Few studies are available on quality of life and treatment satisfaction of patients with type 2 diabetes mellitus (T2DM). Both of them were the primary objectives of the PANORAMA (NCT00916513) study. Metabolic control, treatment patterns, and management by healthcare professionals were also evaluated.
Material and methodsThis multicenter, cross-sectional, observational study randomly recruited >40-year-old patients with T2DM from Spanish healthcare centers. HbA1c was measured using the same technique in all patients, who also completed quality of life (EQ-5D and ADDQoL) and treatment satisfaction (DTSQ) questionnaires and the Hypoglycemia Fear Survey (HFS-II).
ResultsFifty-four investigators recruited 751 patients, 60.3% of whom had HbA1c levels<7%. Approximately 25% of the patients on monotherapy had HbA1c values≥7%, patients with longer disease duration and more complex treatments, especially with insulin, showed the poorer control. Despite good overall treatment satisfaction (mean 29.3±6.1, 0- to 36-point scale), patients with a poorer metabolic control, previous hypoglycemia episodes, and more complex therapies had a worse QoL and a greater fear of suffering hypoglycemia.
ConclusionsDespite advances in metabolic control, there are still areas to improve. Early addition of safe drugs to monotherapy would help achieve control objectives without increasing the risk of hypoglycemia, and delaying the start of insulin therapy. This would also improve QoL and treatment satisfaction.
La evaluación de la calidad de vida y la satisfacción con el tratamiento relacionados con la diabetes tipo 2 han sido poco estudiados. Ambos son los objetivos principales del estudio PANORAMA (NCT00916513). También se evalúa el grado de control metabólico, los patrones de tratamiento y la actuación del profesional sanitario.
Material y métodosEstudio observacional, transversal y multicéntrico que incluyó pacientes diabéticos tipo 2 mayores de 40 años seleccionados de manera aleatorizada entre los centros de salud españoles. Se determinó la HbA1c mediante un mismo sistema y cada paciente completó cuestionarios de calidad de vida (EQ-5D y ADDQoL), satisfacción con el tratamiento (DTSQ) y temor a la hipoglucemia (HFS-II).
ResultadosCincuenta y cuatro investigadores incluyeron 751 pacientes. El 60,3% presenta HbA1c < 7%. El mayor tiempo de evolución de la enfermedad y los tratamientos complejos, especialmente con insulina, se asocian a peor control. Cerca de un 25% de los pacientes en monoterapia presenta HbA1c ≥ 7%. Aunque la satisfacción con el tratamiento en general es buena (media 29,3±6,1, escala de 0 a 36 puntos), los pacientes con peor control metabólico, hipoglucemias previas y tratamientos más complejos refieren significativamente peor calidad de vida y más miedo a sufrir hipoglucemias.
ConclusionesAunque el grado de control metabólico ha avanzado, todavía existen áreas de mejora. La adición precoz a la monoterapia de fármacos seguros ayudaría a lograr los objetivos de control sin aumentar el riesgo de hipoglucemias, y retrasando el inicio del tratamiento con insulina. Esto mejoraría la calidad de vida y la satisfacción con el tratamiento.
Diabetes represents a major health problem because of its high prevalence, morbidity and mortality, its influence on patient quality of life, and its impact on the health system. The age- and sex-adjusted prevalence of diabetes in Spain is 13.8%, while the prevalence of unknown diabetes is 6.0%.1
Poorly controlled type 2 diabetes mellitus (DM) is associated with increased vascular complication rates,2 impaired patient quality of life, less satisfaction with treatment, and greater healthcare expense per patient. Thus, in 2002, DM caused in Europe a total annual cost of approximately 29 billion euros in health care alone.3
By contrast, the achievement of adequate control in patients with DM of recent onset is associated with a decreased incidence of vascular complications.4 This protective effect persists years after treatment discontinuation and is associated with a decrease in the myocardial infarction rate and, for some treatments, with a decreased mortality rate.5 It has also been shown that intensive intervention on risk factors (RFs) decreases the risk of vascular complications by 59% (p<0.001) and cardiovascular mortality by 57% (p=0.04) in diabetic patients with microalbuminuria.6
However, three large trials in patients with long-standing type 2 DM did not show that strict blood glucose control led to a significant decrease in major cardiovascular events except for myocardial infarction.7–9 Moreover, strategies used for treatment intensification were associated with increased rates of hypoglycemia and weight increase8 which markedly impacted on patient quality of life.
Despite this evidence and the recommendations in the most widely disseminated clinical practice guidelines,10–12 many patients, even in early disease stages, do not achieve the recommended glycosylated hemoglobin (HbA1c) goal (<7%).
In recent years, several epidemiological studies have assessed diabetes control, the factors related to such control, and treatment patterns in Spain.13–15 There are however few studies in Europe and Spain assessing quality of life related to diabetes and patient satisfaction with treatment. The DAWN study (Diabetes, Attitudes, Wishes and Needs) was aimed at ascertaining perceptions of diabetic patients about their disease to identify areas for improvement in the psychosocial management of diabetes.16,17 However, this study did not assess quality of life, satisfaction with treatment, or fear of hypoglycemia using validated questionnaires, essential for assessing treatment compliance.
