Activity of the hypothalamic–pituitary–adrenal axis had been studied for the past half century, when some researchers noted that some patients with Cushing's syndrome and severe mood disorders had high baseline cortisol levels, which resulted in an inhibited response in the 1mg dexamethasone suppression test. Altered dexamethasone suppression test results were subsequently found in many psychiatric diseases, including anorexia nervosa, obsessive-compulsive disorder, degenerative dementia, bipolar disorders, and schizophrenia. The relationship between high baseline cortisol levels and stress has also been studied. Some researches on the genesis of borderline personality disorder focused on traumatic childhood backgrounds. Other investigations aimed at elucidating the relationship between traumatic backgrounds and some psychiatric disorders noted that patients with post-traumatic stress disorder and borderline personality disorder showed an enhanced cortisol suppression with low cortisol doses (0.5mg). Recent studies showed that use of an ultra-low dose of cortisol during the dexamethasone suppression test may be helpful for deteting disorders with hyperactivity of the hypothalamic–pituitary–adrenal axis.
Recent advances in neuroimaging support the existence of hyperactivity of the hypothalamic–pituitary–adrenal axis in patients with borderline personality disorder, relating a decreased pituitary gland volume to major traumatic backgrounds and suicidal attempts. The purpose of this paper is to make a narrative review of research using dexamethasone suppression test in psychiatric disorders, in order to ascertain its value as a supplemental diagnostic test or as a prognostic marker.
El interés en el estudio de la actividad del eje hipotálamo–hipofisario–adrenal, en los pacientes con trastornos mentales, surgió a raíz de ver cómo pacientes con trastornos depresivos graves presentaban un hipercortisolismo basal, similar al encontrado en pacientes con síndrome de Cushing. El hipercortisolismo en estos pacientes se veía reflejado por una ausencia de supresión en el test de supresión con dexametasona en dosis única de 1mg. Del mismo modo, con posterioridad se ha visto que numerosas enfermedades psiquiátricas han mostrado algún grado de alteración en esta prueba, como la anorexia nerviosa, el trastorno obsesivo compulsivo, procesos neurodegenerativos, los cuadros maníacos y la esquizofrenia. También en los últimos años se ha estudiado la relación entre el hipercortisolismo basal y el estrés, generando nuevas líneas de estudio en los trastornos de la personalidad, y el trastorno de estrés postraumático. Concretamente en el trastorno límite de la personalidad se estudió la respuesta al estrés, por la importancia que los eventos estresantes y traumáticos tienen en la génesis de este trastorno. Investigaciones realizadas en esta línea demostraron que la utilización de dosis menores de dexametasona podrían ser de una gran utilidad en el estudio de aquellos trastornos que cursaran con una respuesta exagerada al estrés. El objetivo del presente articulo es realizar una revisión narrativa de los estudios neuroendocrinos realizados mediante test de supresión con dexametasona en los trastornos psiquiátricos, y de esta manera conocer su utilidad como prueba complementaria diagnóstica o como marcador pronóstico.
Adrenocorticotropic hormone (ACTH) and cortisol levels increase rapidly in states of physical or psychical stress, hypoglycemia, or fever. Axis regulation mainly depends on three mechanisms. The first mechanism is mediated by pulsatile corticotropin-releasing hormone (CRH) as a function of the endogenous circadian rhythms of the central nervous system. In the second regulatory mechanism, ACTH stimulates the adrenal cortex through the fascicular zone, where glucocorticoids (cortisol and corticosterone) are secreted, and the reticular zone, producing androgens such as dehydroepiandrosterone (DHEA) and androstenedione. Finally, the third regulatory mechanism is the feedback circuit formed by circulating glucocorticoids, by the general stream on the pituitary gland, the hypothalamus, and other areas outside the hypothalamic–pituitary–adrenal axis (HPA), such as the hippocampus.1
Similarly to ACTH, daily cortisol secretion shows a highly marked circadian rhythm (maximum levels in the morning and low levels in the evening). ACTH regulates cortisol release from the adrenal cortex. Hypothalamic CRH is the regulator of ACTH. Cortisol acts through a negative regulation system that affects ACTH and CRH release.1
Measurement of cortisol levelsSince cortisol levels change during the day, blood cortisol levels should be related to sampling time, and since some laboratories do not have their own reference cortisol levels depending on sampling time, an alternative is to measure cortisol at about midnight, which is when the highest level is reached. Blood is the sample of choice, although measurement may also be done in saliva. If cortisol level is high in these tests, there is probably impaired cortisol secretion. Other tests are subsequently requested to ascertain the reason for such increases.
