Hyperglycemic hyperosmolar state (HHS) is a form of diabetes onset or decompensation which is very uncommon in children, although its incidence has increased in recent years, probably due to increased obesity and type 2 diabetes mellitus (T2DM) in this population group.1,2 Among cases of HHS in children reported up to 2008, only one occurred as decompensation of prior type 1 diabetes mellitus (T1DM), while in all other patients HHS was the initial manifestation of both type 1 and, more commonly, type 2 diabetes.2
The main clinical manifestations of HHS, polyuria and polydipsia, may be overlooked for weeks, so delaying the search for medical care and leading to severe dehydration. The significance of HHS lies in the differences in its treatment as compared to diabetic ketoacidosis (DKA), a more common form of diabetes onset and decompensation in childhood which is usually diagnosed earlier because it is associated with more florid symptoms.
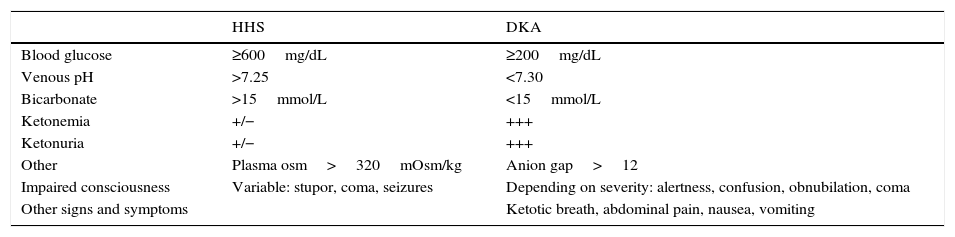
The characteristic features of HHS include marked hyperglycemia, hyperosmolarity, and mild ketosis. Table 1 shows the diagnostic criteria for HHS.3
Diagnostic criteria for HHS and DKA.
| HHS | DKA | |
|---|---|---|
| Blood glucose | ≥600mg/dL | ≥200mg/dL |
| Venous pH | >7.25 | <7.30 |
| Bicarbonate | >15mmol/L | <15mmol/L |
| Ketonemia | +/− | +++ |
| Ketonuria | +/− | +++ |
| Other | Plasma osm>320mOsm/kg | Anion gap>12 |
| Impaired consciousness | Variable: stupor, coma, seizures | Depending on severity: alertness, confusion, obnubilation, coma |
| Other signs and symptoms | Ketotic breath, abdominal pain, nausea, vomiting |
DKA: diabetic ketoacidosis; HHS: hyperglycemic hyperosmolar state.
Source: modified from Wolfsdorf et al.3
The case of a boy aged 13 years and 3 months who attended the emergency room for abdominal pain over the previous 15 days and refractory constipation is reported. He had also experienced polyuria and polydipsia over the previous 2–3 weeks with no weight loss. The patient reported an abundant intake of carbonated drinks to relieve his thirst.
Family history: father with T1DM (poor blood glucose control); mother and two siblings healthy.
Personal history: ADHD treated with methylphenidate.
Initial physical examination: weight, 36kg; BP, 128/80mmHg; temperature, 35.9°C; good general condition, conscious, oriented, cooperative, no focal neurological signs, normal color and hydration.
Supplemental tests:
- -
Chemistry: blood glucose, 1138mg/dL; blood ketones, 0.8mmol/L; sodium, 125mmol/L (corrected for blood glucose: 142mmol/L); potassium, 5.1mmol/L; urea, 25mg/dL; plasma osmolarity, 320mOsm/kg (effective osmolarity, 323.4mOsm/kg); venous pH, 7.31; bicarbonate, 27mmol/L; lactate, 1.3mmol/L; and CRP<0.1mg/dL. All the other test results were normal.
- -
Urine: glycosuria+++ and ketonuria+.
