The reported efficacy of treatments for acromegaly varies depending on reference centers and national registries. The aim of this study was to describe clinical management of this disease and to assess the efficacy of treatments used in standard clinical practice.
Material and methodsAn epidemiological, observational, longitudinal, multicenter study was performed in adult patients with newly diagnosed acromegaly (n=74) seen by 38 Spanish endocrinologists who collected during routine clinic visits data on disease treatment and control during 2 years of follow-up.
ResultsPituitary surgery and treatment with somatostatin analogs were the first choice therapies in 76% and 24% of patients, respectively, with no differences related to tumor size. Surgery achieved disease control in 27% of operated patients. After surgery failure, the preferred therapeutic option was somatostatin analogs, which normalized insulin-like growth factor-1 (IGF-I) in 52% of patients and achieved disease control criteria in more than 40% of patients. At the end of follow-up, normal IGF-I levels were found in 63% and 53% of patients with microadenomas and macroadenomas, respectively. Only 19% of patients with macroadenoma met disease control criteria without requiring drug treatment, which was required by 85% of them at some time during follow-up.
ConclusionsSurgery is the preferred initial treatment for patients with acromegaly, regardless of tumor size. Treatment efficacy in actual clinical practice is far from the success rates reported by reference centers.
La eficacia publicada de las terapias en acromegalia es discordante entre centros de referencia y registros nacionales. El objetivo del presente estudio es describir el manejo clínico de esta enfermedad y la eficacia de los tratamientos implementados en la práctica clínica habitual.
Material y métodosEstudio epidemiológico, observacional, longitudinal y multicéntrico, en pacientes adultos con acromegalia de reciente diagnóstico (n = 74), y con participación de 30 endocrinólogos de la geografía española, que recogieron durante las visitas clínicas rutinarias datos acerca del tratamiento y control de enfermedad durante un periodo de seguimiento de 2 años.
ResultadosLa cirugía hipofisaria fue la terapia de primera elección en el 76% de los pacientes, indicándose en el 24% restante tratamiento de primera línea con análogos de somatostatina, sin diferencias en función del tamaño tumoral. El tratamiento quirúrgico controló la enfermedad en el 27% de sujetos intervenidos. Tras el fracaso de la cirugía inicial, la opción terapéutica preferida fueron los análogos de somatostatina, que normalizaron el factor de crecimiento insulínico tipo 1 (IGF-I) en el 52% de casos, con criterios de control de enfermedad en más del 40% de los sujetos. Al final del seguimiento, un 63% de microadenomas y un 53% de macroadenomas presentaban IGF-I normal. Solo el 19% de los pacientes con macroadenomas cumplía criterios de control sin precisar fármacos. Un 85% de los pacientes requirió tratamiento farmacológico en algún momento del seguimiento.
ConclusionesEl tratamiento inicial preferido en nuestro medio para la acromegalia es la intervención quirúrgica, independientemente del tamaño tumoral. La eficacia del tratamiento en la práctica clínica real se aleja de las tasas de éxito publicadas por centros de referencia.
Acromegaly is an uncommon disease, with an estimated prevalence in Spain of 36 cases per 106 inhabitants,1 but it causes a significant morbidity and mortality in patients who suffer it as a result of the harmful effects of tissue exposure to pathologically increased levels of growth hormone (GH) and its effector, insulin-like growth factor (IGF-1). In patients with acromegaly, mortality2–4 and a shortening of life expectation associated with the disease5 is mainly attributable to increased cardiovascular events, respiratory disease, and the occurrence of secondary neoplasms.6 However, biochemical control of the disease, defined by circulating basal GH levels less than 2.5μg/L4, suppression of GH secretion following a standard oral glucose tolerance test (GH-OGTT) and/or normalization of IGF-1 is associated with a decrease in mortality to levels similar to that of the general population.7,8
The currently agreed treatment for patients with GH-secreting pituitary adenomas is based on either surgical resection, the first choice treatment for patients with microadenoma, non-invasive macroadenoma or local compression symptoms, or on drug treatment (somatostatin analogs [SAs], dopaminergic agonists [DAs], and GH receptor antagonists) combined or not with surgery in tumors where surgery success is unlikely, while conventional radiotherapy or radiosurgery is reserved for patients not controlled with prior treatments.9 Surgery, usually by the transsphenoidal route, achieves biochemical control in approximately 75–90% of microadenomas10–12 and in 40–50% of macroadenomas,10–12 with poorer results achieved in lesions with extrasellar extension.12 Treatment with SAs, the first choice drug therapy, achieves normalization of IGF-1 levels in 70% of selected patients,13 with significant decreases in tumor size in 40–45% of patients.14 It should be noted, however, that the surgical data reported usually come from reference centers, and it is well known that the success of pituitary surgery depends on the number of procedures performed by the surgeon and the surgeon's experience,15 and that the efficacy of drug therapy with SAs, both in terms of biochemical control and tumor mass reduction, is lower when patients who were not previously selected on the basis of their response to subcutaneous octreotide treatment are evaluated.16 Based on the foregoing, it seems natural to think that the scientific literature could overestimate the success of available treatments in a real clinical setting.
