With a prevalence of up to 0.6% in the general population, cerebral calcifications are a common finding in neuroimaging tests, particularly in computed tomography (CT). This proportion increases in autopsy studies, in which microcalcifications are found in the globus pallidus and dentate nucleus in up to 70% of cases, as prevalence increases with age.1
Such calcifications are considered to be pathological when they are extensive or bilateral or occur in people under 40 years of age. When calcifications simultaneously involve the nucleus pallidus, the putamen, the dentate nucleus of the cerebellum, and hemispheric white matter (striato-pallido-dentate calcinosis), the condition is known as Fahr syndrome.2
The most common causes of Fahr syndrome include, in addition to an idiopathic etiology and congenital infections (toxoplasmosis), changes in calcium metabolism, mainly primary hypoparathyroidism, either autoimmune or associated with a polyglandular syndrome, postoperative hypoparathyroidism or pseudohyperparathyroidism, which account for up to 80% of cases with calcifications in the basal ganglia.1–3 It is however an uncommon condition.
Although Delacour in 1850 and Virchow five years later were the first to histologically describe bilateral calcifications in the basal ganglia, it was not until 1939 that Eaton related such calcifications to chronic hypoparathyroidism.3–5
The mechanism by which calcium becomes deposited in those areas has yet to be clearly elucidated. Various hypotheses have been suggested, including chronic changes in intracellular and extracellular calcium and phosphate levels in neuroglial structures, changes in the blood–brain barrier, or a decreased blood flow in the basal ganglia.2,3,6
As regards the clinical consequences, we also do not know why some individuals remain asymptomatic while others experience three types of symptoms: seizures or myoclonus, extrapyramidal choreoathetoid symptoms or parkinsonism and neuropsychiatric disorders.2,3,7
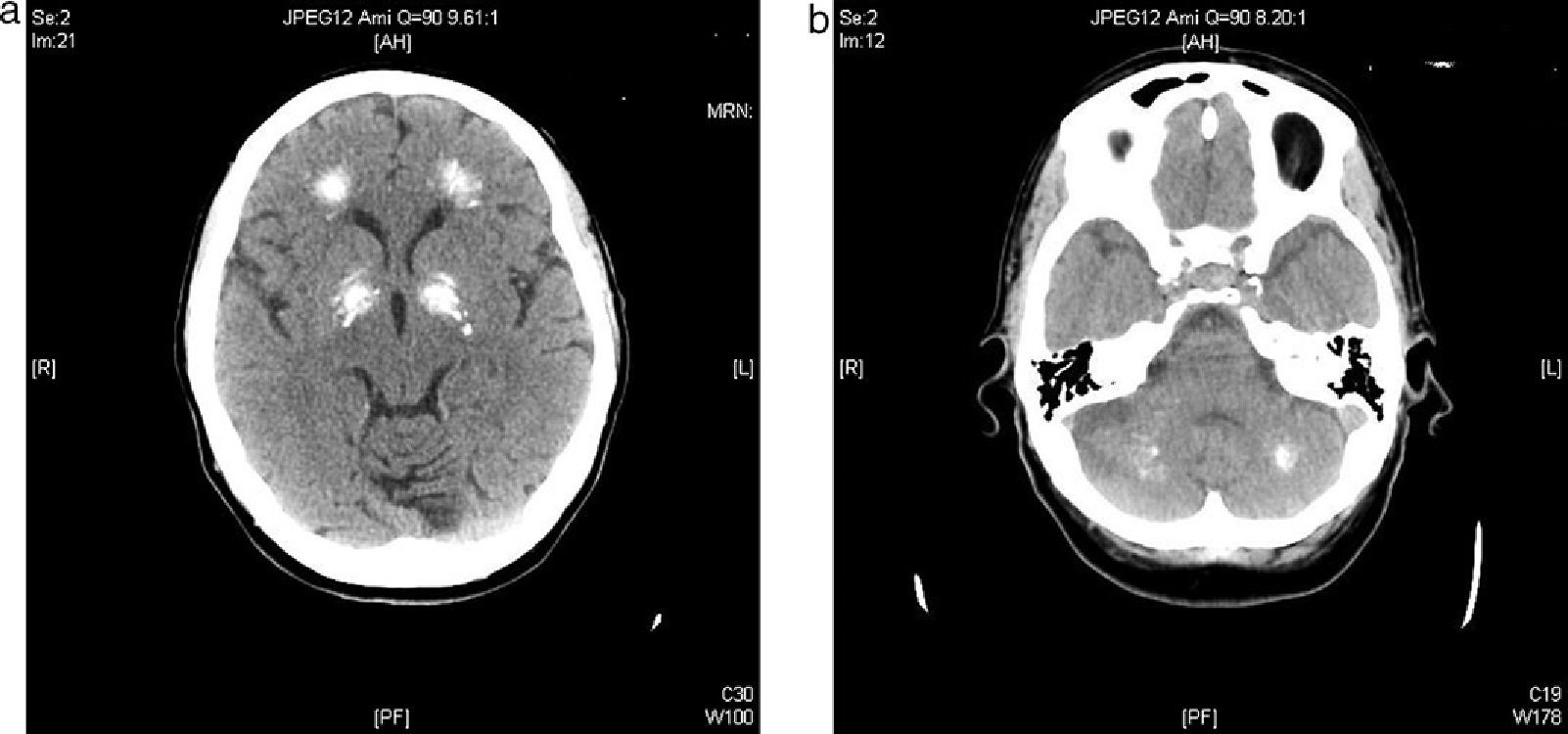
We report the case of a 57-year-old female patient who was admitted to the neurology department complaining of a sensation of stiffening in her lower limbs and progressive gait impairment for approximately 6 months. Her clinical history included dyslipidemia on dietary therapy and a depressive syndrome which had started 6 years before and was being treated with antidepressive and anxiolytic drugs. The patient reported no family history of interest. She had felt pain and a feeling of stiffening in the gastrocnemius area, as well as a difficulty in walking, for approximately the previous 6 months. She had sustained no falls, but there was instability in her posture and ambulation. The patient reported a progressive worsening of her symptoms which had finally caused her to seek help with doing the household chores two months before admission. Cardiopulmonary auscultation revealed no significant changes, and an abdominal examination was unremarkable. Neurological examination found that the patient walked with very short steps and shuffled her feet and had great difficulty in