The primary objective of the PANORAMA study was to assess quality of life and degree of satisfaction with the treatment of patients with type 2 DM in Europe. It was also intended to examine metabolic control and treatment patterns.18,19 This article reports on the results achieved with patients selected in Spain.
Patients and methodsDesignPANORAMA (NCT00916513) was a multicenter, multinational, observational, cross-sectional, epidemiological study in nine European countries (Germany, Belgium, Spain, France, Greece, the Netherlands, Italy, the United Kingdom, and Turkey).19 In order to collect a representative sample, participating investigators were randomly selected.19 In Spain, selection was made from the list of primary healthcare centers of the national health system, with every autonomous community being represented. This article only reports on the study results in Spain.
PopulationThe inclusion criteria were as follows: any patient over 40 years of age diagnosed with type 2 DM at least one year before study entry and with a clinical history at the healthcare center. All types of treatment were included, provided they had not been changed in the three months prior to study start. Patients with type 1 DM, a history of diabetic ketoacidosis, or secondary diabetes, those treated with systemic corticoids (use of the inhaled or topical routes was permitted), pregnant women, patients who could not read and understand the questionnaires, and patients participating in another clinical trial were excluded. All patients signed the relevant informed consent form.
VariablesInformation was collected based on a review of the clinical history of the previous 24 months, including the socio-demographic characteristics of the patients, biological measures, and disease characteristics.19 HbA1c was measured in all participants during the only study visit using the same device (Bayer A1C now, a portable monitor similar to that used to measure blood glucose that provides the HbA1c value), validated by the National Glycosylated Hemoglobin Standardization Program in the United States. Control was considered to be adequate when HbA1c was less than 7%.
Patients completed the following self-administered questionnaires validated into Spanish: Audit of Diabetes-Dependent Quality of Life (ADDQoL), which assesses the impact of diabetes on quality of life; Diabetes Treatment Satisfaction Questionnaire (DTSQ), assessing patient satisfaction with treatment; Hypoglycemia Fear Survey (HFS-II), assessing fear of hypoglycemia; and EuroQol-5 dimensions (EQ-5D), used as a generic measure of health status19 (additional material on the Internet).
Participating physicians also completed several questionnaires to report on why they believed blood glucose goals were not achieved, and the measures they would take if patients were not controlled.
DefinitionsAnalysis of quality of life related to diabetes (ADDQoL)ADDQoL is a questionnaire consisting of 21 items, 19 of which refer to specific life domains (such as social and work life), scored on a 5-point impact scale. The effect of diabetes on each domain is weighed based on its significance for patient quality of life to obtain an average weighted impact (AWI) score. These scores may range from +3 (maximum positive impact of diabetes) to −9 (maximum negative impact of diabetes). The remaining two summary items are computed separately: one measures the impact of diabetes on quality of life (scored from +1 as maximum positive impact to −3 as maximum negative impact), and the other the current quality of life (+3 is excellent and −3 extremely poor).20,21
Diabetes Treatment Satisfaction Questionnaire (DTSQ)This questionnaire consists of eight items, each scored on a 7-point scale. The satisfaction score is the sum of the six questionnaire items. Each item is scored from 0 to 6, and the result may therefore range from very satisfied (36 points) to very dissatisfied (0 points). The remaining two items measure the perceived frequency of hyperglycemia and hypoglycemia and are scored separately. They are scored from 0 (they never perceive it) to 6 (almost all of the time).21
Hypoglycemia Fear Survey (HFS-II)This consists of 18 items assessed on a five-point Likert scale ranging from 0 (never) to 5 (almost always), so that total score ranges from 0 (no fear) to 72 (maximum fear). Aspects related to fear of suffering hypoglycemia being alone or while driving, of fainting in public, etc. are assessed.22
EuroQol 5d questionnaire (EQ-5D)This consists of a visual analog scale (EQ-VAS) followed by five questions related to five dimensions: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. Each dimension has three degrees of intensity (no problems, some problems, and severe problems). A health state value which may range from 0 (corresponding to death) to 1 (perfect state of health) is calculated for each subject.23
Statistical analysisThis paper shows the descriptive data of the PANORAMA substudy in Spain. Continuous variables are given as mean and standard deviation, as is the result of questionnaires, while the remaining descriptive values of the study are given as percentages.
Sample sizeIt was estimated that a sample size of 753 patients per country would provide adequate precision for all primary and secondary study variables. These calculations and the detailed statistical analysis are provided in the publication of the study design.19
ResultsFifty-four investigators, almost all of them (96.3%) primary care physicians, participated in the PANORAMA study in Spain, which enrolled 752 patients. Upon database closure, errors were found in data collection from one patient, who was excluded from the research, so that the final study sample was 751 patients.
