood pressure; P: parametric test; TG: triglycerides.
In bold, p values and the statistical test used.
A high prevalence of obesity, type 2 diabetes mellitus, HBP, and dyslipidemia was found in patients with asymptomatic PHP in our study. Calcium and PTH levels were higher in obese and hypertensive patients not undergoing surgery. However, surgery did not result in an improvement in these cardiovascular risk factors one year after parathyroidectomy.
There are multiple observations relating PHP to a higher cardiovascular risk, but no previous studies are available in our environment. Our data support this relationship and agree with the results of prior studies. We therefore think that the evaluation of these patients should pay special attention to the identification and early treatment of the main cardiovascular risk factors.
An association with HBP has been known for some time, but it is not considered to be a specific manifestation of the disease, and the results as regards the response to hyperparathyroidectomy are conflicting.3,12,13 It has also been shown that PHP induces left ventricular hypertrophy in the absence of HPB, and that PTH levels correlate to left ventricular mass.14 After surgery, regression of left ventricular hypertrophy has been reported in a variable time period ranging from one to five years,15 but subsequent studies do not support this effect in patients with mild PHP.14,16,17 Type 2 diabetes has also been related to PHP, and Richards et al. reported that parathyroidectomy enhanced metabolic control in 77% of patients.18 In this regard, other studies also found improvements in parameters related to blood glucose homeostasis and insulin resistance in non-diabetic patients with PHP after parathyroidectomy.19,20
As regards the causative role of calcium and PTH levels on cardiovascular risk in our study, patients with obesity and HBP from the group under observation were found to have higher serum calcium and PTH levels.
Both serum calcium and PTH have been related to HBP in PHP. Hypercalcemia experimentally induced by the administration of intravenous calcium infusion increases blood pressure values, which return to normal after infusion is discontinued, and the same occurs after continuous PTH infusion.21
The relationship between BMI, calcium, and PTH is more complex. On the one hand, a meta-analysis by Bolland et al.22 showed that, in most studies, patients with mild hyperparathyroidism have greater BMI, which according to the authors could explain the greater presence of cardiovascular risk in this group of patients. The directionality of this relationship has not been elucidated. Increased intracellular calcium levels in adipocytes induce insulin resistance and inhibit lipolysis.23 In addition, adipocytes and osteoblasts have common precursors, and sustained PTH elevation could induce the differentiation of adipose tissue.24 The presence of obesity may also predispose to the development of PHP. In this regard, a positive relationship has been shown between PTH and body weight, even in normocalcemic subjects,25 and the hypovitaminosis D characteristic of obesity has also been related to the development of this disease.26 In our patients, 25-OH-vitamin D levels were below the optimum values. In addition, PHP has recently been shown to influence adipose tissue regulation, which may result in an impaired function of this tissue and influence the development of cardiovascular risk factors.27
The surgical patients in our sample showed no improvement in cardiovascular risk factors at one year of follow-up. As regards HBP, some studies,12,28 but not all,29 have shown blood pressure decreases after surgery. It should be noted that in studies showing no changes, mean baseline blood pressure values were not excessively elevated, as occurred in our study. This may partly be due to the fact that the one-year follow-up in our study is relatively short for showing improvements in these aspects, although it has also been suggested that PHP may cause irreversible vascular damage. This would explain the lack of postoperative improvement in different cardiovascular risk markers such as left ventricular hypertrophy and altered carotid intima-media thickness,17 and also the greater risk of cardiovascular events and mortality in patients with PHP undergoing surgery as compared to controls. No improvement was found either in metabolic control in diabetic or dyslipidemic patients, which agrees with the findings in the Ishay et al. study,30 where surgery had no beneficial effect on metabolic syndrome and insulin resistance markers one year after parathyroidectomy.
Our study has some limitations. The sample size was small, although similar studies previously reported did not enroll greater numbers of patients. This is partly due to the retrospective nature of the study, which only allowed for the inclusion of patients for whom all the clinical and laboratory data required to meet our objectives were available. Moreover, no surrogate or subclinical parameters of cardiovascular disease were assessed, but we think that the analysis of routine clinical and biochemical parameters makes the generalization to daily practice possible. In addition, a one-year follow-up period may be relatively short for assessing significant changes in cardiovascular risk markers. In our clinical practice, the standard follow-up time after parathyroidectomy is one year, when the disease is considered to be cured if surgery has been successful. In the aftermath of this study, we considered extending this period in our surgical patients in order to assess the long-term effects on cardiovascular risk and to observe the potential differences between surgical and non-surgical patients. However, cardiovascular risk and the effects of parathyroidectomy on this risk have not been studied to date in a Spanish cohort of patients with asymptomatic PHP.
To sum up, our results support the high prevalence of obesity, type 2 diabetes mellitus, HBP, and dyslipidemia in patients with asymptomatic PHP. Surgery, however, did not improve these cardiovascular risk factors. We think that larger studies with longer follow-up periods are needed to confirm the irreversible nature of cardiovascular lesions related to PHP.
Conflicts of interestThe authors state that they have no conflicts of interest in relation to this article.
Please cite this article as: García-Martín A, Reyes-García R, García-Castro JM, Quesada-Charneco M, Escobar-Jiménez F, Muñoz-Torres M. Factores de riesgo cardiovascular en pacientes con hiperparatiroidismo primario asintomático. Endocrinol Nutr. 2014;61:516–522.