The aim of this study was to prospectively report on the management of patients with acromegaly secondary to pituitary adenoma in standard clinical practice, and to assess the treatment preferences of specialists in our field and the efficacy of the therapies used.
Patients and methodsA multicenter, open label, observational, longitudinal, epidemiological study was designed. Thirty-eight specialists in endocrinology from 30 centers throughout Spain who usually see patients with acromegaly recruited patients between October 2005 and April 2007. During the two years of follow-up of the study, the investigators were required to collect at all visits made by patients in the setting of standard clinical practice, and applying no external intervention, a number of variables in a predesigned, self-completed electronic case report form.
The study was conducted in accordance with the principles of the Declaration of Helsinki for research in humans, and received administrative and ethical approval from the ethics committees of Hospital Clinic i Provincial in Barcelona and other participating centers where it was required.
Inclusion criteria- –
The patient was aged 18–80 years with acromegaly induced by a pituitary adenoma, documented by a nadir GH-OGTT higher than 1μg/L and IGF-1 levels above the age-adjusted upper limit of normal.
- –
The patient had recently been diagnosed or had undergone surgery in the previous 6 months with or without drug treatment no longer than 3 months prior to surgery.
- –
There was a minimum follow-up of 20 months.
- –
The patient had signed an informed consent to participate in the study.
- –
The patient had not participated in any clinical trial or other studies with drugs.
- –
The patient had been treated with drugs after surgery (before study entry).
- –
The patient had been receiving drug treatment for more than 3 months before surgery.
- –
The patient had any physical or medical condition, which could interfere with data collection or study objectives in the investigator's judgment.
- –
The patient was pregnant, breastfeeding, or of childbearing age and not using reliable contraceptive methods.
The primary objective was to prospectively describe the therapeutic management of patients with acromegaly according to standard clinical practice in Spain. A secondary objective was to establish the efficacy in terms of biochemical control of the different treatment strategies used.
Test variables and disease control definitionData collected included sociodemographic (age and sex) and clinical variables: time of disease diagnosis, type of tumor (microadenoma 10mm or less in size and macroadenoma greater than 10mm); treatments for acromegaly: surgery, drug treatment and/or radiotherapy (date, dose, and scheme), changes in treatment, and biochemical control of acromegaly after interventions. Since recruitment was performed from 2005 to 2007 and ultrasensitive assays for GH measurement were not available in all centers, the disease control criteria for patients without drug treatment were GH-OGTT levels less than 1μg/L and IGF-1 levels within the normal limits adjusted to patient age according to the international consensus applicable at the time.17 The criteria used for patients treated with SAs±DAs included basal GH levels less than 2.5μg/L together with normal IGF-1 levels,4,17 or normal IGF-1 levels regardless of GH levels in subjects treated with pegvisomant at the end of follow-up.
Statistical analysis and description of resultsAs this was an observational, exploratory study, sample size calculation was not based on statistical assumptions. Based on the estimated annual incidence of acromegaly in Spain of 2.5 cases per 106 inhabitants,1 a minimum sample of 60 patients was estimated as being necessary to achieve a representative assessment of standard clinical management.