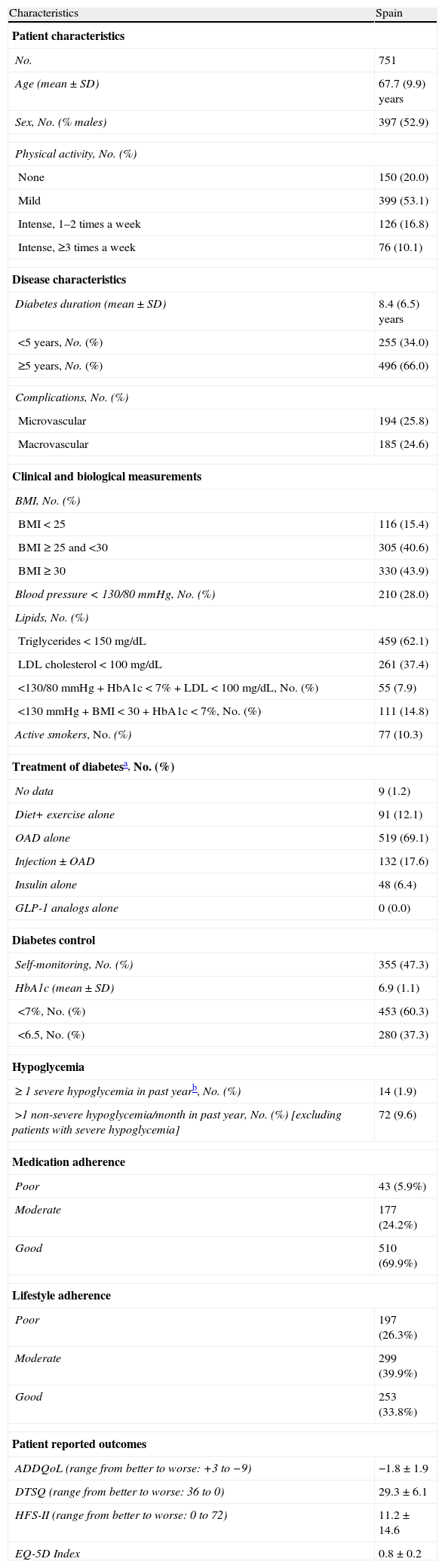
Table 1 shows the characteristics of patients recruited in Spain.
Characteristics of patients recruited in Spain.
| Characteristics | Spain |
| Patient characteristics | |
| No. | 751 |
| Age (mean±SD) | 67.7 (9.9) years |
| Sex, No. (% males) | 397 (52.9) |
| Physical activity, No. (%) | |
| None | 150 (20.0) |
| Mild | 399 (53.1) |
| Intense, 1–2 times a week | 126 (16.8) |
| Intense, ≥3 times a week | 76 (10.1) |
| Disease characteristics | |
| Diabetes duration (mean±SD) | 8.4 (6.5) years |
| <5 years, No. (%) | 255 (34.0) |
| ≥5 years, No. (%) | 496 (66.0) |
| Complications, No. (%) | |
| Microvascular | 194 (25.8) |
| Macrovascular | 185 (24.6) |
| Clinical and biological measurements | |
| BMI, No. (%) | |
| BMI<25 | 116 (15.4) |
| BMI≥25 and <30 | 305 (40.6) |
| BMI≥30 | 330 (43.9) |
| Blood pressure<130/80mmHg, No. (%) | 210 (28.0) |
| Lipids, No. (%) | |
| Triglycerides<150mg/dL | 459 (62.1) |
| LDL cholesterol<100mg/dL | 261 (37.4) |
| <130/80mmHg+HbA1c<7%+LDL<100mg/dL, No. (%) | 55 (7.9) |
| <130mmHg+BMI<30+HbA1c<7%, No. (%) | 111 (14.8) |
| Active smokers, No. (%) | 77 (10.3) |
| Treatment of diabetesa, No. (%) | |
| No data | 9 (1.2) |
| Diet+ exercise alone | 91 (12.1) |
| OAD alone | 519 (69.1) |
| Injection±OAD | 132 (17.6) |
| Insulin alone | 48 (6.4) |
| GLP-1 analogs alone | 0 (0.0) |
| Diabetes control | |
| Self-monitoring, No. (%) | 355 (47.3) |
| HbA1c (mean±SD) | 6.9 (1.1) |
| <7%, No. (%) | 453 (60.3) |
| <6.5, No. (%) | 280 (37.3) |
| Hypoglycemia | |
| ≥ 1 severe hypoglycemia in past yearb, No. (%) | 14 (1.9) |
| >1 non-severe hypoglycemia/month in past year, No. (%) [excluding patients with severe hypoglycemia] | 72 (9.6) |
| Medication adherence | |
| Poor | 43 (5.9%) |
| Moderate | 177 (24.2%) |
| Good | 510 (69.9%) |
| Lifestyle adherence | |
| Poor | 197 (26.3%) |
| Moderate | 299 (39.9%) |
| Good | 253 (33.8%) |
| Patient reported outcomes | |
| ADDQoL (range from better to worse: +3 to −9) | −1.8±1.9 |
| DTSQ (range from better to worse: 36 to 0) | 29.3±6.1 |
| HFS-II (range from better to worse: 0 to 72) | 11.2±14.6 |
| EQ-5D Index | 0.8±0.2 |
ADDQoL: audit of diabetes dependent quality of life; OAD: oral antidiabetic drug; SD: standard deviation; DTSQ: Diabetes Treatment Satisfaction Questionnaire; EQ-5D: EuroQol 5 dimensions; HFS-II: worry subscale of the hypoglycemia fear survey; BMI: body mass index; No.: sample size.
