To assess whether subclinical hypothyroidism is associated to elevations in serum cholesterol and triglyceride levels in patients with type 2 diabetes.
Patients and methodsFrom a total population of 1112 patients with type 2 diabetes screened for thyroid dysfunction (thyrotropin measurement), a group of 325 patients with normal thyroid function and another group of 29 patients with subclinical hypothyroidism were selected. No patient had known dyslipidemia or was taking lipid lowering medication.
ResultsPatients with subclinical hypothyroidism had serum levels of total cholesterol (4.88±0.74mmol/L), HDL cholesterol (1.37±0.34mmol/L), LDL cholesterol (2.94±0.58mmol/L), and triglycerides (1.05 [0.88–1.41] mmol/L) that did not significantly differ from those found in euthyroid patients (4.79±0.83, 1.33±0.36, 2.87±0.76, and 1.11 [0.81–1.43] mmol/L, respectively). Multiple regression analysis showed no association between TSH and serum lipid levels.
ConclusionThese results suggest that, in our population, there are no significant differences in serum cholesterol and triglyceride levels between diabetic patients with normal and reduced thyroid function.
Evaluar si el hipotiroidismo subclínico se acompaña de elevaciones de las concentraciones de colesterol y triglicéridos en pacientes con diabetes tipo 2.
Pacientes y métodosDe un total de 1.112 pacientes diabéticos sometidos a cribado de disfunción tiroidea (determinación de tirotropina), seleccionamos un grupo de 325 pacientes eutiroideos y otro de 29 pacientes con hipotiroidismo subclínico. Ningún paciente presentaba dislipidemia conocida o tratamiento hipolipidemiante.
ResultadosLos pacientes con hipotiroidismo subclínico presentaron unas concentraciones séricas de colesterol (4,88±0,74mmol/l), colesterol-HDL (1,37±0,34mmol/l), colesterol-LDL (2,94±0,58mmol/l) y triglicéridos (1,05 [0,88-1,41] mmol/l) que no difirieron significativamente de las encontradas en los pacientes eutiroideos (4,79±0,83, 1,33±0,36, 2,87±0,76 y 1,11 [0,81-1,43] mmol/l, respectivamente). El análisis de regresión múltiple no mostró una asociación entre los valores de TSH y las concentraciones de lípidos séricos.
ConclusiónEstos resultados evidencian que, en nuestra población, no existen diferencias en las concentraciones de colesterol y triglicéridos entre los pacientes diabéticos tipo 2 con función tiroidea normal o disminuida.
Elevated serum levels of thyroid-stimulating hormone (TSH) in the presence of normal levels of circulating thyroid hormones define the mild thyroid insufficiency known as subclinical hypothyroidism.1 Prevalence of this hormone disorder is high in the general population, particularly in elderly women.2 We have recently shown in patients with type 2 diabetes mellitus that both sexes have an increased risk of hypothyroidism,3 and that female patients have an increased risk of subclinical hypothyroidism.4
Although frank hypothyroidism is associated to elevated serum cholesterol levels,5 the association between subclinical hypothyroidism and dyslipidemia is controversial.6 Some authors have reported increased levels of low density lipoprotein cholesterol (LDL),7,8 which would be related to a potential increase in cardiovascular risk in patients with subclinical hypothyroidism. By contrast, other studies have shown nonsignificant differences in lipid levels between euthyroid subjects and patients with subclinical hypothyroidism.9,10
Subclinical hypothyroidism and dyslipidemia are two factors that may act synergistically in elevation of cardiovascular risk in patients with type 2 diabetes mellitus. It is therefore interesting to analyze whether subclinical hypothyroidism is associated to relevant lipid profile changes. Our aim was to assess whether mild hypothyroidism detected in a thyroid dysfunction screening program is associated to increased cholesterol and triglyceride levels in patients with type 2 diabetes mellitus. For this, we compared lipid values in hypothyroid diabetic patients and diabetic patients with no prior diagnosis of hyperlipidemia and normal thyroid function.
