Iodine is a particularly relevant element in thyroid gland physiology. Although healthy follicular cells have several mechanisms which allow them to adapt to marked changes in iodine provision, both iodine deficiency and excess may cause dysfunction in a diseased thyroid gland.
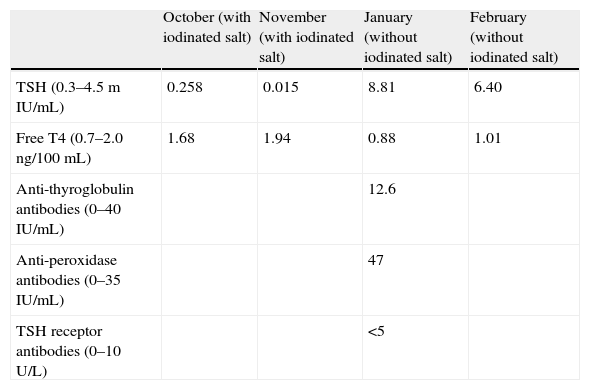
A 26-year-old female patient with an unremarkable history attended her family physician reporting fatigue for several weeks previously. Her clinical symptoms spontaneously resolved, but a blood test showed subclinical hyperthyroidism, with TSH 0.258μU/mL and FT4 1.68ng/100mL. Diagnosis was confirmed one month later by values of TSH 0.015 and FT4 1.94 (Table 1).
Plasma levels of several molecules during the months when the patient was studied. Values in brackets are normal ranges for each parameter.
| October (with iodinated salt) | November (with iodinated salt) | January (without iodinated salt) | February (without iodinated salt) | |
| TSH (0.3–4.5mIU/mL) | 0.258 | 0.015 | 8.81 | 6.40 |
| Free T4 (0.7–2.0ng/100mL) | 1.68 | 1.94 | 0.88 | 1.01 |
| Anti-thyroglobulin antibodies (0–40IU/mL) | 12.6 | |||
| Anti-peroxidase antibodies (0–35IU/mL) | 47 | |||
| TSH receptor antibodies (0–10U/L) | <5 |
A physical examination at the endocrinology clinic revealed a palpable but not visible and nontender goiter of an elastic consistency. Ultrasound examination performed at this same time showed a slightly enlarged gland with no nodules or adjacent adenopathies. The patient had no exophthalmos or signs of eye inflammation, nor edema in the lower limbs, hands, or face. The examination was otherwise unremarkable.
The patient had not had recent contact with iodine-containing products, except that she usually consumed iodinated salt (approximately 3g/day since the age of 18). She was recommended to stop using iodinated salt and a new test was performed two months later. This test showed subclinical hypothyroidism with elevated anti-peroxidase antibodies (Hashimoto's disease). The persistence of this subclinical hypothyroidism was confirmed one month later (Table 1).
The minimum daily iodine intake recommended for adults is 150μg (220μg for pregnant women).1 This amount is usually provided by diet in coastal areas, but daily intake is frequently lower in inland areas. Salt iodination (with 76μg iodine per gram) is a public health measure aimed at relieving this deficiency.2 Apart from food, iodine provision may come from several drugs and X-ray contrast media.
Of all the regulatory mechanisms protecting the body from changes in exogenous iodine provision, the best known is the Wolff-Chaikoff effect,3 a phenomenon that allows healthy follicular cells to decrease iodine organification when plasma iodine levels increase and which remains active for approximately 4 weeks. Several thyroid gland diseases may alter this and other regulatory mechanisms and lead to a predisposition to hyperthyroidism or hypothyroidism in iodine excess conditions.4
Endemic goiter is the thyroid disease that promotes the development of hyperthyroidism when iodine provision is increased.5 This occurs through two mechanisms:
- 1.
Approximately 20% of these cases are actually patients with undiagnosed Graves-Basedow who remain euthyroid before iodine provision because the scarcity of this element limits thyroxine production by the thyroid gland.6
- 2.
The remaining 80% are patients who have developed one or several autonomous glands within the gland and who often already have subclinical hyperthyroidism before iodine provision.
Hashimoto's disease and subtotal thyroidectomy (regardless of their origin) promotes the development of hypothyroidism in conditions of iodine excess7 by indefinitely prolonging the above-described Wolff-Chaikoff effect in these patients.
The use of drugs or the administration of iodinated contrast media was ruled out in our patient. Her only additional iodine intake was the salt she used for cooking. The thyroid hormone response to salt removal suggested a salt influence upon the development of hyperthyroidism. This effect was particularly surprising because the amount of iodine provided by salt was close to the recommended daily amount of this element.
Graves-Basedow disease (a condition the diagnosis of which is based on the triad of hyperthyroidism, goiter, and ophthalmopathy) or nodular goiter, conditions which are both associated with the occurrence of hyperthyroidism secondary to iodine provision, were ruled out. The only thyroid disease in this patient was Hashimoto's disease, which according to the literature causes hypothyroidism in iodine excess conditions, rather than hyperthyroidism as occurred in the reported case.
The temporal proximity of the cessation of iodinated salt use and thyroid function changes does not show a definite cause-effect relationship between the two. Despite the marked influence of iodine on thyroid gland physiology, it cannot be ruled out that the condition reported was independent of changes in iodine provision. The measurement of urinary iodine levels before and after iodinated salt use could have helped support one or the other option. An attractive possibility is the one suggested by recent classifications of autoimmune thyroiditis, which include both Graves’ and Hashimoto's diseases under the same heading, as the two extremes of the same condition.8 This patient may have had a type 3 autoimmune thyroiditis which had evolved (spontaneously or as a result of a change in iodine consumption) to type 2 autoimmune thyroiditis.
Please cite this article as: Penín M, et al. Hipertiroidismo subclínico inducido por yodo en una paciente con enfermedad de Hashimoto. Endocrinol Nutr. 2012;59:69–83.