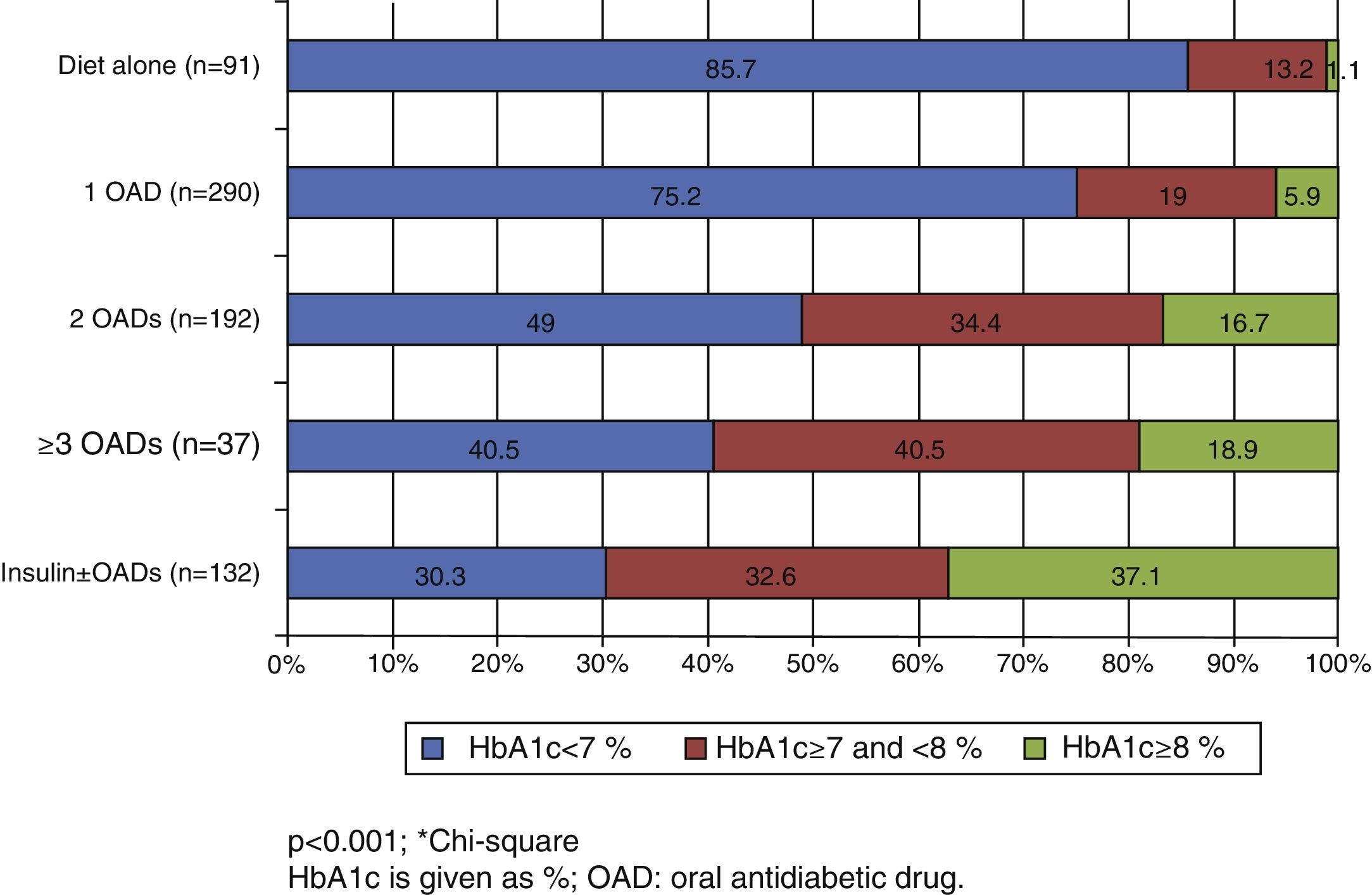
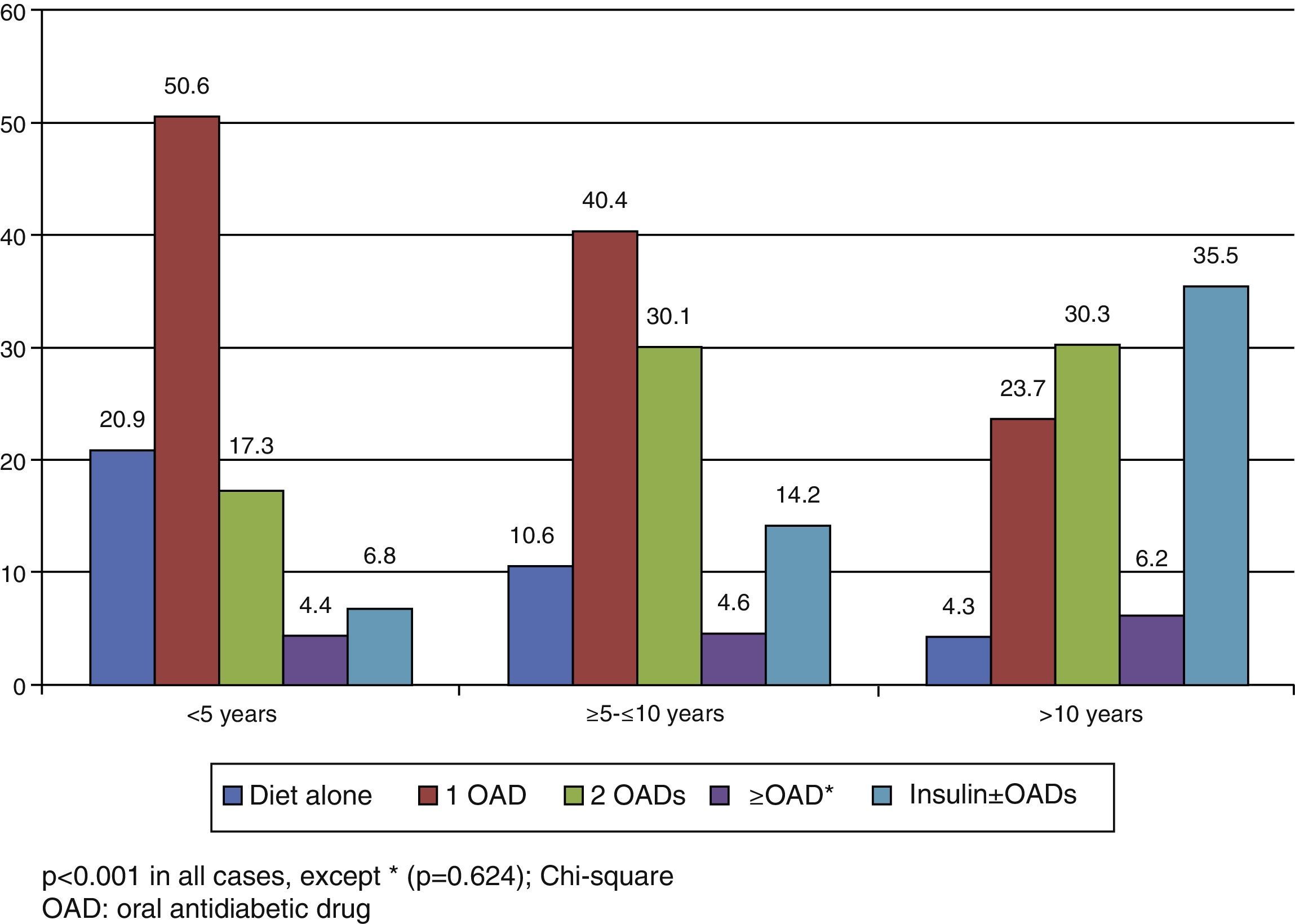
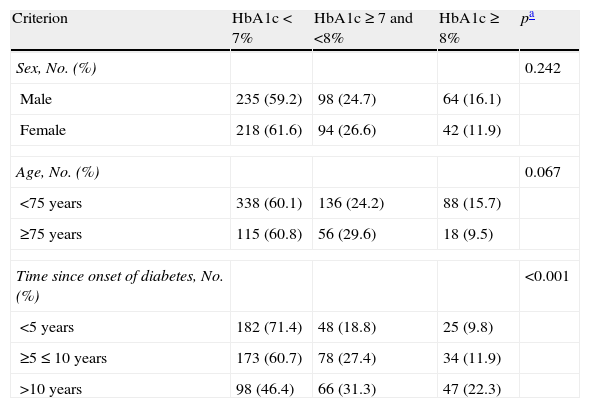
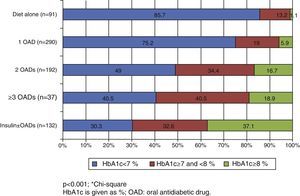
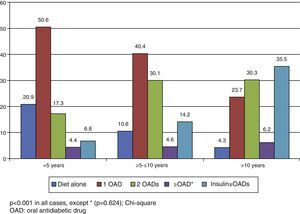
In the Spanish sample, no significant differences related to sex or age (older and younger than 75 years) were found in blood glucose control, but differences were seen in the proportion of patients with adequate control, which was associated with shorter diabetes duration (p<0.001) and less treatment complexity (Table 2 and Fig. 1). There was also a significant direct relationship (p<0.001) between disease duration and the proportion of patients treated with injectable drugs, mainly insulin (Fig. 2).