Cortisol in 24-h urineThis is also known as urinary free cortisol, and is often used to assess total cortisol production.
Corticotropin-releasing hormone stimulation testIn this test, CRH is injected and ACTH and cortisol levels are measured at different times: just before CRH administration (basal level) and serial over time, for example 30 and 60min after CRH administration. The normal response represents maximum ACTH levels followed by maximum cortisol levels. Most patients with Cushing's syndrome, due to either adrenal tumors or tumors inducing ectopic ACTH secretion, do not respond to the administration of CRH. ACTH may be measured in blood samples taken through a catheter placed in the inferior petrosal sinuses, which are venous formations carrying blood from the pituitary gland.
Dexamethasone suppression testDexamethasone is a synthetic steroid that mimics cortisol action, causing feedback inhibition of CRH and ACTH production. There are different modalities of this suppression test which are used to confirm the diagnosis of Cushing's syndrome. The normal response to dexamethasone consists of the suppression of cortisol secretion. Patients with Cushing's syndrome do not show adequate suppression of cortisol secretion after a single low dexamethasone dose administered at bedtime, and the dexamethasone suppression test (DST) is then considered positive.2 Higher dexamethasone doses may also be administered over 48h to differentiate an ACTH-secreting pituitary tumor from other potential causes of Cushing's syndrome.
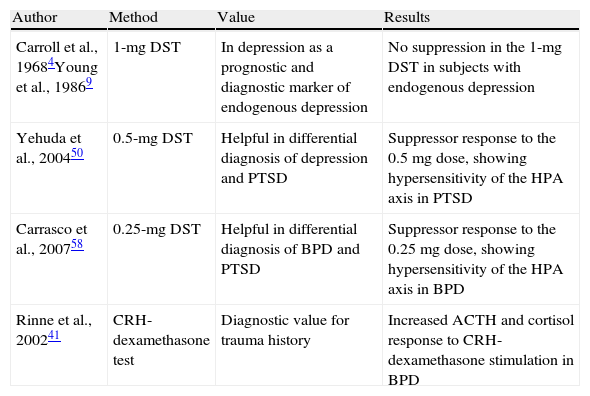
Studies in mood disordersTo speak of depression, at least two clinical symptoms should be found: low mood and anhedonia, or loss of interest in normally pleasurable activities.3 The current psychiatric classifications divide depression into two large groups: major depression, encompassing severe depressions lasting for at least two weeks, having a greater biological substrate and impairing overall functioning of the subject; and dysthymia, encompassing mild depressions lasting for at least two years, having a greater neurotic substrate and impairing overall functioning of the subject to a lesser extent. Studies of depression and activity of the HPA axis started in the late 60s when a group of researchers headed by Carroll et al.4–6 reported increases in cortisol secretion refractory to dexamethasone suppression in the 1-mg DST in patients with severe depression. This had previously been seen in patients with Cushing's syndrome (Table 1). Based on these studies, it was concluded that the 1-mg DST had 67% sensitivity and 96% specificity for identifying patients with depressive symptoms of a melancholic type.4–8 Melancholic depression is considered a highly heritable subtype of major depression with a significant biological substrate, hence the name of endogenous or melancholic depression. However, the sensitivity of the DST in patients with major depression was estimated at only 44%, although it was found to increase with the presence of psychotic symptoms (67–78%) and symptoms of mixed mania. In other words, sensitivity increased when there were more variables of an endogenous or biological substrate.4–8 This finding was made in both children and adults irrespective of sex. One of the practical applications of the DST in affective disorders such as depression was as a predictor of response to drug treatment. Patients with a positive suppression test (those showing no suppression in the DST using 1mg) were seen to benefit less from placebo treatment and more from drug treatment as compared to patients with a negative test. The diagnostic value of the 1-mg DST cannot be ensured in patients with major depression criteria because it only has 44% sensitivity, and because major depression is a heterogeneous condition comprising several types of depression, including psychotic, endogenous, post-partum, and anancastic depression, amongst others. It may however be of value as a prognostic marker because patients with a positive 1-mg DST have a better response to drug treatment than those with a negative DST. In the 90s, in agreement with the data recorded, Young et al.9–11 showed hyperactivity of hypothalamic CRH-releasing hormone with a flattening of ACTH response in depressive patients (Table 1).