Data are given as mean±SD and crude numbers (percentages). For descriptive analysis of the variables, two treatment groups were established based on the first line treatment decided (surgery or drug treatment). Differences in treatment and therapeutic efficacy depending on tumor size were also recorded and analyzed. The tumor could not be classified as micro or macroadenoma in three patients because no adequate data were available in the electronic case report form. These patients were therefore excluded from the subanalyses performed based on tumor size. Quantitative variables were compared using a Student's t-test and univariate general linear models. Generalized linear models for repeated measures were used to assess the impact of surgery on hormone measurements. Normality of variables was previously verified in all cases using a Kolmogorov–Smirnov test, and logarithmic transformations were performed when required. Qualitative variables were compared using a Chi-square test or a Fisher's exact test as required. To avoid type I errors derived from missing data, an intent-to-treat analysis was performed imputing to the missing variable the last value previously reported during the assessment visits.
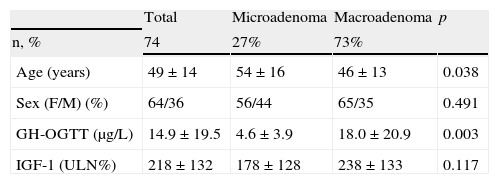
ResultsBaseline characteristics of the sampleEighty-six patients were recruited, 12 of whom were excluded because they did not meet all the inclusion criteria or had any exclusion criteria. Data from the remaining 74 patients, followed up for 28±5 months and collected data at 6±2 visits, were used for the description of the results. Table 1 shows the baseline characteristics of the recruited patients.
Baseline characteristics of the sample.
| Total | Microadenoma | Macroadenoma | p | |
| n, % | 74 | 27% | 73% | |
| Age (years) | 49±14 | 54±16 | 46±13 | 0.038 |
| Sex (F/M) (%) | 64/36 | 56/44 | 65/35 | 0.491 |
| GH-OGTT (μg/L) | 14.9±19.5 | 4.6±3.9 | 18.0±20.9 | 0.003 |
| IGF-1 (ULN%) | 218±132 | 178±128 | 238±133 | 0.117 |