Blood glucose control by sex, age, and disease duration in Spain.
| Criterion | HbA1c<7% | HbA1c≥7 and <8% | HbA1c≥8% | pa |
| Sex, No. (%) | 0.242 | |||
| Male | 235 (59.2) | 98 (24.7) | 64 (16.1) | |
| Female | 218 (61.6) | 94 (26.6) | 42 (11.9) | |
| Age, No. (%) | 0.067 | |||
| <75 years | 338 (60.1) | 136 (24.2) | 88 (15.7) | |
| ≥75 years | 115 (60.8) | 56 (29.6) | 18 (9.5) | |
| Time since onset of diabetes, No. (%) | <0.001 | |||
| <5 years | 182 (71.4) | 48 (18.8) | 25 (9.8) | |
| ≥5≤10 years | 173 (60.7) | 78 (27.4) | 34 (11.9) | |
| >10 years | 98 (46.4) | 66 (31.3) | 47 (22.3) | |
Table 1 shows the Spanish data on the control of other risk factors (RFs) usually associated with diabetes, such as hypertension, dyslipidemia, and obesity. The proportion of patients with simultaneous control of blood pressure (BP) (<130/80mmHg), HbA1c (<7%), and LDL cholesterol (<100mg/dL) was 7.9%. Simultaneous control of systolic BP (<130mmHg), HbA1c, and weight (body mass index [BMI]<30kg/m2) was seen in 14.8% of the sample.
Physicians’ opinion and attitudesAn additional significant study objective was to assess the reasons hindering the achievement of the glucose control goal in the opinion of Spanish primary care physicians. The reasons reported (more than one could be selected) by physicians were as follows (in decreasing order of frequency): low patient adherence to advice on diet and physical exercise (54.2%), ineffectiveness of medication (23.8%), low patient adherence to glucose self-monitoring (10.7%), patient reluctance to treatment intensification (10%), low patient adherence to advice on hypoglycemic medication (9.2%), and physician reluctance to intensify treatment due to fear of hypoglycemia (3.7%), fear of interactions with other drugs (1.5%), fear of weight increase (1.3%), cost of treatment (0.9%), and fear of adverse effects, including gastrointestinal effects or peripheral edema (0.7%).
Actions that Spanish physicians would take (they could select more than one) to solve this problem were as follows: 59.1% would intensify educational measures, 22.8% would increase the dosage of the prescribed medication, 13.7% would add a new oral antidiabetic, 4.4% would refer the patient to a specialist, 2.9% would start insulin therapy, 4.1% would start other actions, and 6.5% would not take any specific action.
Patient reported outcomesTable 1 shows the opinion of Spanish patients with regard to the impact of diabetes on their quality of life, satisfaction with treatment received, perception of their state of health, and fear of hypoglycemia.
In Spain, 75% of the patients answered the ADDQoL question “If I had no diabetes, my quality of life would be…” that it would improve “a little”, “much”, or “very much” if they had no diabetes. They also reported that the aspects of their lives most affected by diabetes were freedom to eat (mean score −4.4±3.2) and freedom to drink (mean score −3.0±3.1).
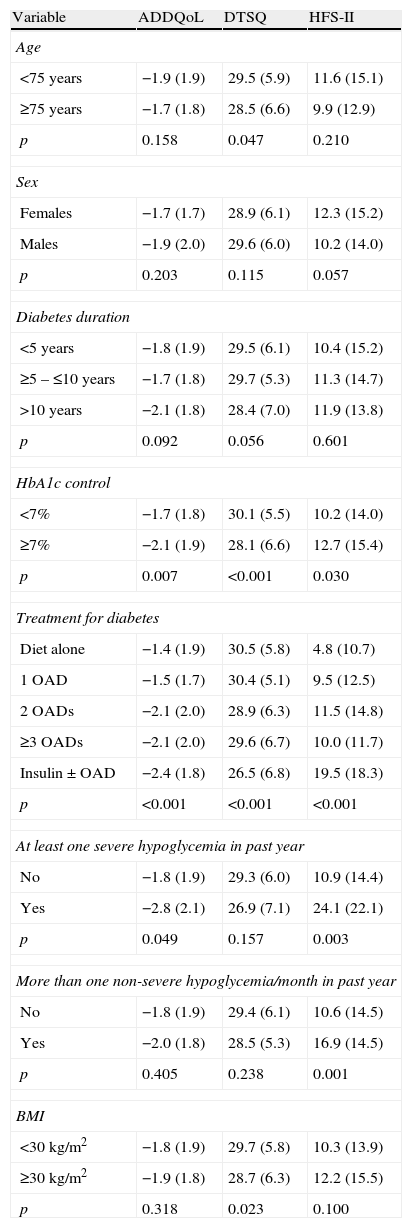
Patients with poorer glucose control (p=0.007), more complex treatment patterns (p<0.001), and experience of severe hypoglycemia (p=0.049) had a poorer quality of life as compared to patients without these factors (Table 3).