Main researches conducted with the dexamethasone suppression test in mental diseases.
| Author | Method | Value | Results |
| Carroll et al., 19684Young et al., 19869 | 1-mg DST | In depression as a prognostic and diagnostic marker of endogenous depression | No suppression in the 1-mg DST in subjects with endogenous depression |
| Yehuda et al., 200450 | 0.5-mg DST | Helpful in differential diagnosis of depression and PTSD | Suppressor response to the 0.5mg dose, showing hypersensitivity of the HPA axis in PTSD |
| Carrasco et al., 200758 | 0.25-mg DST | Helpful in differential diagnosis of BPD and PTSD | Suppressor response to the 0.25mg dose, showing hypersensitivity of the HPA axis in BPD |
| Rinne et al., 200241 | CRH-dexamethasone test | Diagnostic value for trauma history | Increased ACTH and cortisol response to CRH-dexamethasone stimulation in BPD |
CRH: corticoid releasing hormone; HPA: hypothalamic–pituitary adrenal axis; PTSD: post-traumatic stress disorder; BPD: borderline personality disorder; DST: dexamethasone suppression test.
In atypical depression, in contrast to basal hypercortisolism seen in melancholic depression, patients have a lower basal level and a higher proportion of normal levels. In 2002, Levitan et al.12 noted lower cortisol levels after dexamethasone administration in atypical depression as compared to other types of depression (melancholic and psychotic). In studies conducted by Asnis et al. in 1995,13,14 increased cortisol levels were also seen in atypical depression after the administration of desipramine 75mg (a selective norepinephrine reuptake inhibitor), suggesting less dysfunction of the noradrenergic system.
The results in patients with dysthymia showed a non-suppressor rate similar to normal subjects, but significantly lower as compared to depressive patients.15,16 However, a study conducted by Brambilla et al. in 198917 found no differences between dysthymia and major depression.
Studies in neurotic disordersNeurosis was traditionally defined as a group of mental diseases with no evidence of organic lesion, preserved reality testing, and a high level of anxiety. According to DSM-IV-TR, neurosis encompasses mood, anxiety, somatomorphic, dissociative, factitious, and personality disorders, amongst others.3 Disagreement exists in DST studies in obsessive-compulsive disorder (OCD), in which the number of non-suppressors greatly varies in the different reports (0–41%). While studies conducted in the late 80s by Insel et al., Cottraux et al., and Schlesser et al. reported a non-suppressor rate similar to that found in melancholic depression (higher than 25%),18–21 other studies conducted in the same decade by Lieberman et al., Monteiro et al., Vallejo et al., and Curtis et al. reported markedly lower rates.21–25 Curtis et al. analyzed in 198225 such discrepancies in a sample of 29 obsessive patients and concluded that DST abnormalities in these patients depended on added affective disorder, because three of the five non-suppressors (17%) met the DSM-III-R criteria for major depressive disorder, and they all had scores higher than 17 in the Hamilton test for depression. In addition, a high correlation was noted between these scores and cortisol levels after dexamethasone administration. All of this suggests that depression mediates the results of the DST.