Patients and methodsStudy designA cross-sectional study enrolling all euthyroid and hypothyroid patients found in a thyroid function screening program conducted during 2004–2010 at our center was designed.3,4 All patients had been diagnosed with type 2 diabetes mellitus according to criteria of the American Diabetes Association11 and were studied on an outpatient basis. Patients with recent acute disease and treated with drugs able to alter thyroid function were excluded. The study was approved by the local ethics committee, and patients gave their informed consent before blood sampling.
Variables recorded included demographic and anthropometric data, time since onset of diabetes, presence of goiter, high blood pressure, hyperlipidemia, microvascular and macrovascular complications of diabetes, antidiabetic treatment used (diet, oral antidiabetic drugs, insulin), and other laboratory data (glucose, cholesterol, triglycerides, and hemoglobin A1c). Serum TSH levels were measured in all patients with previously known thyroid disease. When TSH levels were higher than 5mU/L, free T4 levels were also measured. Autoimmunity was assessed by measuring thyroid peroxidase and thyroglobulin antibodies. Thyroid autoimmunity was considered to be negative when both autoantibodies were negative, and positive when one of the antibodies was positive.
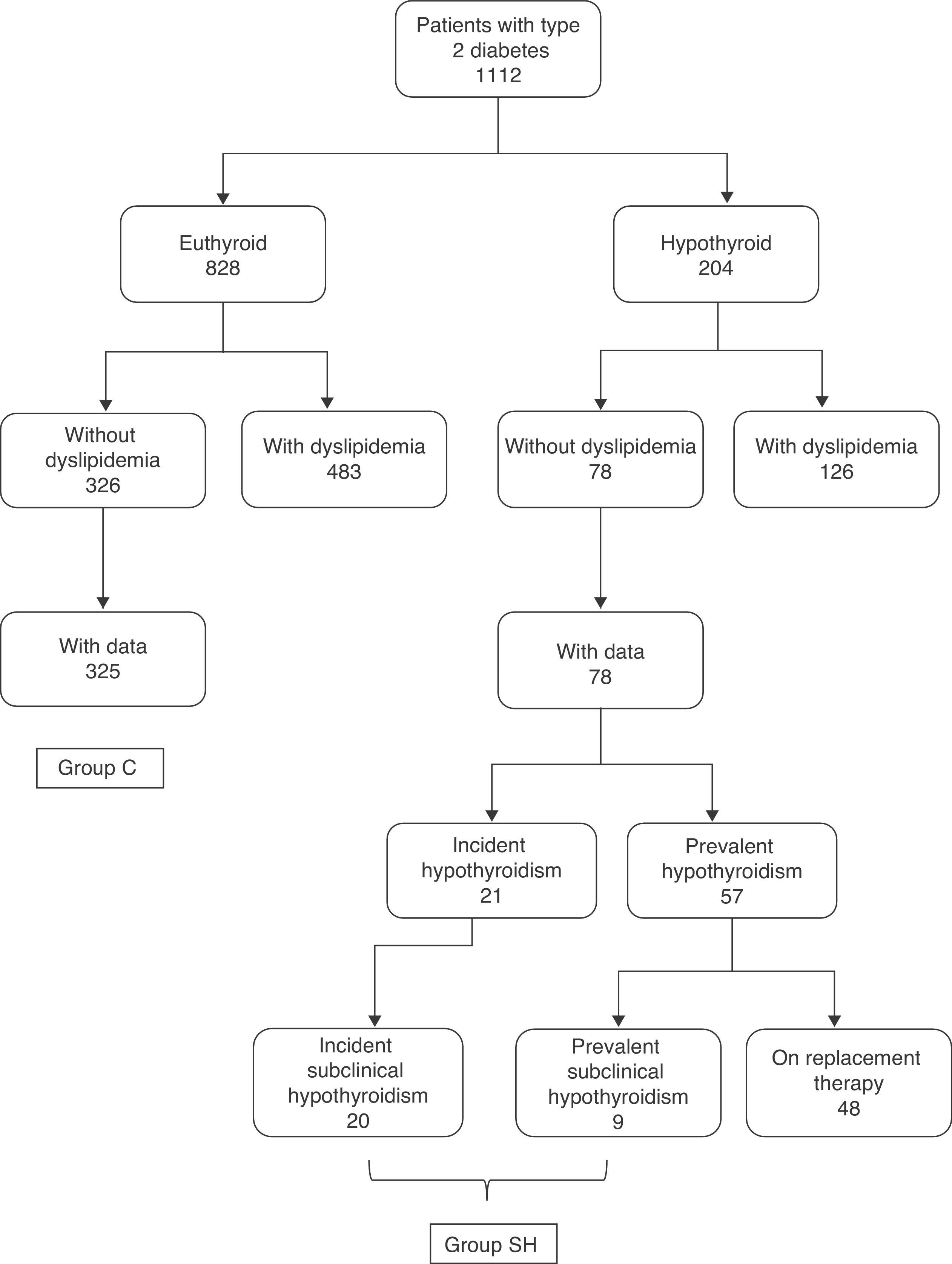
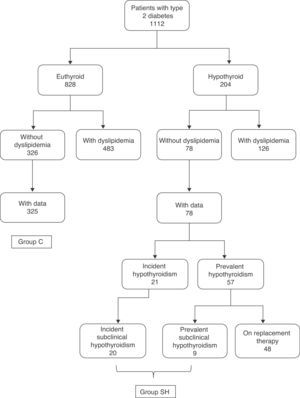
PatientsAmong the 1112 study patients, there were 828 euthyroid and 204 hypothyroid subjects3 (Fig. 1). Subclinical hypothyroidism was defined as TSH levels higher than 5mU/L with normal free T4, while frank hypothyroidism required both TSH levels higher than 5mU/L and free T4 levels less than 9pmol/L.
In the group of 828 euthyroid diabetic patients, 483 patients had a prior diagnosis of hyperlipidemia, while 326 patients had no known prior hyperlipidemia (no information about prior diagnosis of hyperlipidemia was available for 19 patients). Lipid profile data (at least cholesterol and triglycerides) were available for 325 of the 326 patients without hyperlipidemia. The control group (group C) consisted of these 325 patients (164 males and 161 females with a mean age of 65.2±13.4 years).