GH-OGTT, growth hormone levels 2h after a standard oral glucose tolerance test; IGF-1, insulin-like growth factor-1; F, female; %ULN, proportion of IGF-1 concentration above the age-adjusted normal range; M, male.
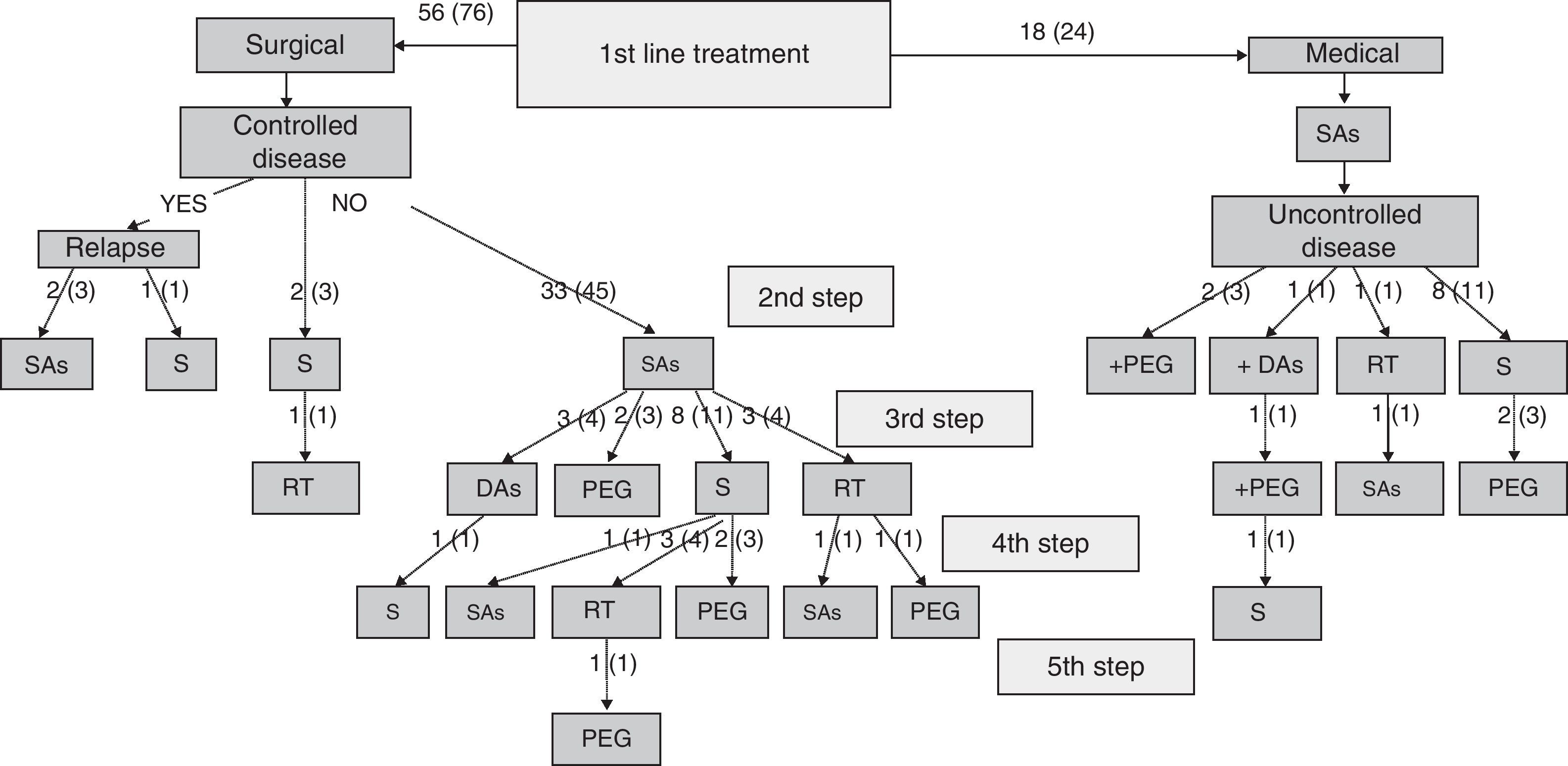
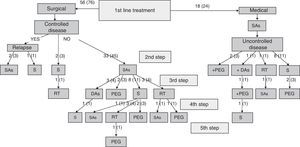
Specialists in charge of patients with acromegaly selected surgery as their first treatment option for 56 patients (76%), and first line treatment with SAs for the remaining 18 patients (24%). There were no significant differences in baseline characteristics between patients indicated for surgery or medical treatment as first line therapy (Table 2). Surgery was the preferred option for both microadenomas and macroadenomas. Thirty-six percent of patients undergoing surgery as first line treatment had been previously treated with SAs (37% of microadenomas and 35% of macroadenomas). Fig. 1 shows the stepwise treatment used in patients with uncontrolled disease and the proportions of patients given each treatment modality. The option most commonly selected following the failure of initial surgery was treatment with SAs followed by surgery.
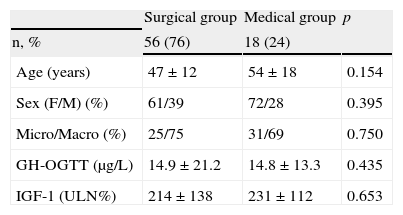
Baseline characteristics of groups receiving first line surgical and medical treatment.
| Surgical group | Medical group | p | |
| n, % | 56 (76) | 18 (24) | |
| Age (years) | 47±12 | 54±18 | 0.154 |
| Sex (F/M) (%) | 61/39 | 72/28 | 0.395 |
| Micro/Macro (%) | 25/75 | 31/69 | 0.750 |
| GH-OGTT (μg/L) | 14.9±21.2 | 14.8±13.3 | 0.435 |
| IGF-1 (ULN%) | 214±138 | 231±112 | 0.653 |
GH-OGTT, growth hormone levels 2h after a standard oral glucose tolerance test; IGF-1, insulin-like growth factor-1; F, female; %ULN, proportion of IGF-1 concentration above the age-adjusted normal range; M, male.
Stepwise treatment in patients with uncontrolled disease and proportion of patients receiving each treatment modality.
Numbers of patients are given as crude numbers (proportions of all patients).
SAs, somatostatin analogs; DA, dopaminergic agonists; PEG, pegvisomant; S, surgery; RT, pituitary radiotherapy.