Studies conducted in the 80s on panic disorder by Curtis et al., Sheehan et al., Lieberman et al., and Goldstein et al., amongst others, found a low proportion (approximately 15%) of non-suppressors in the DST.25–34 A study conducted by Sheehan et al. in 198326 on subjects with panic disorder found only three abnormal DSTs among 20 patients diagnosed with panic attacks or agoraphobia, and they also appeared to be attributable to causes other than panic or agoraphobia. An additional study conducted by Lieberman et al. in 198327 reported a 7.8% non-suppression rate among 51 patients with panic attacks. On the other hand, Goldstein et al. in 198733 found abnormal DSTs in three out of 35 patients with anxiety (two with agoraphobia and panic attacks and one with panic attacks without agoraphobia).
However, studies conducted by Grunhaus et al. and Ceulemans et al. in 1987 and 1985 respectively detected rates similar to those found in depressions.35,36 In any case, as pointed out by Ceulemans et al., the impact of stress on the DST should be considered. In the 90s, Coryell et al.29 found no suppression in 47.5% of 40 presurgical subjects with no psychiatric history. The high non-suppression rates in these patients probably reflected non-specific stress. This may be the reason why these authors found a relationship between non-suppression and the presence of more anxiety symptoms, greater social and occupational disability, and increased frequency of comorbid depression.
Studies in schizophreniaStudies conducted in the 90s in patients with schizophrenia are conflicting. The absence of cortisol suppression in the DST has largely been noted, but its etiology is not clear. It could be related to depression or to negative symptoms, as suggested by Ismail et al.37 However, Pivac et al. found similar non-suppression rates in schizophrenic patients with positive (56%) and negative (53%) symptoms.38 A relationship between suicidal behavior and an absence of suppression, as suggested by Lewis et al.,39 was not found either.
Studies in chronic fatigue syndromeStudies conducted by Gaab et al. in 2002 of patients with chronic fatigue syndrome found normal cortisol levels, showing increased and prolonged dexamethasone suppression.40 This increase in negative feedback of the HPA axis may be an explanation for changes in HPE axis function previously reported in these patients.
Studies with the dexamethasone suppression test at doses of 0.5mg and 0.25mgThe turning point leading to the recent revival of neuropsychiatric interest in dexamethasone were the studies conducted on differential diagnosis of post-traumatic stress disorder (PTSD) and major depression, which showed hypersensitivity of the HPA axis in patients with PTSD. Initial studies showed that urinary free cortisol was increased in depression and decreased in PTSD; that plasma cortisol levels after dexamethasone 1mg were increased in depression and decreased in PTSD; that, as suggested by Rinne et al.41 in 2002, ACTH response to CRH was decreased in both conditions; and, most importantly, that the number of glucocorticoid receptors in lymphocytes was decreased in depression and increased in PTSD (Table 1). This demonstrated the hypersensitivity of the HPA axis to low dexamethasone doses in subjects with PTSD. The increased glucocorticoid receptor density in lymphocytes from patients with PTSD seen using cytosolic radioligand assay would appear to suggest an increased sensitivity to feedback inhibition systems of the axis at pituitary glucocorticoid receptor level.