In the group of 204 hypothyroid diabetic patients, 126 had a prior diagnosis of hyperlipidemia and 78 had no such diagnosis. In the latter group, 52 patients had prevalent hypothyroidism (with a disease duration of 5 [3–12.8] years) and 21 incident hypothyroidism (detected at screening). Of these 21 hypothyroid patients, one had frank hypothyroidism, and the remaining 20 patients had subclinical hypothyroidism. In the group of 57 patients with prevalent hypothyroidism, 48 patients were being treated with levothyroxine and 9 took no replacement therapy because they had slight subclinical hypothyroidism. As the primary objective of our study was to analyze differences between euthyroid patients and those with subclinical hypothyroidism, group C data (euthyroid control patients) were compared to data from the group of patients with either incident or prevalent subclinical hypothyroidism. This latter group (SH group) consisted of the 20 patients with incident subclinical hypothyroidism and the 9 patients with untreated prevalent subclinical hypothyroidism (Fig. 1).
Laboratory measurementsFor laboratory measurements, basal blood samples were taken between 8 and 9 AM. TSH and free T4 levels were measured using a commercial immunochemiluminescent assay (Immulite®, Diagnostic Products Corporation, Los Angeles, CA, USA). Sensitivity of the TSH assay was 0.004mU/L. Intra- and inter-assay coefficients of variation were less than 10%. Normal TSH values were 0.4–5.0mU/L.
Glucose, total cholesterol, high density lipoprotein (HDL) cholesterol, and triglyceride levels were measured using an Architect ci8200 multichannel analyzer (Abbot Diagnostics, Berkshire, United Kingdom). LDL cholesterol levels were calculated using the Friedewald formula [LDL cholesterol=total cholesterol−HDL cholesterol−(triglycerides/5)], provided HDL cholesterol levels were available and patient had triglyceride levels less than 4.52mmol/L. Hemoglobin A1c was measured by high performance liquid chromatography (Diamat, Bio-Rad, Wien, Austria) using a Merk-Hitachi model L9100 autoanalyzer. Thyroid peroxidase and thyroglobulin antibodies were tested using a chemiluminescent immunoassay (Immulite® Thyroid Autoantibodies, Siemens Medical Solutions Diagnostic Ltd., Llanberis, Gwynedd, United Kingdom). Titers higher than 100U/mL for thyroid peroxidase antibodies and higher than 340U/mL for thyroglobulin antibodies were considered positive.
