To provide practical and up-to-date recommendations for evaluation, differential diagnosis, and treatment of prolactinoma and hyperprolactinemia in various clinical settings.
ParticipantsMembers of the Neuroendocrinology Working Group of the Spanish Society of Endocrinology.
MethodsRecommendations were formulated according to the Grading of Recommendations, Assessment, Development, and Evaluation system (GRADE) to describe both the strength of recommendations and the quality of evidence. A systematic search was made in Medline (Pubmed) for each subject, and authors’ considerations were added in areas where the literature provided scarce evidence. Finally, recommendations were jointly discussed by the Working Group.
ConclusionsThe document provides evidence-based practical and updated recommendations for diagnosis and management of hyperprolactinemia and prolactinoma, including drug-induced hyperprolactinemia, treatment options for prolactinoma (drugs, surgery, and radiotherapy), prolactinoma in pregnancy, adverse effects of dopaminergic agents, and drug-resistant and malignant prolactinomas.
Proporcionar recomendaciones prácticas y actualizadas para la evaluación, diagnóstico diferencial y tratamiento del prolactinoma y la hiperprolactinemia en diversos contextos clínicos.
ParticipantesMiembros del Grupo de Neuroendocrinología de la Sociedad Española de Endocrinología y Nutrición.
MétodosLas recomendaciones se formularon de acuerdo al sistema Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) para establecer tanto la fuerza de las recomendaciones como el grado de evidencia. Se realizó una búsqueda sistemática en Medline (Pubmed) para cada apartado, y se añadieron consideraciones de los autores en los aspectos en los que la bibliografía ofrece escasa evidencia. Tras la formulación de las recomendaciones estas se discutieron de forma conjunta en el Grupo de Trabajo.
ConclusionesEl documento establece unas recomendaciones prácticas y actualizadas del diagnóstico y tratamiento de la hiperprolactinemia y el prolactinoma incluyendo la hiperprolactinemia inducida por fármacos, diversas modalidades del tratamiento de los prolactinomas (fármacos, cirugía y radioterapia), prolactinoma y gestación, efectos adversos de los fármacos dopaminérgicos, y prolactinomas resistentes a fármacos y malignos.
These guidelines were based on a literature review of original papers, meta-analyses, and clinical guidelines prepared by expert groups of recognized competence, supplemented by our views and personal experiences. Whenever possible, the available evidence on each subject has been categorized based on the grades of recommendation (GRADE system) used by the Endocrine Society in preparing its clinical guidelines. This system includes two grades of recommendation (1: strong, or 2: weak) and classifies the quality of evidence into one of four categories (⊕⊕⊕⊕: high, ⊕⊕⊕○: moderate, ⊕⊕○○: low, or ⊕○○○: very low). As with other subjects related to neuroendocrinology, it should be taken into account that little high-quality evidence is available in many aspects.
Introduction: prolactinoma and hyperprolactinemiaProlactinoma is a pituitary adenoma that secretes prolactin (PRL). Prolactinomas account for 40% of all pituitary adenomas and occur more commonly in women. Based on size, they are classified as microprolactinoma (<10mm) and macroprolactinoma (≥10mm). Eighty percent of prolactinomas are intrasellar microadenomas which will not grow during follow-up.1 Although they are rarely hereditary in nature, they may be part of multiple endocrine neoplasia (MEN1) and of the so-called familial isolated pituitary adenomas.2 Malignant prolactinoma is uncommon.
PRL is subject to the inhibitory effect of dopamine. Hyperprolactinemia may therefore be not only due to tumor hypersecretion, but also because of various physiological and pathological conditions, and also due to multiple drugs which alter dopamine production, transport, or action.
Together, prolactinoma and hyperprolactinemia are a common cause of visits to endocrinology outpatient clinics.
Clinical signsRegardless of its cause, hyperprolactinemia interferes with pulsatile GnRH secretion and inhibits LH and FSH secretion, thus causing hypogonadism in both sexes and infertility. In addition, the mass of a prolactinoma may cause compressive effects on parasellar structures and hypopituitarism.
In women, most prolactinomas are microadenomas and induce menstrual changes (oligoamenorrhea), galactorrhea, and infertility. In postmenopausal women, the clinical signs are mainly due to the mass effect of the adenoma. In men, 80% of prolactinomas are macroadenomas. Thus, clinical signs due to a mass effect (headache, vision loss) and/or the involvement of other pituitary axes usually occur. Hyperprolactinemia causes decreased libido, erectile dysfunction, oligospermia and infertility and, less frequently, gynecomastia and galactorrhea. Hypogonadism induced by hyperprolactinemia is associated with decreased mineral bone density in both sexes.1
Prolactinoma is rare in children and adolescents, and causes delayed puberty and/or clinical signs due to a mass effect.
Diagnosis of hyperprolactinemiaFor most laboratories, normal serum PRL levels are less than 25ng/mL in women and less than 20ng/mL in men (1ng/mL is equivalent to 21.2mIU/L).
As recommended by the most recent clinical guidelines, the measurement of PRL in a single sample is sufficient for diagnosis if vein puncture has not been traumatic (1⊕⊕⊕⊕). When the results are doubtful (mild PRL elevations) or inconsistent with the clinical picture, the measurement may be repeated on samples taken at 15–20min intervals to minimize the effect of pulsatility.3,4
Dynamic tests (TRH, l-dopa, domperidone, etc.) have no advantage over basal PRL measurement, and are therefore not recommended3–5 (1⊕⊕⊕⊕).