It was then hypothesized that subjects with PTSD could show suppression in the DST with doses lower than 1mg of dexamethasone, and 0.5mg of dexamethasone (half the usual dose) started to be used. At this dose, patients with PTSD continued to show suppression, a response that was not initially expected, while depressive patients had no suppressor response. This new version of the classical test was considered to differentiate depression from PTSD. However, the difference could be due to prior history. Thus, after a traumatic event, subjects with post-traumatic stress and those with major depression but significant prior trauma develop greater suppression. Depressive patients with no prior trauma have no suppressor response. However, Lindley et al., in 2004, were not able to show the previous findings of decreased basal plasma and hypersuppression in post-traumatic stress by measuring free cortisol in saliva from war veterans.42
Based on these studies showing the significance of a history of trauma in the genesis of personality disorders and because of the significant comorbidity between PTSD and borderline personality disorder (BPD), response to cortisol started to be studied in these patients. Thus, some researchers43 conducted studies with the DST in patients diagnosed with BPD and comorbid diagnosis of PTSD. Studies with the 0.5-mg DST reported a higher suppression rate in DST in patients with BPD and comorbid diagnosis of PTSD, but not in patients with BPD but no PTSD. These studies demonstrated a cortisol suppression response to low dexamethasone doses, although a significant number of control subjects also had high suppression rates (70–80% at a dose of 0.5mg).
Continuing with the research line of childhood traumas, patients with BPD were reported to have increased ACTH and cortisol responses to stimulation with CRH-dexamethasone, while if they developed post-traumatic stress, their response to ACTH was attenuated41 (Table 1). A history of child abuse had a greater impact on this response than a diagnosis of BPD itself. A subsequent study reported a reduction of this hyperresponse of the HPA axis in women with BPD who had sustained child abuse by the use of drug treatment with fluvoxamine.44
In this same line, a group of researchers tried to increase the specificity of the DST by decreasing the dexamethasone dose to 0.25mg.45 They assumed that the normal population would not suppress plasma cortisol, i.e. their plasma levels would not exceed 5mcg/dL. By excluding subjects with no disease, the detection of a suppressor hyperresponse in subjects with BPD would be facilitated.
The study found a significantly higher suppression rate in patients diagnosed with BPD and with a history of childhood trauma as compared to patients with BPD but no history of trauma and controls. The dexamethasone dose used in this study was 0.25mg, instead of the 0.5mg dose used in previous studies. The study therefore proposed the existence of hypersensitivity of the feedback inhibition systems of the axis at pituitary glucocorticoid receptor level. The purpose of all these studies was to confirm the inhibitory hypersensitization of stress response mechanisms in patients with BPD by testing the cortisol suppression response using a minimum stimulus with dexamethasone 0.25mg.45
Some studies have found a high prevalence of childhood abuse in the history of patients with eating disorders and have related it to a greater severity of the disorder and to the presence of more bulimic symptoms, suggesting that changes in stress response mechanisms and in HPA axis function may play a significant role in the pathophysiology of these disorders. Preliminary findings suggest that in some subtypes of eating disorders there is hypersensitivity of the HPA axis with increased sensitivity to dexamethasone.46–48 This is similar to what occurs in PTSD and BPD, A study of 25 female patients diagnosed with eating disorders similarly found that 12% of these patients had a history of trauma, although no relationship was found with the eating disorder subtype.48 The patients most impulsive and with more borderline traits had a significantly higher number of prior traumatic events. A significant relationship was also found between cortisol suppression and a history of trauma, so that patients more impulsive and with more borderline traits had significantly lower cortisol levels after 0.25mg of dexamethasone.
There are however authors who disagree with the existence of this HPA hypersensitivity in BPDs. These studies suggest that the weight of a history of trauma on HPA axis dysfunction depends on comorbid PTSD. However, there is no evidence that these changes occur in patients with BPD.
Hypersensitivity of the HPA axis has also been shown using a combined dexamethasone and CRH test. A higher suppression rate was seen in patients diagnosed with BPD who had a history of childhood abuse trauma as compared to those with no such history.43,45,49,50
In neuroimaging studies, hypersensitivity of the HPA axis has been related to pituitary gland volume.45 In magnetic resonance imaging studies, patients with BPD and a higher number of suicidal attempts showed a decreased volume.51,52 A decreased pituitary volume has also been seen in patients with BPD and a significant history of trauma.52
Relationship with dimensional traitsStudies in healthy populations conducted by McCleery et al. in 2001 and Zobel et al. in 2004 found that subjects with low neuroticism showed a significantly greater cortisol response to the CRH-dexamethasone test (high reactivity) as compared to subjects with high neuroticism.53,54
Other authors, such as Rosenblitt et al. in 2001,55 conducted similar studies in university students using the Zuckerman sensation seeking scale and measuring testosterone and cortisol. As would be expected, men had higher scale scores than women. Results support the existence of a significant inverse relationship between cortisol and sensation seeking in men, but not in women. This difference is maintained even after adjusting for testosterone levels and age.