Statistical analysisResults are given as mean±standard deviation for normal variables, and as median (interquartile range) for nonparametric variables. Normal distribution of variables was verified using a Kolmogorov test. Qualitative variables are reported as proportions or percentages. To compare means between the two groups of patients, a Student's t test was used for normal data, and a Mann–Whitney test was used for nonparametric variables. A Chi-square test and a Fisher's exact test were used to compare proportions. To analyze the relationship between thyroid function values and lipid profile in the different patient groups, simple and multiple regression analysis was used. All statistical tests were two-sided, and differences were considered to be significant with values of p<0.05.
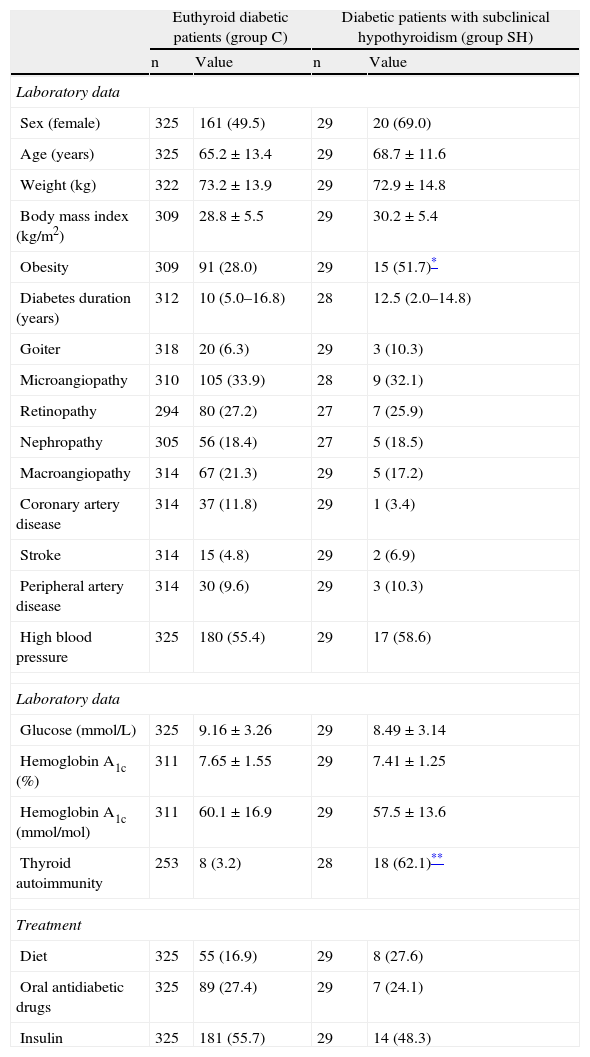
ResultsLipid profile in patients with subclinical hypothyroidismNo significant differences were seen between group C and group SH in age, diabetes duration, presence of goiter, chronic complications of diabetes, or basal glucose and hemoglobin A1c values. The antidiabetic treatment used was also similar in both groups. The group of patients with subclinical hypothyroidism had a greater proportion of obese people. Positive thyroid autoimmunity was also common in hypothyroid patients (Table 1).
Clinical and laboratory characteristics of euthyroid diabetic patients and diabetic patients with subclinical hypothyroidism (incident and prevalent).