turning. Cogwheel axial rigidity. No tremor. Stereotypic, continuous involuntary movements of the hands. No impairments were found in the higher functions. Achilles and patellar reflexes were abolished. An electromyogram showed spontaneous, continuous muscle activity in the lower limbs. A CT scan of the head showed a ventricular system with a size and morphology within normal limits and multiple intra-axial parenchymal calcifications bilaterally and symmetrically involving the dentate nuclei, the basal ganglia, and the semioval centers (Fig. 1a and b).
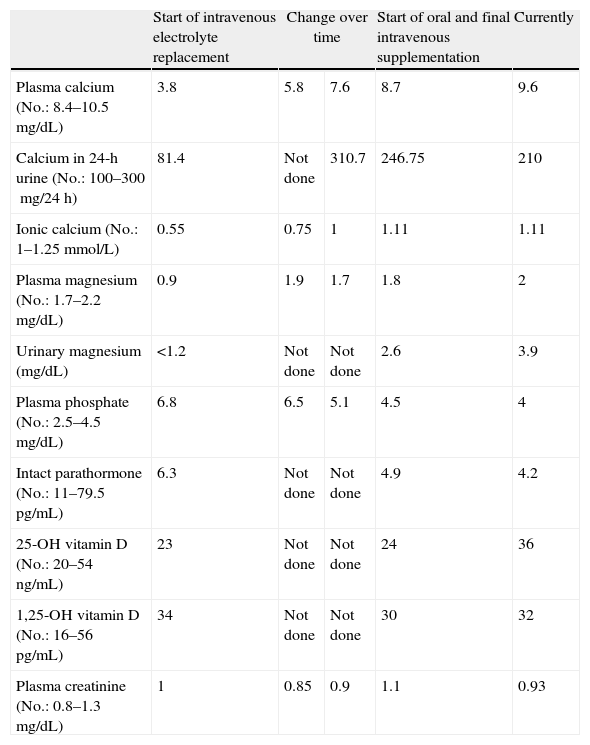
Gait impairment was attributed to multiple calcifications in the basal ganglia. Laboratory tests showed normal results in the complete blood count and liver and kidney function tests; sodium 140mEq/L (No.: 133–145); potassium 3.5mEq/L (No.: 3.5–5); calcium 3.8mg/dL (No.: 84–10.5); ionic calcium 0.55mmol/L (No.: 1–1.25); phosphate 6.8mg/dL (No.: 2.5–4.5); magnesium: 0.9mg/dL (No.: 1.7–2.2); CK 693U/L (No.: 20–135); albumin 4.2g/dL (No.: 4.2–6). Because of electrolyte changes, the endocrinology department was consulted, and intravenous calcium and magnesium replacement was started. The following measurements were also requested: 25(OH) vitamin D 23ng/mL (No.: 20–54); 1,25(OH) vitamin D 34pg/mL (No.: 16–56); intact parathormone (PTH) 6.3pg/mL (No.: 11.1–79.5); calcium in 24-h urine 81.4mg/24h (No.: 100–300) (Table 1). Chvostek and Trosseau signs were positive. No other signs of hypocalcemia such as subcapsular cataract, prolongation of the QT interval in the electrocardiogram, or pseudotumor cerebri were found. Based on a diagnosis of primary hypoparathyroidism, tests for anti-parathyroid tissue and anti-CASR (calcium-sensing receptor) antibodies were performed and reported to be negative. Tests for Huntington chorea and serologic viral tests were also negative. Other endocrine conditions such as Wilson's disease and hemochromatosis, and gland infiltration by a tumor were also ruled out. After gradual correction of calcium, magnesium, and phosphate, clinical improvement was shown by a decreased occurrence of myoclonus and the negativization of Chvostek and Trousseau signs. Electromyogram findings and creatine kinase levels also were normalized. However, there was no improvement in gait. Despite correction of hypomagnesemia, PTH levels remained low, and this was ruled out as the cause of hypoparathyroidism. The patient was discharged on levodopa and calcium supplementation (calcium lactogluconate 1000mg/8h), magnesium lactate (500mg/24h), and calcitriol (0.5mcg/day), and on her standard treatment. Six months later, the patient had normal electrolyte values and had regained independence in performing everyday activities, although her parkinsonian-like gait disorder persisted despite treatment. One year later, she was readmitted for gradual gait impairment with frequent falls despite calcium and magnesium levels within the normal range and repeated modifications of the specific treatment for the parkinsonian disorder. She also reported impaired memory, which was considered as early cognitive impairment.