Impact of different variables on patient reported outcomes in Spain.
| Variable | ADDQoL | DTSQ | HFS-II |
| Age | |||
| <75 years | −1.9 (1.9) | 29.5 (5.9) | 11.6 (15.1) |
| ≥75 years | −1.7 (1.8) | 28.5 (6.6) | 9.9 (12.9) |
| p | 0.158 | 0.047 | 0.210 |
| Sex | |||
| Females | −1.7 (1.7) | 28.9 (6.1) | 12.3 (15.2) |
| Males | −1.9 (2.0) | 29.6 (6.0) | 10.2 (14.0) |
| p | 0.203 | 0.115 | 0.057 |
| Diabetes duration | |||
| <5 years | −1.8 (1.9) | 29.5 (6.1) | 10.4 (15.2) |
| ≥5 – ≤10 years | −1.7 (1.8) | 29.7 (5.3) | 11.3 (14.7) |
| >10 years | −2.1 (1.8) | 28.4 (7.0) | 11.9 (13.8) |
| p | 0.092 | 0.056 | 0.601 |
| HbA1c control | |||
| <7% | −1.7 (1.8) | 30.1 (5.5) | 10.2 (14.0) |
| ≥7% | −2.1 (1.9) | 28.1 (6.6) | 12.7 (15.4) |
| p | 0.007 | <0.001 | 0.030 |
| Treatment for diabetes | |||
| Diet alone | −1.4 (1.9) | 30.5 (5.8) | 4.8 (10.7) |
| 1 OAD | −1.5 (1.7) | 30.4 (5.1) | 9.5 (12.5) |
| 2 OADs | −2.1 (2.0) | 28.9 (6.3) | 11.5 (14.8) |
| ≥3 OADs | −2.1 (2.0) | 29.6 (6.7) | 10.0 (11.7) |
| Insulin±OAD | −2.4 (1.8) | 26.5 (6.8) | 19.5 (18.3) |
| p | <0.001 | <0.001 | <0.001 |
| At least one severe hypoglycemia in past year | |||
| No | −1.8 (1.9) | 29.3 (6.0) | 10.9 (14.4) |
| Yes | −2.8 (2.1) | 26.9 (7.1) | 24.1 (22.1) |
| p | 0.049 | 0.157 | 0.003 |
| More than one non-severe hypoglycemia/month in past year | |||
| No | −1.8 (1.9) | 29.4 (6.1) | 10.6 (14.5) |
| Yes | −2.0 (1.8) | 28.5 (5.3) | 16.9 (14.5) |
| p | 0.405 | 0.238 | 0.001 |
| BMI | |||
| <30kg/m2 | −1.8 (1.9) | 29.7 (5.8) | 10.3 (13.9) |
| ≥30kg/m2 | −1.9 (1.8) | 28.7 (6.3) | 12.2 (15.5) |
| p | 0.318 | 0.023 | 0.100 |
ADDQoL: audit of diabetes dependent quality of life; OAD: oral antidiabetic drug; DTSQ: Diabetes Treatment Satisfaction Questionnaire; HFS-II: worry subscale of the Hypoglycemia Fear Survey; BMI: body mass index.
Overall patient satisfaction with treatment was good (mean score, 29.3±6.1). Factors associated with less satisfaction included age (≥75 years) (p=0.047), poor blood glucose control (p<0.001), treatment complexity (p<0.001), and BMI (≥30kg/m2) (p=0.023) (Table 3).
Although fear of suffering hypoglycemia was not a significant concern for our participants (mean score, 11.2±14.6 out of a maximum of 72), insecurity was increased by the risk of suffering hypoglycemia while being alone and having nobody near to help (13.2% of patients had worried often/most of the time in the previous six months about having nobody near at hand to help them, and 14.7% about being alone).
In addition, patients with poorer metabolic control (p=0.030), more complex treatment (p<0.001), and a history of hypoglycemia (both severe and non-severe) were more worried about the risk of hypoglycemia (Table 3).
DiscussionThe PANORAMA sub-study in Spain was the first national observational, epidemiological study conducted to assess the impact of diabetes on patient quality of life and satisfaction with antidiabetic treatment using international questionnaires validated into Spanish, and to assess metabolic control and treatment patterns used.