| Euthyroid diabetic patients (group C) | Diabetic patients with subclinical hypothyroidism (group SH) | |||
| n | Value | n | Value | |
| Laboratory data | ||||
| Sex (female) | 325 | 161 (49.5) | 29 | 20 (69.0) |
| Age (years) | 325 | 65.2±13.4 | 29 | 68.7±11.6 |
| Weight (kg) | 322 | 73.2±13.9 | 29 | 72.9±14.8 |
| Body mass index (kg/m2) | 309 | 28.8±5.5 | 29 | 30.2±5.4 |
| Obesity | 309 | 91 (28.0) | 29 | 15 (51.7)* |
| Diabetes duration (years) | 312 | 10 (5.0–16.8) | 28 | 12.5 (2.0–14.8) |
| Goiter | 318 | 20 (6.3) | 29 | 3 (10.3) |
| Microangiopathy | 310 | 105 (33.9) | 28 | 9 (32.1) |
| Retinopathy | 294 | 80 (27.2) | 27 | 7 (25.9) |
| Nephropathy | 305 | 56 (18.4) | 27 | 5 (18.5) |
| Macroangiopathy | 314 | 67 (21.3) | 29 | 5 (17.2) |
| Coronary artery disease | 314 | 37 (11.8) | 29 | 1 (3.4) |
| Stroke | 314 | 15 (4.8) | 29 | 2 (6.9) |
| Peripheral artery disease | 314 | 30 (9.6) | 29 | 3 (10.3) |
| High blood pressure | 325 | 180 (55.4) | 29 | 17 (58.6) |
| Laboratory data | ||||
| Glucose (mmol/L) | 325 | 9.16±3.26 | 29 | 8.49±3.14 |
| Hemoglobin A1c (%) | 311 | 7.65±1.55 | 29 | 7.41±1.25 |
| Hemoglobin A1c (mmol/mol) | 311 | 60.1±16.9 | 29 | 57.5±13.6 |
| Thyroid autoimmunity | 253 | 8 (3.2) | 28 | 18 (62.1)** |
| Treatment | ||||
| Diet | 325 | 55 (16.9) | 29 | 8 (27.6) |
| Oral antidiabetic drugs | 325 | 89 (27.4) | 29 | 7 (24.1) |
| Insulin | 325 | 181 (55.7) | 29 | 14 (48.3) |
Data are numbers of patients (percentage), mean±standard deviation for normal data, and median (interquartile range) for nonparametric data. Numbers of patients used to calculate mean, medians, and percentages are indicated by n.
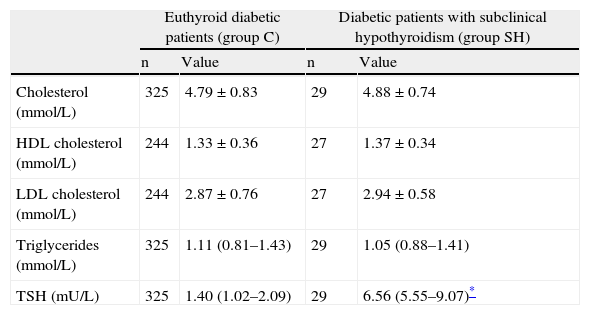
Patients with subclinical hypothyroidism had cholesterol, HDL cholesterol, LDL cholesterol, and triglyceride levels that were not significantly different from those found in euthyroid patients (Table 2). When lipid levels were tested in the subgroups of patients with incident and prevalent subclinical hypothyroidism, and in the subgroups of females and patients with obesity, no significant differences were also found from values of euthyroid patients (data not shown).
Lipid profile and TSH values in diabetic patients with subclinical hypothyroidism as compared to euthyroid diabetic patients.
| Euthyroid diabetic patients (group C) | Diabetic patients with subclinical hypothyroidism (group SH) | |||
| n | Value | n | Value | |
| Cholesterol (mmol/L) | 325 | 4.79±0.83 | 29 | 4.88±0.74 |
| HDL cholesterol (mmol/L) | 244 | 1.33±0.36 | 27 | 1.37±0.34 |
| LDL cholesterol (mmol/L) | 244 | 2.87±0.76 | 27 | 2.94±0.58 |
| Triglycerides (mmol/L) | 325 | 1.11 (0.81–1.43) | 29 | 1.05 (0.88–1.41) |
| TSH (mU/L) | 325 | 1.40 (1.02–2.09) | 29 | 6.56 (5.55–9.07)* |
Data given as mean±standard deviation or median (interquartile range). The numbers of patients used to calculate these values in each group is indicated by n.