Changes in laboratory parameters over time.
| Start of intravenous electrolyte replacement | Change over time | Start of oral and final intravenous supplementation | Currently | ||
| Plasma calcium (No.: 8.4–10.5mg/dL) | 3.8 | 5.8 | 7.6 | 8.7 | 9.6 |
| Calcium in 24-h urine (No.: 100–300mg/24h) | 81.4 | Not done | 310.7 | 246.75 | 210 |
| Ionic calcium (No.: 1–1.25mmol/L) | 0.55 | 0.75 | 1 | 1.11 | 1.11 |
| Plasma magnesium (No.: 1.7–2.2mg/dL) | 0.9 | 1.9 | 1.7 | 1.8 | 2 |
| Urinary magnesium (mg/dL) | <1.2 | Not done | Not done | 2.6 | 3.9 |
| Plasma phosphate (No.: 2.5–4.5mg/dL) | 6.8 | 6.5 | 5.1 | 4.5 | 4 |
| Intact parathormone (No.: 11–79.5pg/mL) | 6.3 | Not done | Not done | 4.9 | 4.2 |
| 25-OH vitamin D (No.: 20–54ng/mL) | 23 | Not done | Not done | 24 | 36 |
| 1,25-OH vitamin D (No.: 16–56pg/mL) | 34 | Not done | Not done | 30 | 32 |
| Plasma creatinine (No.: 0.8–1.3mg/dL) | 1 | 0.85 | 0.9 | 1.1 | 0.93 |
Serum albumin was normal at all times.
The clinical consequences of calcifications in the basal ganglia are of three types: seizures or myoclonus, extrapyramidal symptoms, or neuropsychiatric disorders.
Seizures are secondary to hypocalcemia and are characterized by their poor response to anticonvulsants and their resolution when plasma calcium levels are normalized. The same occurs with myoclonus, as found in our patient. This suggests that the cause of myoclonus is acute hypocalcemia.
Movement disorders occur in 20–30% of cases, and may be choreoathetoid or parkinsonian in nature. Interestingly, they are caused by different mechanisms; the first is by dopamine deficiency and the second is by dopamine excess. Parkinsonism is characterized by being resistant to standard levodopa treatment, and in early cases may occasionally respond to calcium therapy.1,2
Neuropsychiatric changes may also occur. Among these, widely diverse symptoms that may range from depression to psychosis have been reported. Their course usually runs parallel to adequate electrolyte replacement, and improves with sustained normalization of calcemia. Of all of these conditions, dementia is the one with the worst response to the normalization of phosphorus and calcium metabolism.
We do not know the extent to which the dormant course of phosphorus and calcium disorder could have influenced the depressive syndrome in our patient. However, the patient continued to require her standard antidepressive treatment despite electrolyte replacement.1,2 The early cognitive impairment found at the last visit could also be another manifestation of Fahr syndrome.
The cause of the hypomagnesemia experienced by the patient could not be determined with absolute certainty despite an adequate differential diagnosis and was finally attributed to chronic omeprazole treatment because when levels were normalized and this treatment was discontinued, normal magnesium levels were maintained with minimum supplementation.8
Primary hypoparathyroidism may be part of type I polyglandular autoimmune syndrome, associated with primary adrenal insufficiency and mucocutaneous candidiasis. Measurements of anti-parathyroid tissue antibodies are of little value for diagnosis because they are only positive in 40% of cases when they are associated with type I polyglandular autoimmune syndrome and in 30% of sporadic cases. The same occurs with anti-CASR antibodies, which are positive in approximately 50% of cases.
As reported in the literature, an adequate control of phosphorus and calcium metabolism in Fahr syndrome is not usually associated with an improvement in gait disorder or cognitive impairment. Only the early diagnosis of hypoparathyroidism can prevent calcium deposition in the basal ganglia and, as a result, the changes are resulting from such a cerebral calcification.1,3,9
Please, cite this article as: Nicolau Ramis J, et al. Calcinosis estriado-pálido-dentada como causa de extrapiramidalismo en una paciente con hipoparatiroidismo primario. Endocrinol Nutr. 2012;59:69-83.