PRL levels usually correlate to prolactinoma size. In large adenomas with only slightly elevated PRL levels, the “hook effect”6 should be ruled out, although this is virtually nonexistent with some of the new immunoassays.3 To detect this effect, it is recommended that PRL be tested after 1:100 serum dilution3,4 (1⊕⊕⊕⊕). The possibility that the tumor does not secrete PRL should also be considered (see section “Causes of hyperprolactinemia”).
Causes of hyperprolactinemiaThere are multiple conditions that may induce hyperprolactinemia (Table 1). Physiological causes include pregnancy, lactation, intercourse, sleep, exercise, and stress.
Causes of hyperprolactinemia.
| Physiological: Pregnancy, lactation, nipple stimulation, intercourse, sleep, exercise, stress |
| Pharmacological |
| Neuroleptics/antipsychotics: phenotiazines, haloperidol, butyrophenones |
| Antidepressants: tricyclic antidepressants, monoamine oxidase inhibitors, serotonin reuptake inhibitors |
| Antihistamines H2 |
| Estrogens: oral contraceptives |
| Antihypertensives: verapamil, methyldopa |
| Anesthetics |
| Anticonvulsants |
| Opiates: cocaine, morphine, heroin |
| Benzodiazepines |
| Dopaminergic blockers: metoclopramide, sulpiride, domperidone, cisapride |
| Serotonin, norepinephrine |
| Hypothalamic-pituitary conditions |
| Pituitary conditions: prolactinoma, acromegaly, plurihormonal adenomas, surgery, radiotherapy, trauma, hypophysitis |
| Hypothalamic conditions/pituitary stalk compression: tumors (craniopharyngioma, germinoma, meningioma, metastasis, Rathke cyst, etc.), granuloma, infiltrative diseases, trauma with stalk section |
| Other conditions |
| Chronic renal failure and hepatic insufficiency |
| Primary hypothyroidism |
| Polycystic ovary syndrome |
| Neurogenic: chest trauma, herpes zoster |
| Idiopathic hyperprolactinemia |
| Macroprolactin |
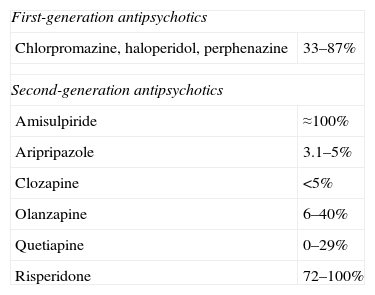
The most common cause of non-tumoral hyperprolactinemia is the action of drugs,3 mainly antipsychotic and neuroleptic drugs (Table 2), but also antihypertensives, calcium channel blockers (verapamil), antidepressants, antemetics (metoclopramide), etc.
Prevalence of hyperprolactinemia with the different antipsychotic agents.
| First-generation antipsychotics | |
| Chlorpromazine, haloperidol, perphenazine | 33–87% |
| Second-generation antipsychotics | |
| Amisulpiride | ≈100% |
| Aripripazole | 3.1–5% |
| Clozapine | <5% |
| Olanzapine | 6–40% |
| Quetiapine | 0–29% |
| Risperidone | 72–100% |
Paliperidone is an active metabolite of risperidone associated with a high prevalence of hyperprolactinemia.
In addition to prolactinoma, various hypothalamic-pituitary conditions may cause hyperprolactinemia through damage to dopaminergic neurons, brainstem compression, or hormone co-secretion (GH- and PRL-secreting adenoma).
In kidney and liver failure, PRL levels may be increased due to decreased clearance.
Highly prevalent endocrine diseases cause hyperprolactinemia. Thus, primary hypothyroidism may be associated with mild hyperprolactinemia that reverts upon the normalization of thyroid function with levothyroxine. In addition, up to 30% of women with polycystic ovary syndrome have mild PRL elevations.5
Macroprolactin (larger PRL molecules, usually due to PRL binding to an IgG antibody, or to the dimerization or glycosylation of monomeric PRL), which accumulates due to a decreased clearance, is another cause of hyperprolactinemia. Macroprolactinemia is detected by precipitation of the sample with polyethylene glycol.
Differential diagnosis of hyperprolactinemiaThe first steps in differential diagnosis in a patient with hyperprolactinemia include a thorough clinical history aimed at detecting potential secondary causes of hyperprolactinemia (including drug use) and a complete physical examination. These initial steps may already suggest a secreting tumor with neurological involvement or a functional change with little impact.
Liver, kidney, and thyroid function tests are recommended in all patients with hyperprolactinemia, and pregnancy should be ruled out in women of childbearing age3–5 (1⊕⊕⊕⊕).
Macroprolactinemia is a benign change occurring in up to 20% of patients studied for hyperprolactinemia.7 Macroprolactin molecules are less bioactive, and the typical symptoms of hyperprolactinemia are therefore usually absent. Endocrine Society guidelines suggest that macroprolactin levels be tested in patients with asymptomatic hyperprolactinemia.3 The routine measurement of macroprolactin in patients with classical symptoms is controversial.8 The test is simple and inexpensive, and by using it unnecessary procedures and treatments can be avoided. In addition, the presence of macroprolactin has been documented in patients with tumors. We therefore suggest the measurement of macroprolactin in patients with asymptomatic hyperprolactinemia.
For any hyperprolactinemia of unknown cause, pituitary magnetic resonance imaging (MRI) with gadolinium should be performed3,4 (1⊕⊕⊕⊕).