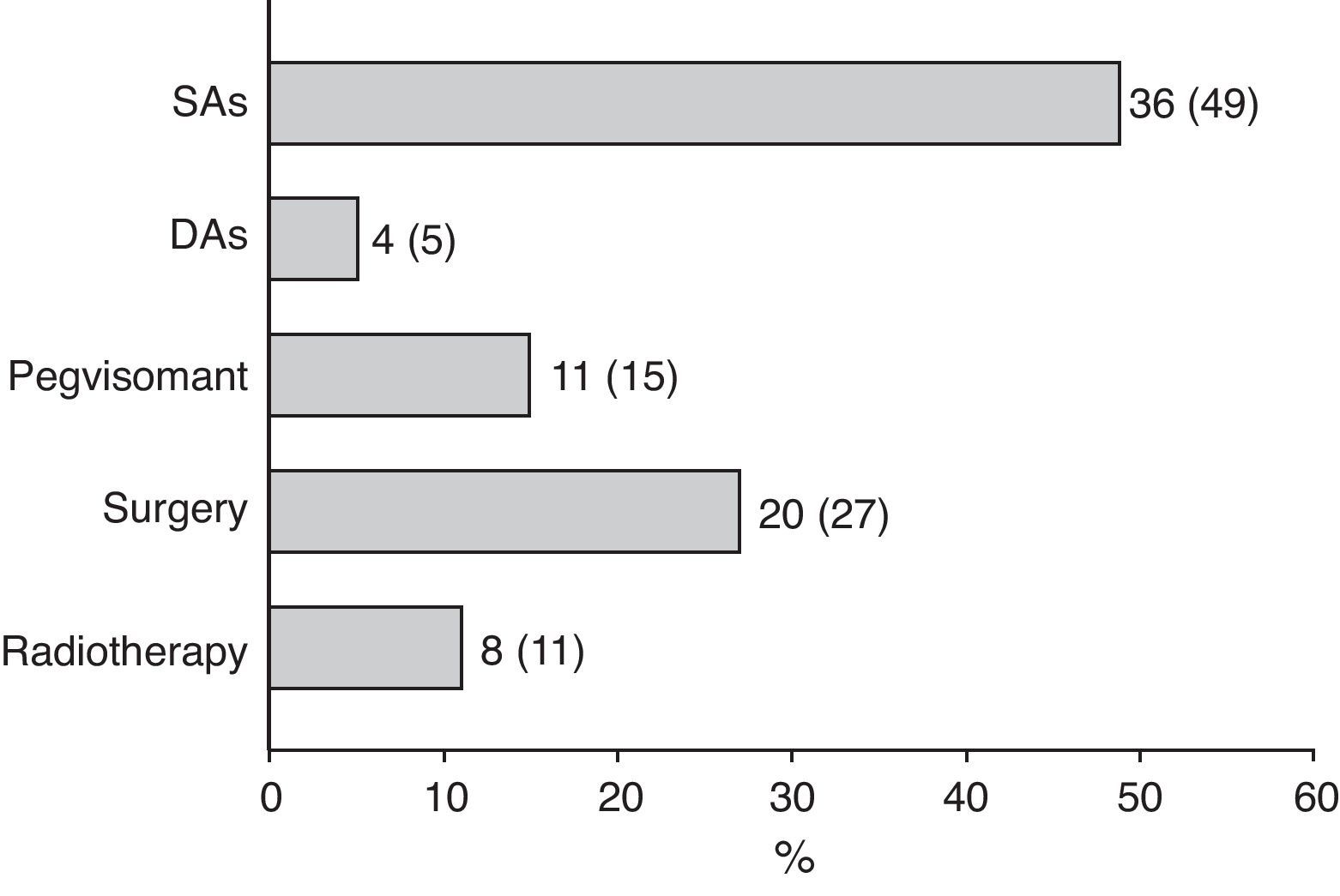
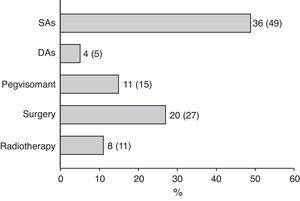
Surgery was recommended for 9 of the 18 patients (50%) prescribed first line treatment with SAs due to poor disease control after 13±6 months of drug treatment. Of the remaining 9 patients who continued treatment with SAs until the end of the follow-up period, two were prescribed concomitant pegvisomant and one received radiotherapy due to the presence of concurrent diseases contraindicating surgery. Fig. 2 shows the frequency of use of the different second line treatment modalities in the overall patients. SAs were more commonly required as second line treatment in patients with macroadenoma as compared to those with microadenoma (56% versus 26%; Chi-square test2: 5.48; p=0.019). Similarly, second line surgical treatment was also more common in patients with macroadenoma (37% versus 5%; Chi-square test: 6.73; p=0.009). However, no significant differences were found in treatment with DAs, pegvisomant, or pituitary radiotherapy by tumor size.