Based on a suspicion of diabetes onset, volume replacement was started in the emergency room with physiological saline (initial bolus of 10mL/kg, followed by 14mL/kg/h) with the provision of potassium (1mEq/L), which gradually decreased the blood glucose level and normalized electrolyte levels. At 4h, when glucose was <300mg/dL, a 5% dextrose solution was added. In addition, based on the patient's good general condition, adequate tolerance of oral intake, and minimal ketosis, subcutaneous regular insulin was started (0.7IU/kg/day) based on capillary blood glucose. Twelve hours after admission, this was replaced by multiple doses of subcutaneous insulin (aspart and glargine) at 0.6IU/kg/day with a good clinical response. He was put on a diet with controlled carbohydrate intake by servings. The patient and his family were instructed in diabetes management, and the patient was discharged a week later.
The results of baseline tests for DM were as follows: HbA1c, 12.3% (normal, <6%); C-peptide, 0.03mmol/L (normal, >0.16nmol/L); insulin, 8mIU/L (normal, 2–16mIU/L); antibodies: anti-IA2 1537.6U/mL (normal, <1U/mL); anti-GAD-65 68.34U/mL (normal, ≤1U/mL), and negative anti-insulin antibodies. The diagnosis of T1DM was confirmed.
The patient currently attends an outpatient clinic for regular visits and maintains good blood glucose control (the most recent HbA1c measurement, 6.5%).
Early identification of diabetic decompensation may be difficult, particularly when it corresponds to the onset of unsuspected diabetes. The early detection and management of the condition may avoid severe complications later.
Because HHS is an uncommon form of diabetes onset in childhood, it is the less likely to be suspected. (It is more common in elderly patients, patients with overweight or obesity, and as the form of onset or decompensation of T2DM.) In addition, HHS usually causes greater morbidity and mortality than DKA, depending on the severity of dehydration and hyperosmolarity and patient age4. Our patient did not have the typical characteristics for diabetes onset as HHS, which was probably determined by his heavy intake of carbonated drinks on the previous days and the existence of some pancreatic reserve of insulin which was sufficient to avoid a ketogenic response, but not to prevent hyperglycemia or affect tissue sensitivity to insulin.5
Unlike in DKA, intravenous fluid administration and electrolyte replacement are important in HHS,6 in which the infusion rate of solutions should be faster than that recommended for DKA. Intravenous fluid replacement achieves rehydration and decreases the blood glucose level, so that when the decrease achieved with solutions is less than 50mg/dL/h, continuous insulin infusion (after correcting hypokalemia and bicarbonate levels, if impaired) should be considered. The initial dose to be administered should be lower than that recommended for DKA: 0.025–0.050U/kg/h3. During this process, plasma sodium levels should be monitored to ensure progressive correction. The early diagnosis and adequate management of HHS are essential to prevent complications.
The adequate classification of the type of decompensation of the patient leads to consideration of the most adequate treatment, because the management of DKA and HHS differs in regard to the treatment sequence (and also in the infusion rate of solutions and the recommended insulin doses). In fact, in our patient, the initial treatment was based on a suspicion of DKA, using saline infusions at a rate different from that recommended, and higher insulin doses than those indicated for HHS management (although subcutaneous, instead of intravenous, administration was decided upon because of the good general condition of the patient). This decreased his blood glucose level from 1138mg/dL to 119mg/dL within 12h, after which the patient had symptoms consistent with hypoglycemia. This could have been because the patient had had much higher glucose levels on the previous days, so that a sharp decrease in blood glucose to almost normal levels triggered the typical symptoms of hypoglycemia. If HHS had been suspected from the start, management would have been more focused on patient rehydration and lower insulin doses would have been used, which would probably have resulted in a more gradual decrease in blood glucose, so perhaps preventing the reported episode and associated symptoms. Moreover, adequate treatment of HHS in children is essential because of the greater risk of brain edema in childhood when HHS is treated as DKA7.
In conclusion, it may be stated that, despite only a few cases having been reported of HHS as a form of diabetes onset in children, its characteristic laboratory parameters should be known in order that, if necessary, adequate treatment can be started. Additional studies are also needed to clarify why this should be the form of onset in some pediatric patients eventually found to have T1DM.
Please cite this article as: Hernández Moreno A, Sanz Fernández M, Ballesteros Pomar MD, Rodríguez Sánchez A. Estado hiperglucémico hiperosmolar: una forma poco frecuente de inicio de la diabetes mellitus tipo 1 en la infancia. Endocrinol Nutr. 2016;63:252–253.