Hyperprolactinemia is considered to be idiopathic when its secondary causes have been ruled out and pituitary MRI is normal. Approximately 10% of these patients are subsequently diagnosed with a microadenoma, while spontaneous normalization of PRL levels occurs in 30%.4
Most secondary causes and macroprolactinemia induce PRL levels ranging from 25 to 100ng/mL. When hyperprolactinemia is due to hypothalamic damage or pituitary stalk compression, PRL levels are usually less than 100–150ng/mL. The findings in all these conditions are superimposable on those of microprolactinoma. PRL values higher than 250ng/mL normally suggest the presence of a prolactinoma (usually a macroprolactinoma), but it should be taken into account that some drugs such as metoclopramide, risperidone, or phenotiazines may induce PRL levels higher than 200ng/mL.1 To sum up, PRL levels help guide diagnosis, but superimposition exists between the different etiologies.
Drug-induced hyperprolactinemiaIn patients treated with drugs that may cause hyperprolactinemia, we suggest that, if at all possible, a new PRL measurement be performed at least 72h after drug discontinuation3,4 (2⊕⊕○○). For some drugs, the time required for the normalization of PRL levels after discontinuation is unknown. It should be noted that this time may be longer when depot drug formulations are used.
In the event of psychotropic medication, the drug should be discontinued by the psychiatrist, and the possibility of administering an alternative treatment with a lower impact on PRL levels should be considered. An additional option is the use of aripripazole, an atypical antipsychotic with dopamine agonist and antagonist activity that may decrease PRL levels.3
If drug discontinuation is not possible, MRI is advised to rule out the presence of pituitary or hypothalamic tumors3,4 (1⊕○○○).
The treatment of drug-induced hyperprolactinemia should be restricted to symptomatic patients in whom the concerned drug cannot be discontinued or replaced. Replacement therapy with estrogens in women and testosterone in males with symptomatic hypogonadism and/or decreased bone density is suggested3,4,9 (2⊕○○○).
Treatment with dopamine agonists of patients with hyperprolactinemia induced by antipsychotics is highly controversial because of the risk of exacerbating clinical psychotic signs.9 In our view, this option should only be considered, under strict control, in patients with symptomatic hyperprolactinemia in whom the antipsychotic drug cannot be replaced and replacement therapy with estrogens/testosterone is absolutely contraindicated, or in the special case of women who want to become pregnant within this context.
Imaging studiesThe imaging test indicated for pituitary lesions is MRI. Examination may be performed after hyperprolactinemia is detected and secondary causes have been ruled out, or may be requested for other reasons (headache, campimetric changes, etc.), with the finding of a pituitary lesion, and the subsequent functional study revealing hyperprolactinemia. MRI should consist of T1- and T2-weighted images and after the injection of a paramagnetic contrast agent (gadolinium), using thin slices (2–3mm).10 If this examination is not possible, high-resolution computed tomography should be performed (1⊕⊕⊕○).
Since the initial management of microprolactinoma and persistent idiopathic hyperprolactinemia will be similar, a more intensive search for microadenomas is not required when they are not evident in this examination. Three-Tesla MRI, as studied in other microadenomas (e.g. ACTH-secreting tumors, whose identification is crucial for treatment11), in which however it did not improve localization sensitivity, has therefore not been assessed. Positron emission tomography with various tracers such as FDG, 11Cmethionine, or F18-dopa12 (the latter studied in other diseases affecting dopamine pathways, such as Parkinson's disease) has not been routinely assessed either.
The recommended timing of imaging tests is discussed in the section “Treatment monitoring”.
Supplemental testsIn intrasellar microadenomas there is no need to assess visual field or pituitary function, except when hypogonadism does not disappear after the normalization of serum PRL levels. In macroadenomas, overall adenohypophysial evaluation is recommended (thyrotropic, gonadotropic, corticotropic, and somatotropic axes). Moreover, if the tumor is in contact with or compresses the chiasm, an initial complete neuro-ophthalmological evaluation is advised, including visual acuity, computed campimetry, extrinsic eye movements, and pupil and eye fundus examination.3,4 The subsequent course may of course condition the treatment approach.
On the other hand, if a long-term history of hypogonadism is found at the time of diagnosis, there may be bone mineral density (BMD) loss, which should initially be assessed using dual photon densitometry. In our view, it is advisable to monitor the course of BMD and to consider the use of specific treatment if no recovery occurs when gonadal function is restored.
Genetic studyMost pituitary tumors, including prolactinomas, are sporadic in nature. Some of them, however, are part of well defined familial syndromes such as MEN1 (MEN1 gene) or Carney's complex (PRKAR1A gene), and also of other syndromes of familial predisposition to the development of pituitary tumors (familial isolated pituitary adenomas) identified in recent years in relation to the tumor suppressor gene AIP (encoding the AIP-aryl hydrocarbon receptor-interacting protein).2,13 Pituitary tumors, but not prolactinomas, related to so-called MEN4 (due to mutations in the CDKN1B gene (p27kip1), have been reported in humans. Genetic study should be considered in patients with prolactinomas with a history of familial clustering, association with other endocrine neoplasms, or aggressive forms and/or forms starting at an early age. Patient phenotype and family history will define the testing sequence of the different genes. No evidence is yet available of the potential impact of this information on disease management and prognosis.
These sophisticated tests are only available at some centers. The laboratories performing the tests in Spain and the relevant contact persons may be consulted at the SEEN website (www.seen.es), at the section Endocrinology/List of tests/Genetics.