At the end of follow-up, 33 patients (45%) were on drug treatment, with no significant differences in regard to adenoma size, and 63 patients (85%) had received drug treatment at some time during the study.
Secondary objective: efficacy of the therapeutic strategies usedPatients undergoing surgery as first line treatmentAfter initial surgery, patients in this group experienced a significant decrease in GH-OGTT levels (17.0±21.8 versus 8.3±17.1μg/L, F: 32.76; p<0.001), with no significant differences in regard to tumor size. GH-OGTT levels after surgery were less than 1μg/L in 71% of patients with microadenoma as compared to 15% of patients with macroadenoma (Chi-square test: 16.32; p<0.001). Surgery resulted in a significant decrease in IGF-1 levels as compared to presurgical levels (249±170 versus 129±120 ULN%; F: 12.40; p=0.001), with the type of adenoma having no significant influence. Normal age-adjusted basal IGF-1 levels were achieved in 79% of microadenomas and 32% of macroadenomas (Chi-square test: 9.32; p=0.002). After initial surgery, 15 patients (27%) met the criteria for disease control. The surgical success rate was significantly higher in patients with microadenomas as compared to those with macroadenomas (71% versus 12%; Chi-square test: 18.46; p<0.001). The administration of SAs before surgery had no influence on the surgical success rate in the patients overall (18% versus 28% in untreated and treated patients, respectively; Chi-square test: 0.393; p=0.531) or regarding tumor size (microadenomas: 57% versus 86% in untreated and treated patients, respectively; Chi-square test: 1.40; p=0.559); macroadenomas (13% versus 11% in untreated and treated patients, respectively; Chi-square test: 0.35; p=1.000). After the failure of initial surgery or the recurrence of acromegalic activity, the most commonly used therapeutic option was the administration of SAs, which achieved normalization of IGF-1 levels in 52% of patients, “safe” basal GH levels less than 2.5μg/L in 47% of patients, and disease control criteria in 43% of patients, none of whom required additional treatment.
At the end of the follow-up, 34 patients (61%) had basal IGF-1 levels within normal limits (86% of microadenomas and 54% of macroadenomas; Chi-square test: 4.54; p=0.033), 17 patients (30%) met disease control criteria and did not require medical treatment for acromegaly (64% of microadenomas and 20% of macroadenomas; Chi-square test: 9.80; p=0.005), and 24 (43%) were on drug treatment. Among the latter, 10 patients (42%) had control criteria, with no significant differences regarding tumor size.
Patients receiving first line drug treatmentAfter a mean follow-up of 28±5 months, 7 (39%) of the 18 patients had IGF-1 levels within normal limits, with no differences depending on adenoma size or the use of surgery during the study period. Only 2 (11%) of the 9 patients (50%) who underwent surgery in this group had cure criteria (2 macroadenomas), and 4 patients (22%) met the disease control criteria on drug treatment.