Simple regression analysis showed no linear correlation of TSH and free T4 values with lipid levels in patients with subclinical hypothyroidism. In euthyroid patients, a significant correlation was found between TSH and triglyceride levels (rho=0.165; p=0.003).
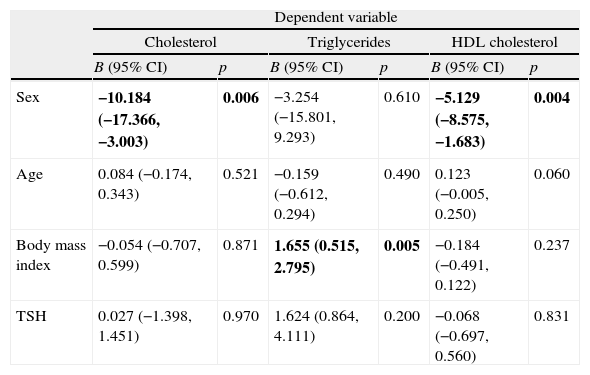
A multiple regression analysis was performed to see if there was an association between thyroid function, represented by TSH levels, and plasma lipid levels in the whole group of diabetic patients. When age, sex, body mass index and TSH were considered as independent variables, a significant association was found between sex and total cholesterol and HDL cholesterol, and also between body mass index and triglycerides, but no association was found between TSH and serum lipid levels (Table 3). In a second model where TSH levels were replaced by the patient group (C or SH), results were very similar (data not shown).
Multiple regression analysis to study the relationship between plasma lipids and TSH levels in the diabetic patients studied.
| Dependent variable | ||||||
| Cholesterol | Triglycerides | HDL cholesterol | ||||
| B (95% CI) | p | B (95% CI) | p | B (95% CI) | p | |
| Sex | −10.184 (−17.366, −3.003) | 0.006 | −3.254 (−15.801, 9.293) | 0.610 | −5.129 (−8.575, −1.683) | 0.004 |
| Age | 0.084 (−0.174, 0.343) | 0.521 | −0.159 (−0.612, 0.294) | 0.490 | 0.123 (−0.005, 0.250) | 0.060 |
| Body mass index | −0.054 (−0.707, 0.599) | 0.871 | 1.655 (0.515, 2.795) | 0.005 | −0.184 (−0.491, 0.122) | 0.237 |
| TSH | 0.027 (−1.398, 1.451) | 0.970 | 1.624 (0.864, 4.111) | 0.200 | −0.068 (−0.697, 0.560) | 0.831 |
Statistically significant values appear in bold.
Implementation of a thyroid dysfunction screening program in our population with type 2 diabetes gave us the opportunity to compare a group of 29 diabetic patients with untreated subclinical hypothyroidism to a large group of euthyroid diabetic patients from the same population. Our results show that cholesterol and triglyceride levels are not significantly different in hypothyroid diabetic patients and diabetic patients with normal thyroid function. In both groups, patients with prior diagnosis of dyslipidemia and who were therefore being treated for this metabolic disorder were excluded.