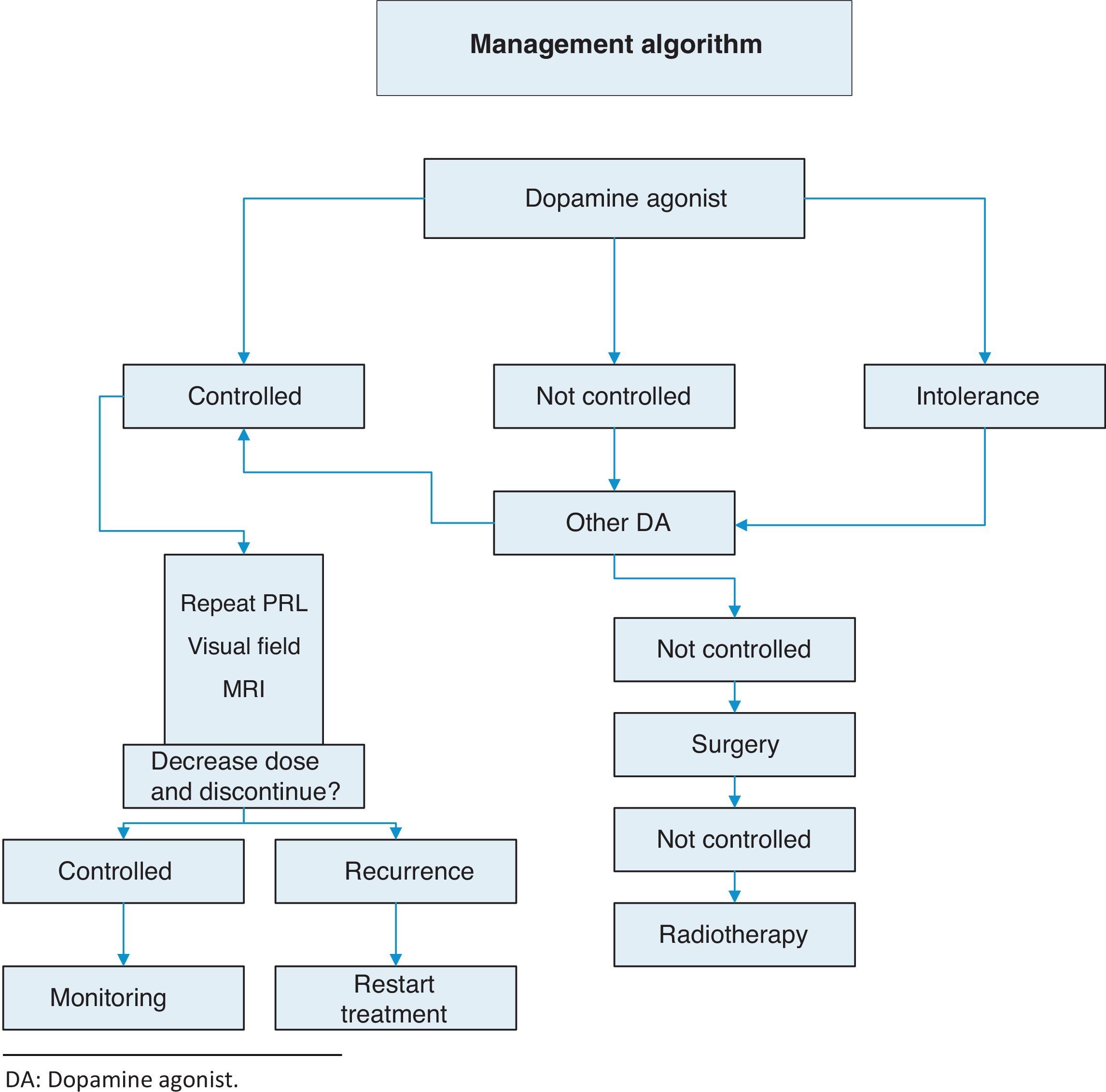
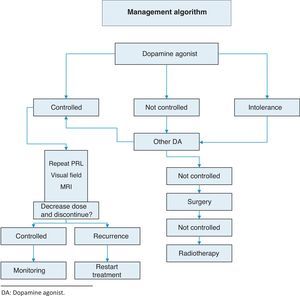
TreatmentThis is summarized in Annex 1: Treatment algorithm.
- A.
Objectives: we recommend treatment of prolactinomas to3,4,14:
- 1.
Decrease PRL levels and reverse clinical signs.
- 2.
Decrease tumor size.
- 3.
Restore gonadal function and other pituitary hormone deficiencies.
- 4.
Prevent tumor recurrence and progression.
- 1.
- B.
Indications: to avoid the effects of tumor size and hyperprolactinemia. We recommend treatment of patients with symptomatic microprolactinomas and macroprolactinomas (1⊕⊕⊕⊕). Symptoms derived from hyperprolactinemia which represent indications for treatment include: hypogonadism, bothersome galactorrhea, infertility, and BMD decrease.4
Since microprolactinomas rarely grow,15 patients with asymptomatic microprolactinoma do not mandatorily require treatment. We therefore suggest that they should not be treated (2⊕OOO). This may be the case for postmenopausal women (previously treated or untreated) in whom hyperprolactinemia no longer conditions hypogonadism.