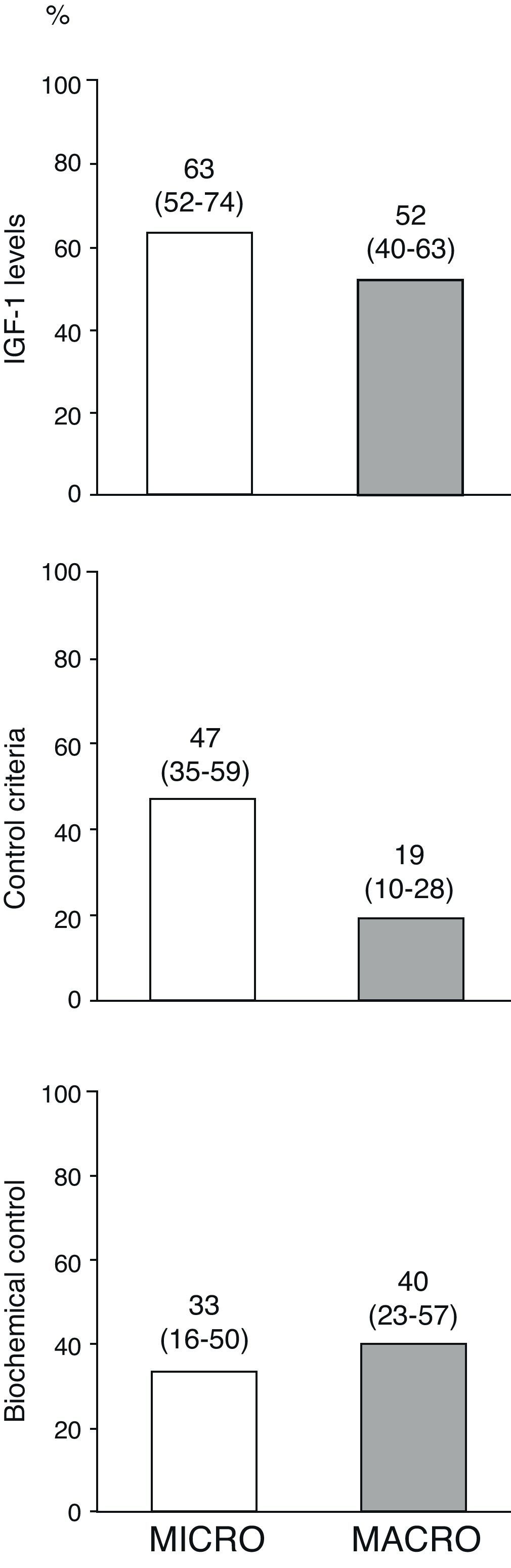
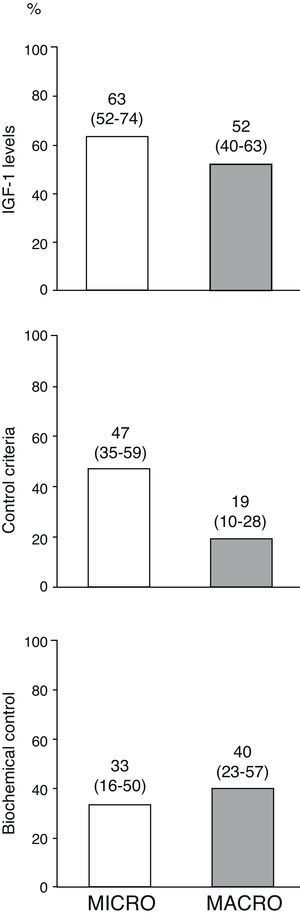
Fig. 3 shows the percentages of the overall patients who had normal age-adjusted IGF-1 levels, cure criteria (disease control with no drugs), and biochemical control (disease control with drugs) at the end of follow-up by type of adenoma. Of the 10 patients treated with pegvisomant at the end of follow-up, 5 (50%) had age-adjusted basal IGF-1 levels above the normal limit.
Disease control in overall patients at the end of the follow-up period by type of adenoma.
The term “disease cure” refers to patients who meet the disease control criteria (GH-OGTT less than 1μg/L together with normal IGF-1 levels) and do not require medical treatment for acromegaly, and “biochemical control” refers to patients who meet disease control criteria among those who completed follow-up on treatment with somatostatin analogs, dopaminergic agonists and/or pegvisomant (n=34). Numbers above the bars represent proportions of patients (95% confidence interval, lower–upper limit).
The prospectively collected data reported in this paper, combined with the data already published in the Spanish Registry of Acromegaly (REA),1,18 provide a quite reliable and informative picture of the current management of acromegaly in Spain.