Some recent population studies have shown increases in total and LDL cholesterol levels, usually modest, in patients with subclinical hypothyroidism as compared to euthyroid patients,12–14 while other studies only found increased lipid values in female patients.15 Our results agree with those from a wide group of studies showing nonsignificant differences in lipid levels between euthyroid subjects and patients with subclinical hypothyroidism taken from the general population.9,10,16–18 An observational study specifically aimed at assessing lipid profile in hypothyroid patients and including 4886 euthyroid subjects and 1055 with subclinical hypothyroidism found no significant differences between both groups in total cholesterol, LDL cholesterol, HDL cholesterol, and triglyceride levels.19 An in-depth analysis of data from more than 8000 subjects assessed in the Third National Health and Nutritional Examination Survey showed that subclinical hypothyroidism in the general US population was not related to elevations in total cholesterol, LDL cholesterol, or triglyceride levels or with decreases in HDL cholesterol levels.20
While high quality studies in the general population are abundant in the literature, we could not find large scale studies comparing lipid levels in diabetic patients with subclinical hypothyroidism and normal thyroid function. Wang et al.21 reported a greater prevalence of elevated total and LDL cholesterol levels, or decreased HDL cholesterol levels, in diabetic women with subclinical hypothyroidism as compared to euthyroid women, but this study enrolled elderly women with poorly controlled dyslipidemia only. In a prior study conducted on a patient cohort different from the one included in this study, presence of thyroid dysfunction was unrelated to complications of diabetes, including hyperlipidemia.22
A recent study found a positive correlation between TSH and lipid levels in a patient cohort with various degrees of thyroid dysfunction, although data suggest that slight TSH increases only have a marginal impact on lipid profile.23 Our regression analysis showed no relationship between TSH and lipid levels in hypothyroid diabetic patients. A positive linear correlation was however found between TSH and triglycerides in the euthyroid subject sample. Such correlation, possibly dependent on body mass index, was not seen in the multiple regression analysis. A similar finding was made in women in the Tromsø study, an epidemiological analysis of 5143 subjects from the general population.15
The mechanisms relating thyroid function to lipid levels in diabetic patients are complex. Some studies show that the known associations between dyslipidemia and frank hypothyroidism may be valid even in subjects with TSH levels within the reference range.24,25 Chubb et al.24 showed significant associations between TSH and serum lipids in euthyroid women with type 2 diabetes which occurred in insulin-resistant patients, but were absent in those with greater insulin sensitivity. Even in patients with type 1 diabetes, it has recently been shown that TSH is associated to LDL cholesterol alone in patients with less insulin sensitivity.26 That is, interaction between thyroid function and insulin resistance may play a significant role in the genesis of diabetic dyslipidemia.
Our study has the limitations inherent to a screening study which was not initially designed to assess lipid levels in patients with thyroid dysfunction. Because of this, HDL cholesterol levels were not available for some patients. The sample of 29 patients with subclinical hypothyroidism may appear initially small. However, recruitment of a prospective sample of patients with type 2 diabetes not treated with lipid lowering agents and with mild thyroid dysfunction not on replacement therapy, and another sample of euthyroid diabetic patients does not appear to be currently easy. Although our analysis may not have the same value as a prospective analysis, it allows for avoiding the great difficulties involved by the latter. The sample of 325 euthyroid patients acting as the control group did not significantly differ from the study sample, except for a greater proportion of obese people. However, subgroup analysis also showed no significant differences in cholesterol and triglyceride levels in obese subjects. Insulin resistance of diabetic patients could not be estimated in our study. Thus, the effects of insulin sensitivity on the relations between thyroid function and serum lipids cannot be analyzed.
The clinical significance of the slight cholesterol and triglyceride elevation that may occur at diagnosis of mild hypothyroidism in a diabetic patient is uncertain. First of all, because subclinical hypothyroidism may revert spontaneously.27 Second, because it has not been shown that replacement therapy with levothyroxine induces a relevant improvement of lipid profile in patients with subclinical hypothyroidism. Different interventional studies have shown that treatment with levothyroxine causes no significant changes in serum lipids in patients with subclinical hypothyroidism.28,29 Other studies have shown decreases in total and LDL cholesterol levels after treatment with thyroxine,7,8 but decreases were more marked in patients with increased cholesterol levels and in those with inadequately replaced frank hypothyroidism. A meta-analysis of 13 interventional studies showed that levothyroxine replacement therapy caused cholesterol decreases proportional to the severity of hypothyroidism and to the increase in serum lipid levels.30
In conclusion, our results show that, in our population, there were no differences in cholesterol and triglyceride levels between patients with type 2 diabetes with normal or decreased thyroid function. No correlation was also found between TSH and lipid levels in diabetic patients. These results would not support the start of early replacement therapy with levothyroxine in diabetic patients with mild hypothyroidism for prevention of diabetic dyslipidemia. This conclusion can only be reliably drawn from adequate prospective clinical studies on a sufficiently large patient sample.
Conflicts of interestThe authors state that they have no conflicts of interest in relation to this article.
Please cite this article as: Díez JJ, Iglesias P. Concentraciones séricas de colesterol y triglicéridos en pacientes diabéticos con hipotiroidismo subclínico. Endocrinol Nutr. 2014;61:419–425.