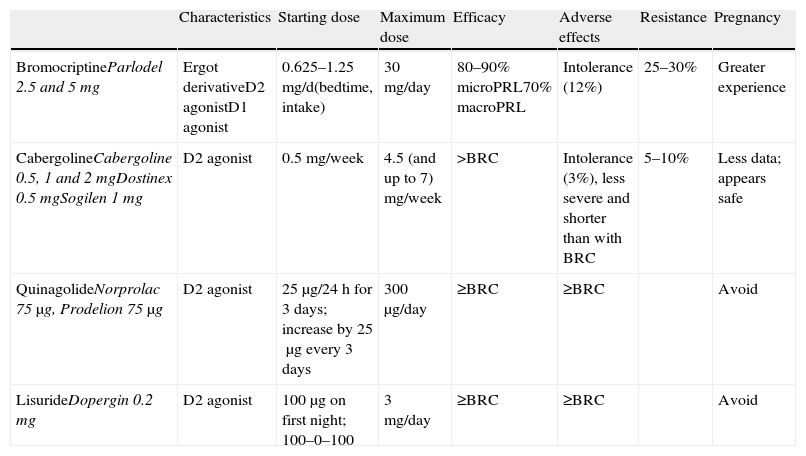
Medical treatment: dopamine agonistsThis is the first choice in all patients in whom treatment is indicated, even in those with campimetric deficiency.4 Its characteristics are summarized in Table 3.
Characteristics and available dosage forms of dopamine agonists.
| Characteristics | Starting dose | Maximum dose | Efficacy | Adverse effects | Resistance | Pregnancy | |
| BromocriptineParlodel 2.5 and 5mg | Ergot derivativeD2 agonistD1 agonist | 0.625–1.25mg/d(bedtime, intake) | 30mg/day | 80–90% microPRL70% macroPRL | Intolerance (12%) | 25–30% | Greater experience |
| CabergolineCabergoline 0.5, 1 and 2mgDostinex 0.5mgSogilen 1mg | D2 agonist | 0.5mg/week | 4.5 (and up to 7) mg/week | >BRC | Intolerance (3%), less severe and shorter than with BRC | 5–10% | Less data; appears safe |
| QuinagolideNorprolac 75μg, Prodelion 75μg | D2 agonist | 25μg/24h for 3 days; increase by 25μg every 3 days | 300μg/day | ≥BRC | ≥BRC | Avoid | |
| LisurideDopergin 0.2mg | D2 agonist | 100μg on first night; 100–0–100 | 3mg/day | ≥BRC | ≥BRC | Avoid |
BRC: bromocriptine; MacroPRL: macroprolactinoma; MicroPRL: microprolactinoma.
Several dopamine agonists, as described below, have been used to date
- A.
Cabergoline: this is an ergot dopamine agonist with a long half-life which allows for once or twice weekly oral administration. The starting dose is 0.25–0.5mg/week and is increased weekly until normal PRL levels have been achieved. Mean dose is 0.5–1mg/week. It is recommended as the first choice because of its greater efficacy (both in terms of normalization of PRL levels and tumor size decrease (1⊕⊕⊕⊕)) and better tolerability.3,16 The only exceptions could be tumor growth during pregnancy or for economic reasons. In these cases, the first choice would be bromocriptine.
- B.
Bromocriptine: this was the first drug introduced for the treatment of hyperprolactinemia more than 30 years ago. It is a D2 selective dopamine agonist and D1 antagonist. It is usually administered 2–3 times daily because of its short half-life, but one dose is sufficient in some patients. Bromocriptine should be started at a dose of 0.62–1.25mg/day, which is increased by 1.25mg every week. The therapeutic margin usually ranges from 2.5 to 7.5mg/day. However, some patients eventually need daily doses of 20–30mg. Side effects such as nausea, vomiting, postural arterial hypertension, and headache are very common, increase with high doses, and often limit treatment compliance. Bromocriptine is the drug of choice in pregnancy because of the greater cumulative experience with it, but it is less well tolerated than cabergoline.
- C.
Quinagolide: this is a non-ergot dopamine agonist. It is administered as a single daily dose; 25μg/day are initially given for 3 days, which are increased to 50μg/day for another 3 days, and to 75μg/day from day 7 onwards. Fifty percent of patients refractory to bromocriptine respond to quinagolide.
- D.
Lisuride: this drug has characteristics similar to quinagolide. It is administered twice daily; treatment is started with 0.1mg at night, and the dose is gradually increased. Mean dose is 0.3mg/day.
- E.
Pergolide: this drug has been withdrawn in the US because of a greater rate of valve disease as compared to all other dopaminergic drugs. Its use is not recommended.
These mainly occur at the start of treatment; initial doses should therefore be very low, and be gradually increased:
- •
Gastrointestinal (most common): nausea, vomiting, constipation, reflux, and dyspepsia.
- •
Neurological: headache, dizziness, dyskinesia, and confusion.
- •
Cardiovascular: postural hypertension, syncope, and finger vasospasm.
- •
Cerebrospinal fluid fistula: potential complication of treatment of big adenomas.17
- •
Other: dry mouth, muscle cramps, psychosis, and mania.
Treatment response is defined as the normalization of PRL levels (biochemical response) and tumor size reduction.3
Treatment monitoringGeneral recommendations should be adapted to the aggressiveness of the disease, so that invasive macroprolactinomas require close monitoring, even weekly, and microprolactinomas may be monitored at even longer periods than discussed.
- •
Serum PRL: one month after treatment start and regularly thereafter (depending on response) for dose adjustment.3
- •
MRI: at 3 months in macroadenoma. This has not been established as being required for microprolactinoma unless PRL increases or new symptoms occur.3 For macroadenomas, MRI should be performed annually thereafter.
- •
Campimetry: baseline assessment in macroadenoma with the risk of compression of the optic chiasm, and then depending on the course.
- •
BMD: at baseline if there is a long-term history of hypogonadism. Subsequently, repeat testing should be considered if it is affected.
- •
Rest of pituitary function: in macroadenoma at diagnosis and depending on the course thereafter. It should also be assessed in microadenomas in which gonadal function is not restored upon the normalization of PRL.