One of the strengths of our study, differentiating it from other studies, is that HbA1c was assessed using one and the same device, validated by the National Glycosylated Hemoglobin Standardization Program in the United States, while in all other studies, HbA1c was assessed using different laboratories/methods.
Blood glucose control data from the PANORAMA study in Spain suggested improved control in patients with type 2 DM seen in primary care. Thus, while in 1996 only 43% of patients had HbA1c values less than 7%, the proportion of well-controlled patients in more recent studies ranged from 59% to 66.4%.15,24
Discrepancies exist between the different studies as regards relationship of sex and age with degree of blood glucose control, but it appears to be clear that a longer disease duration and insulin treatment are associated with a greater probability of unsatisfactory glucose control.16,24,25
It is well known that intensive blood glucose control decreases the incidence of vascular complications in patients with a recent onset of type 2 DM.4 Moreover, this protective effect persists years after the discontinuation of intensive treatment.6 In this regard, current guidelines11–13 recommend the early addition of a second antidiabetic drug if the patient is poorly controlled on a monotherapy regimen. The results of this study show that almost 25% of Spanish patients on monotherapy have HbA1c values≥7%. Another study26 conducted in Spain showed that patients on monotherapy remained with poor control for a median of two years (range, 0.0–29.7) before a second oral antidiabetic drug was added. Both studies suggest that measures are needed to avoid treatment inertia in order to improve the degree of control of patients.
Other aspects to be emphasized regarding this study are the inadequate control of other cardiovascular RFs and the low proportion of patients (approximately 8–15%) who have all these factors, as well as HbA1c, controlled at the same time. Although the Steno-2 study7 showed that overall RF control significantly decreases cardiovascular morbidity and mortality, and scientific bodies11–13 recommend a multifactorial approach in the treatment of type 2 DM, the results of the PANORAMA and other studies15,24 in Spanish diabetic patients of similar characteristics only showed improvements in glucose, lipid, and BP control. Moreover, they also reported increases in the proportion of obese people and in mean BMI, as well as inadequate simultaneous control of all these RFs. In the Vinagre et al. study,27 adequate control of HbA1c, BP, and LDL cholesterol was seen in 12.9% of patients in primary prevention and in 12.1% in secondary prevention.
Data from the PANORAMA study suggest that Spanish patients with hypoglycemic episodes have poorer quality of life and more fear of hypoglycemia than patients from elsewhere. Similar results were reported in previous studies,28,29 where hypoglycemia was also associated with less satisfaction with treatment and acted as a barrier to adequate treatment adherence.30 As severity of hypoglycemia symptoms is also associated with lack of HbA1c control30 and in the PANORAMA study many Spanish primary care physicians did not optimize treatment for fear of side effects, the use of drugs that do not increase the risk of hypoglycemia could enhance adherence and improve patient quality of life, satisfaction with treatment, and even glucose control.
The study has some limitations inherent to cross-sectional design, such as the impossibility of establishing causality (although association could be established), the lack of temporal relationship between drug exposure and effect, and the difficulty of establishing baseline values for comparison purposes. The PANORAMA study was based on prevalent cases, who usually tend to be patients with longer disease duration. However, this study has been helpful in characterizing the general health status of the diabetic population.
In conclusion, although a comparison of the PANORAMA study with prior studies shows that metabolic control in Spain has improved, it also shows that there are still some areas in need of improvement, especially in patients on monotherapy. It is important to break the therapeutic inertia of Spanish physicians. The early addition of safe drugs may help to achieve control goals without increasing the risk of hypoglycemia, and possibly delaying the start of insulin treatment. This could also contribute to improvements in both patient quality of life and treatment satisfaction.
AuthorshipPedro DePablos-Velasco, Beatriz DeRivas-Otero, and Pablo Viguera-Ester participated in manuscript conception and design, in data analysis and interpretation, and in writing, review, and approval of the manuscript submitted. Emilio Salguero-Chaves, Julio Mata-Poyo y Ricardo García-Sánchez participated in writing, review, and approval of the manuscript submitted.
FundingData used in this analysis come from the PANORAMA study funded by the Bristol-Myers Squibb/AstraZeneca Alliance.
Conflicts of interestBeatriz Derivas-Otero and Pablo Viguera-Ester are employees of AstraZeneca Farmacéutica Spain. Ricardo García Sánchez is an employee of Bristol-Myers Squibb.
The authors want to thank all the physicians who participated in the study. Unfortunately, the list is very long and cannot be fully transcribed. The authors also acknowledge the collaboration of the Medical Writing Unit of TFS in manuscript writing (funded by the Bristol-Myers Squibb/AstraZeneca Alliance).
Please cite this article as: DePablos-Velasco P, Salguero-Chaves E, Mata-Poyo J, DeRivas-Otero B, García-Sánchez R, Viguera-Ester P. Calidad de vida y satisfacción con el tratamiento de sujetos con diabetes tipo 2: resultados en España del estudio PANORAMA. Endocrinol Nutr. 2014;61:18–26.