An additional study by Schweitzer et al., conducted in 2001,56 used the DST and the revised version of the Millon Clinical Multiaxial Inventory (MCMI-II) to analyze patients diagnosed with major depressive disorder with a clinically relevant personality disorder, and found that suppressors had significantly higher scores as compared to non-suppressors in six of the 13 MCMI-II scales: borderline, passive-aggressive, schizoid, schizotypal, avoidant, and self-defeating.
ConclusionStudy of cortisol response may be helpful for identifying or predicting the prognosis of some psychiatric disorders.
Depression has traditionally been the most widely studied disease in this field, and is associated with basal hypercortisolism similar to that seen in some endocrine disorders such as Cushing's syndrome.2 Patients with major depression have shown an absence of cortisol suppression after the DST using a low dose of 1mg dexamethasone. The sensitivity of the DST for diagnosing major depression was greater the higher the number of melancholic, endogenous, and psychotic symptoms. Studies conducted in this area concluded that the DST could have prognostic value, because subjects with a positive DST had a more favorable response to drug treatment as compared to psychotherapy and placebo.5–7 However, some impairment in this test has been seen in many psychiatric diseases such as anorexia nervosa, OCT, degenerative dementia, manic disorders, and schizophrenia, thus providing discordant data regarding the involvement of cortisol in their pathophysiological mechanisms. Interest in the relationship between basal hypercortisolism and stress revived when differences were found between major depression and post-traumatic stress disorder. These studies showed that urinary free cortisol was increased in depression and decreased in PTSD, and that serum cortisol levels after the 1-mg DST were increased in depression and decreased in PTSD. ACTH response to CRH was decreased in both conditions, and the number of glucocorticoid receptors in lymphocytes was decreased in depression and increased in PTSD despite basal hypercortisolism.
The most recent studies appear to suggest that glucocorticoid receptors are responsible for the sensitization of feedback inhibition of the HPA axis.
In disorders where a relationship exists with trauma history, an exaggerated cortisol response to minimum dexamethasone stimuli has been seen as an increased suppression in response to the 0.25-mg DST. In patients with borderline personality disorder, in whom the weight of their trauma history is responsible for the condition according to various authors, significantly lower plasma cortisol levels are seen as compared to healthy subjects or other patients. As occurs in depression, where the 1-mg DST has prognostic value, in this case due to lack of cortisol suppression, it appears that suppressor response to low doses of 0.25mg in the DST may also have prognostic value in BPD, because high suppression could be correlated to greater affective instability, impulsiveness, and clinical severity. This could reflect poorer social, familial, and occupational functioning, and could become clinically manifest as an increased number of suicidal attempts.57,58
Finally, DST studies in relation to personality traits found that personality dimensions related to impulsiveness and lack of emotional control appeared to be related to a hypersensitive cortisol response to suppression, although the relationship to neuroticism was not absolutely clear.53,54 This dimension of neuroticism could have two different and possibly opposite components with regard to their relationship with the HPA axis: a panic-inhibition component (probably related to hypercortisolism) and an emotional lability component (probably related to hypocortisolism and excess suppression in the DST).55
Conflicts of interestThe authors state that they have no conflicts of interest.
Please cite this article as: Tajima-Pozo K, Montes-Montero A, Güemes I, González-Vives S, Díaz-Marsá M, Carrasco JL. Aportaciones de los tests de supresión de cortisol al conocimiento de los trastornos psiquiátricos: revisión narrativa de la literatura. Endocrinol Nutr. 2013;60:396–403.