Resistance to dopamine agonists is defined as failure to achieve normal PRL levels with the maximum tolerated dose of dopamine agonists and the lack of a 50% reduction in tumor size.3 In this situation, we recommend:
- •
Reaching the maximum tolerated dose3 (1⊕⊕⊕⊕).
- •
Switching to cabergoline in patients refractory to bromocriptine3 (1⊕⊕⊕⊕) or, as a second option, using some of the other dopamine agonists. A response to bromocriptine has been achieved in some patients refractory to cabergoline.18
- •
Transsphenoidal surgery in patients who do not respond to any of the above regimens.3
- •
If surgery fails, radiotherapy.
The cause of resistance is not fully known, but a decreased number of D2 receptors have been reported, among other mechanisms. Ten percent of microprolactinomas and 18% of macroprolactinomas are resistant to cabergoline.19
Prolactinomas are more frequently refractory in males as compared to females.20
EchocardiographyThe risk of cardiac valve disease seen in Parkinson's disease treated with very high doses of dopamine agonists was recently studied in patients with hyperprolactinemia. Pergolide was withdrawn from the market for this reason. Studies were conducted with cabergoline, but no clear evidence of either an association at the standard dose or a potential impact of the total cumulative dose was found, which may be important in treatments lasting decades.21–24
Although the cumulative risk dose has not yet been established, we suggest that performing routine echocardiographic controls in the first few years of treatment in patients receiving cabergoline 1–2mg weekly is not indispensable,3 unless advised by the presence of coexistent clinical factors. On the other hand, we suggest echocardiography in patients who require high initial cabergoline doses (>2mg/week) or after >5 years of treatment. In any case, because of the duration of these treatments (lifetime administration is sometimes required), it is advisable to keep a record of the cumulative doses of dopamine agonists.
Other alternativesWomen with microprolactinomas who do not want to become pregnant may not require treatment with dopamine agonists. Women with amenorrhea may be treated with estrogens and should be measured for PRL levels annually. MRI should be repeated if tumor expansion signs occur or PRL levels increase.4
Discontinuation of dopamine agonistsControversy still exists about optimal treatment duration and whether treatment should be definitively discontinued.25 The discontinuation of dopamine agonists should be considered in carefully selected patients because disease relapse may lead to the recurrence of hyperprolactinemia and tumor re-expansion.
The recurrence of hyperprolactinemia upon the discontinuation of dopaminergic treatment in microprolactinoma and macroprolactinoma is a common event, which increases in frequency the longer the follow-up (up to approximately 80% at 8 years). Recurrence is correlated to the duration of treatment with dopamine agonists, PRL levels at diagnosis, and initial tumor size. The most recent clinical guidelines suggest that drug treatment should be discontinued in patients who show for at least two years persistent normal prolactin levels and have no remaining tumor visible in MRI or a marked decrease in the initial tumor size (Table 4) (2⊕○○○). Barber et al. suggest that the duration of dopaminergic treatment should be longer than two years, mainly in macroprolactinomas.26 When treatment discontinuation has been decided upon, we suggest that the dosage of dopamine agonists be gradually reduced to a dose that still allows PRL to be maintained within normal levels (2⊕⊕○○) until the drug is finally discontinued.
Criteria for the discontinuation of treatment with dopamine agonists.
| Favorable | Exclusive |
| Treatment duration ≥2 yearsNormal prolactin levelsNo radiographic image of adenomaTumor size decrease:- >50% compared to baseline- Macroadenoma size decreased to <10mmPregnancyPostmenopausal statePossibility of adequate medical monitoring | Treatment duration <2 yearsHigh prolactin levelsTumor size increasePersistent tumor size >10mmTumor margin very close to optic chiasmCavernous sinus invasionImpossibility of adequate medical monitoring |
Since PRL levels may continue to be suppressed until 120 days after drug discontinuation,27we suggest that the first assessment of PRL levels after discontinuation be delayed for three months. PRL should subsequently be monitored every three months for the first year and annually thereafter for at least five years, particularly in patients with macroadenoma. If PRL levels significantly increase to values higher than 100ng/mL or either neurological symptoms or vision loss occur, MRI should be requested, especially if the patient initially had a macroadenoma.
We recommend that treatment with dopamine agonists should not be discontinued in patients in whom the upper margin of adenoma is less than 5mm from the optic chiasm, there is invasion of cavernous sinuses,5 or high PRL levels and/or tumor size increase or non-reduction are seen (Table 4). An exception could be microadenomas with no decrease in tumor size but with radiographic changes suggesting necrosis, provided PRL levels are kept controlled with low doses of dopamine agonists.
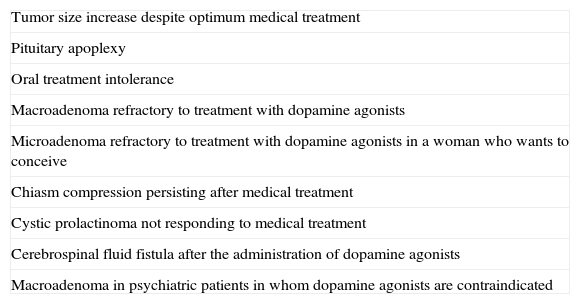
SurgeryOnly a minority of patients require surgery. We usually recommend transsphenoidal surgery when treatment with dopamine agonists does not decrease PRL levels or macroadenoma size. In microadenomas, surgery is usually limited to patients with no response to drug treatment and persistent clinical signs (2⊕○○○) (Table 5).
