Hypomagnesemia is defined as plasma magnesium levels less than 1.8mg/dL. Levels less than 1.2mg/dL are considered severe hypomagnesemia. The first diagnostic step to ascertain the cause of hypomagnesemia is to assess the fractional excretion of magnesium (FEMg) in urine. The most common causes include nutritional problems and gastrointestinal or renal losses, while isolated malabsorption is a less common cause. In the past year, alerts related to chronic administration of proton pump inhibitors (PPIs) have increasingly been reported.1 Renal response to increased gastrointestinal losses of this cation is the maintenance of a FEMg less than 2%, and severe hypomagnesemia per se impairs PTH secretion, leading to hypokalemia in most cases.2,3 We report the case of a male patient who experienced severe hypomagnesemia and hypokalemia secondary to chronic diarrhea. He had been treated with omeprazole for 10 years. The side effect of this drug, uncommon but with significant clinical consequences and whose etiology has not been fully elucidated yet, has prompted us to report this causal relationship in the scientific literature. Diagnosis is confirmed by the reversibility of electrolyte disorders upon drug discontinuation once other causes have been ruled out, which occurred in our patient.
A 64-year-old male patient with a history of high blood pressure, grade II obesity, sleep apnea syndrome, and type 2 diabetes mellitus with good metabolic control who had been treated with omeprazole 20mg/day for gastroesophageal reflux for 10 years is reported. The patient attended the emergency room for diarrhea over the previous month with no fever, blood, or mucus associated with severe asthenia, fatigue, and generalized muscle weakness. Laboratory test results at the emergency room showed hypocalcemia (6.8mg/dL), ionic calcium 0.62, severe hypomagnesemia (0.30mg/dL), and normal potassium and phosphorus. Electrocardiographic changes consistent with this severe deficiency and clinical signs of latent tetany were found. Electrolyte disorders were therefore corrected, and the patient was admitted for work-up. Supplemental gastrointestinal tract examinations (bowel transit, abdominal ultrasound, gastrointestinal endoscopy, serum tests for celiac disease, among others) were normal, and malabsorption was therefore ruled out. Vitamin D, 25 OHD, 1–25 OHD, thyroid profile, and cortisol levels, as well as the renin–angiotensin–aldosterone system, were normal in our patient. The patient was discharged with no symptoms to the endocrinology outpatient clinic for monitoring. He was being treated with oral supplements of calcium, magnesium, and calcitriol. The possibility of a disorder related to PTH or a primary magnesium deficiency was first considered. During monitoring, based on a FEMg less than 2%, and ruling out tubular disorders leading to increased magnesium excretion (Gitelman or Bartter syndrome), it was decided, because of the reported cases of severe magnesium deficiency related to proton pump inhibitors, to discontinue treatment with omeprazole, which was replaced by ranitidine 150mg/day. This led to the changes in laboratory parameters shown in Table 1. The clinical course of the patient was very satisfactory. He gradually started to feel well, showing electrolyte levels within the normal range, and oral supplementation could progressively be decreased until now, when the patient is asymptomatic and untreated and magnesium, calcium, and parathyroid hormone levels have returned to normal.
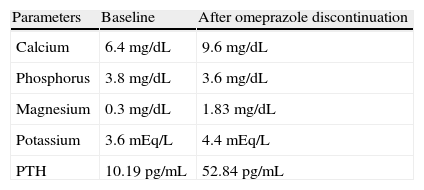
Plasma levels at baseline and six months after omeprazole discontinuation without replacement treatment.
| Parameters | Baseline | After omeprazole discontinuation |
| Calcium | 6.4mg/dL | 9.6mg/dL |
| Phosphorus | 3.8mg/dL | 3.6mg/dL |
| Magnesium | 0.3mg/dL | 1.83mg/dL |
| Potassium | 3.6mEq/L | 4.4mEq/L |
| PTH | 10.19pg/mL | 52.84pg/mL |
Severe hypomagnesemia is recognized as a cause of hypoparathyroidism due to impaired parathyroid hormone secretion, with resultant hypocalcemia. Nine cases of severe hypomagnesemia induced by omeprazole have recently been reported in the literature, to which we now add the present case.1,4 Magnesium homeostasis is the result of a balance between intestinal absorption and renal clearance. Although various drugs have been related to hypomagnesemia, this is due to increased renal loss. By contrast, hypomagnesemia induced by omeprazole and other inhibitors is due to deficient magnesium absorption in the bowel and to an imbalance between passive and active transport in the intestinal lumen. It has been postulated that patients treated with proton pump inhibitors have impaired active transport due to intestinal pH changes that alter channel functions or because sensitive patients are heterozygous carriers of mutations of TRPM6 (the protein of the receptor family of the transport canals in the intestinal lumen). In our patient, the resolution of electrolyte disorders after drug discontinuation, six months later, and the maintenance of normal electrolyte levels without supplementation suggest the need for managing this adverse effect in patients on long-term treatment with this drug class, as has already been reported.
Hospital Infanta Cristina, Badajoz.
Please cite this article as: Rodríguez Ortega P, et al. Hipomagnesemia grave e hipoparatiroidismo secundario a omeprazol. Endocrinol Nutr. 2013;60:156–7.