Indications for surgery in prolactinomas.
| Tumor size increase despite optimum medical treatment |
| Pituitary apoplexy |
| Oral treatment intolerance |
| Macroadenoma refractory to treatment with dopamine agonists |
| Microadenoma refractory to treatment with dopamine agonists in a woman who wants to conceive |
| Chiasm compression persisting after medical treatment |
| Cystic prolactinoma not responding to medical treatment |
| Cerebrospinal fluid fistula after the administration of dopamine agonists |
| Macroadenoma in psychiatric patients in whom dopamine agonists are contraindicated |
Surgical cure rates range from 75% to 90% for microadenomas and from 18% to 80% for macroadenomas. Surgical results depend on neurosurgeon experience and skills, tumor size, and PRL levels.28 Cure rates of both microadenomas and macroadenomas decrease when presurgical PRL levels are higher than 200μg/L.29 Some studies suggest that prior treatment with dopamine agonists may worsen the surgical outcome by inducing fibrosis.30
In the hands of experienced surgeons, surgical mortality is very low (0.2%) and few complications occur in the early postoperative period.28 We therefore suggest that pituitary surgery be performed at hospitals where at least 25 pituitary surgeries are performed annually (2⊕○○○).
PRL levels <20μg/L the morning after surgery suggest remission in patients who have not received dopamine agonists for four weeks before surgery.31,32 In a series of 409 women, the best predictor of cure was a PRL level <5μg/L on the first postoperative day. When cabergoline is maintained until surgery, measurement of PRL levels should be delayed until three months after surgery to obviate the effects of the drug.27
Tumor recurrence is uncommon in microadenomas, but occurs in more than 80% of patients with macroprolactinoma.33 The time to a recurrence of hyperprolactinemia after surgery may be long, but most studies report a recurrence within three years.34 We suggest that patients be monitored every three months in the first year after surgery and annually thereafter for at least five years, particularly in cases with macroprolactinoma.
No evidence is yet available on the superiority of new endoscopic procedures over prior techniques in transsphenoidal surgery.
RadiotherapyThe efficacy of radiotherapy should always be weighed against the complications derived from the treatment. Standard radiotherapy achieves a normalization of PRL levels in only 34.1% of patients, and most often after a long latency period with significant side effects (the risk of hypopituitarism, the occurrence of secondary intracranial neoplasms, cerebral injury, optic nerve damage). We suggest that radiotherapy only be used in patients with big tumors who are not candidates for surgery and who do not respond to dopamine agonists or cannot be treated with them, and in patients with aggressive prolactinoma or carcinoma (2⊕○○○).
No scientific evidence is available to support the recommendation by some authors to discontinue dopamine agonists before radiotherapy is administered to improve its results.35
It is not known whether stereotactic radiotherapy is superior to standard radiotherapy in decreasing the side effects and/or decreasing hormone levels and tumor size more rapidly. Tumors with suprasellar extension or those in which the separation between tumor margins and optic chiasm is less than 5mm should not be treated with a single radiotherapy dose.36 Prolactinomas invading the cavernous sinus may receive a single radiotherapy dose because the cranial nerves at this level are relatively radiation-resistant.
Reproductive ageIn women of childbearing age, the use of barrier contraceptives should be advised when drug treatment is started because ovulation and fertility may rapidly be recovered after the normalization of PRL levels.
Premenopausal women with microprolactinoma and hypogonadism who do not want to become pregnant and do not tolerate or respond to dopamine agonists may be treated with oral contraceptives to prevent bone mass loss. There is no evidence that estrogen therapy increases tumor size in these patients, but prospective studies would be needed to clarify this.
In men, treatment with dopamine agonists usually causes an improvement in sperm quality together with the restoration of gonadal function and decreased tumor size.37
Planning of pregnancyAchieving a normalization of PRL levels and a tumor size <10mm is recommended before conception (1⊕○○○). Transsphenoidal surgery may be an option in women with microadenoma or macroadenoma intolerant or refractory to dopamine agonists,4 and in those with macroadenoma that does not decrease in size with drug treatment38 (2⊕○○○). However, transsphenoidal surgery may also cause hypopituitarism, requiring the subsequent use of assisted reproduction techniques.
When ovulation cannot be restored in women with microprolactinomas who want to become pregnant, the use of clomiphene citrate or gonadotropin therapy is suggested.
In planning before pregnancy, cabergoline should be discontinued and bromocriptine should be introduced, although this also crosses the placental barrier39 and the use of dopamine agonists is not recommended during pregnancy.
PregnancyWhen the first menstrual period is missed and a pregnancy test is positive, we recommend the discontinuation of bromocriptine to prevent fetal exposure (2⊕⊕○○).
During normal pregnancy, PRL levels increase more than 10 times,40 and treatment decisions in women with prolactinoma should therefore be taken based on their symptoms and signs, rather than on plasma PRL levels. Moreover, PRL levels should not be routinely measured during pregnancy (1⊕⊕⊕⊕).
In normal pregnancy, the pituitary gland may double in size due to lactotropic cell hyperplasia secondary to high estrogen levels.41 The risk of symptomatic tumor growth during pregnancy is 2.2–5% in women with microprolactinoma, but may be up to 31% in macroprolactinoma. It would therefore be appropriate to continue treatment with dopamine agonists in women with macroprolactinoma who have achieved pregnancy while on this treatment, particularly if the tumors are invasive or close to the optic chiasm. If patients with macroadenoma have previously been treated with surgery or radiotherapy, the risk of symptomatic growth is only 2.8–4.3%, similar to that of women with microadenoma.
In women with microprolactinoma, the clinical evaluation at each trimester of pregnancy is only suggested. In women with macroprolactinoma, a campimetric study should also be performed every three months, or even more frequently if evidence of suprasellar growth was already available before pregnancy.
If neurological symptoms or visual changes occur, an ophthalmological and morphological MRI study (without gadolinium) is recommended in pregnant women with both microprolactinoma and macroprolactinoma (1⊕⊕○○).
If symptomatic tumor size increase occurs, treatment with bromocriptine should be restarted, if previously discontinued (1⊕⊕○○). It has been reported that the use of bromocriptine did not increase the risk of fetal malformations in more than 2500 pregnancies.42 Experience with cabergoline is more limited, but the data appear to be similar.42,43 However, some recent findings43 suggest the need for larger series assessing and monitoring the subsequent neurological development of children exposed in utero to cabergoline.
Surgery may also be considered in patients with tumor growth. The benefits and risks of surgery as compared to medical treatment should be discussed with each patient, because no controlled studies demonstrating what the best therapeutic option is are currently available. Resumption of the dopamine agonist is probably less harmful for the mother and fetus than surgery. If drug treatment is selected, it should be monitored continuously. Transsphenoidal surgery or labor induction, if the fetus is almost at term, is recommended when there is no response to dopamine agonists and progressive vision impairment38 (1⊕⊕○○).
Postpartum and lactationAfter birth, treatment with a dopamine agonist is not restarted in women with microadenomas who want to breast-feed. Although there is no evidence that breast-feeding induces tumor size increase,14 it is usually not advised in macroadenoma (1⊕○○○). It may however be considered if the mother wants to breast-feed her child and no tumor size increase has been found during pregnancy.
In normal pregnancies, normal prolactin levels are restored approximately six weeks after birth, and PRL measurements may therefore be resumed from that time.
The spontaneous remission of hyperprolactinemia has only been reported in women with microprolactinomas.44 In these cases, the long-term discontinuation of treatment with dopamine agonists after birth with regular monitoring for at least five years may be considered.
Prolactinoma in children and adolescentsProlactinoma is the most common pituitary adenoma in children and adolescents. Virtually all prolactinomas diagnosed in children are asymptomatic, and should therefore be mandatorily treated.45 The use of dopamine agonists is the first treatment option in the absence of complications requiring immediate surgery. Radiotherapy should be limited to the more aggressive tumors not responding to dopamine agonists.
Prolactinoma during menopauseFew studies are available regarding the treatment of prolactinoma in postmenopausal patients. The management of macroprolactinoma does not differ from that in other age groups, but the treatment of microadenomas is probably unnecessary (1⊕○○○). Cases where menopause may be related to the resolution of hyperprolactinemia have also been reported.46 If discontinuation of drug treatment is decided upon, patients should be monitored regularly for at least five years.
Treatment of malignant prolactinomaThe incidence of malignant prolactinoma is very low. Malignant prolactinoma should be suspected in patients with no response to drug treatment or recurrence after surgery. A diagnosis of malignancy is only made based on tumor extension to non-contiguous areas of the central nervous system or with the presence of metastasis. Pathological examination is not conclusive, but there are atypical parameters suggesting malignancy such as the presence of multiple mitoses, atypias and nuclear polymorphism, positive p53 immunoreactivity, a Ki-67 proliferation index higher than 3%, and invasion.47
Only 60% of patients with PRL-secreting carcinoma survive for longer than one year after the development of metastases. In most cases, treatment with surgery, radiotherapy, chemotherapy, or dopamine agonists, either alone or in combination, achieves no results or only a partial response.48
Temozolomide has been used with encouraging results in several cases of PRL-secreting pituitary carcinomas49 that do not respond to treatment with dopamine agonists and in benign but invasive prolactinomas.50 We therefore suggest that temozolomide be used in patients with malignant or invasive prolactinomas not controlled with surgery and radiotherapy (1⊕○○○).
Histological study. New technologiesProlactinomas account for more than 50% of pituitary tumors, but are much less common in surgical series because they are adequately man×aged with drug treatment. However, some of them are clinically more aggressive, either because they are refractory to dopamine agonists or because they have a large size at diagnosis and a high recurrence rate after surgery. The above discussed classical histopathological information should therefore be supplemented with data that may predict for increased aggressiveness, invasiveness, and even malignancy (defined in pituitary tumors as the presence of distant metastases).47 Conventional data suggesting aggressiveness or malignancy (nuclear polymorphism, atypia, necrotic areas) have a limited value in these tumors. The finding of a Ki-67 proliferation index >3% or immunoreactivity to p53 >3% is considered to predict for increased invasiveness and recurrence. Research is ongoing on other cell cycle and adhesion markers (cyclins D1 and E, proliferating cell nuclear antigen [PCNA], polysialylated neural cell adhesion molecule [PNCAM], reduced E-cadherin/catenin expression) and the altered expression of genes regulating angiogenesis (FGF, VEGF, and others), which could have clinical value in the future.51 It has recently been reported that the immunoexpression of O6-methylguanine-DNA-methyltransferase (MGMT), associated with an aggressive behavior in pituitary tumors (especially prolactinomas), may predict a positive response to temozolomide.50,52 Finally, molecular studies on the expression and function of dopamine and estrogen receptors may also provide clinically relevant information. All these procedures, only available at some laboratories, are optimally developed in the setting of multicenter studies such as the Molecular Registry of Pituitary Adenomas recently promoted in Spain by the SEEN. (For contact and information, see: http://www.remahnacional.com).
Conflicts of interestThe authors state that they have no conflicts of interest.
Please cite this article as: Halperin Rabinovich I, et al. Guía clínica de diagnóstico y tratamiento del prolactinoma y la hiperprolactinemia. Endocrinol Nutr. 2013;60:308–19.