The initial treatment of choice in approximately 80% of newly diagnosed patients in our study was surgical resection by the transsphenoidal route, which agrees with prior REA data updated as of 2004 and other more recent similar national registries.19,20 Surgery is the fastest way to decrease GH and IGF-1 levels in patients with acromegaly, and reference neurosurgical centers have reported surgical success rates higher than 75% in intrasellar microadenomas and macroadenomas and of approximately 40% in invasive lesions.12 However, disease control rates achieved with surgery are lower in the different national registries,19,21 including the REA, where postoperative cure criteria are reported in only 40% of patients,1 which agrees with our results. This substantial disagreement between surgical series and national registries as regards efficacy could be due to two main reasons. These are publication bias favoring positive or superior results and the non-reporting of series from centers with less “brilliant” results, on the one hand, and factors inherent to pituitary surgery on the other hand. The success of pituitary surgery is highly dependent on the learning curve and experience of the neurosurgeon,22 with the best results being achieved at centers performing higher numbers of surgical procedures done by a lower number of surgeons,15,22 while the worst results are found at centers where procedures are performed by a higher number of surgeons.15,23 In agreement with this evidence, the 2004 REA report1 cited a 25% greater surgical success rate in centers with the largest numbers of patients. Based on this premise and making a quite rough but illustrative calculation, according to data from the Spanish Society of Neurosurgery there are currently 72 accredited neurosurgery departments in Spain,24 17 of which are in a single province. Assuming that pituitary neurosurgery is performed by a single specialist in each of these departments and based on the estimated annual incidence of acromegaly in Spain,1 each neurosurgeon would see 1.6 patients with newly diagnosed acromegaly every year, which gives an idea of the difficulties involved in completing an adequate learning curve.
It should also be noted that more than one-third of the patients in whom pituitary surgery was indicated were treated with SAs before the procedure, probably to decrease morbidity and improve surgical success. However, the benefit of drug treatment before surgery is controversial.25,26 According to prospective studies, it does not appear to provide a clear benefit in patients with microadenoma, but was reported in some series as increasing the chance of surgical success in patients with macroadenomas, especially of an invasive nature.26 In our series, drug administration before surgery was not associated with any benefit in patients with both microadenomas and macroadenomas.
After first line surgery, the treatment most commonly used in both patients relapsing after initial disease control and those with persistent acromegalic activity after surgery was administration of SAs, prescribed to more than 90% of such patients and which was the only postoperative treatment in 54% of patients who required it. In patients with not fully resectable macroadenoma, partial surgical resection27,28 appears to improve the efficacy of a subsequent treatment with SAs in terms of disease control, particularly when more than 75% of the tumor mass is resected.27 In our study, this therapeutic approach achieved disease control and normalization of IGF-1 levels in more than 40% and 50% of patients, respectively, in agreement with the results of prior retrospective series.27 This supports the choice of this treatment strategy because of its high efficacy in a high proportion of patients.
The use of radiotherapy or radiosurgery in our series was quite marginal. Its introduction into the therapeutic armamentarium for acromegaly of long-acting SAs and pegvisomant, its moderate efficacy in terms of disease control, its long latency period from administration to achievement of maximum effect, and concern about the resulting mid- to long-term morbidity have caused this treatment modality to be relegated to a second plane after drug treatment. Radiotherapy is mainly indicated for the few patients not controlled after surgery plus drug treatment and/or tumors with a tendency to expand despite these treatments.9,18 However, the short follow-up time in our series does not allow for drawing additional conclusions, because its administration may continue to be an adequate strategy in the high proportion of patients not controlled at study end.
Some experts and the most recent treatment guidelines recommend medical treatment as the first therapeutic option for patients in whom complete initial surgical resection is unlikely.9,29 First choice medical treatment with SAs is effective regardless of tumor size and invasiveness,30 and meta-analyses of clinical trials with long-acting SAs as primary treatment show normalization of GH and IGFD-1 in approximately 50–60% of patients with both microadenomas and macroadenomas.16 Control rates of GH and IGF-1 hypersecretion reported are, however, not so high in real clinical practice when SAs are used in unselected patients,19,20 and the significant economic burden they represent should also be taken into account, not least because this is a lifetime treatment.31,32 In our reported series, first line drug therapy was prescribed to 24% of newly diagnosed patients, and such prescription was not influenced by patient age or tumor size. On the other hand, the associated comorbidities were only responsible for the exclusion of surgery in a previously mentioned patient treated with SA followed by conventional radiotherapy, and causal inferences about this indication cannot therefore be drawn either. However, two variables not collected in the study, patient functional status and the presence of invasive adenoma with few chances of complete surgical resection, could partially explain the first line drug indication, although this is a merely speculative hypothesis considering that 50% of these patients initially treated with SAs finally underwent surgery.
In our series, only 22% of patients receiving first line medical treatment had disease control criteria at the end of follow-up. This figure is similar to those reported at the REA1 and the Belgian national registry,19 and slightly lower than that reported in the German registry.20 With regard to drug treatment, it should be noted that only 5 (50%) of the 10 patients on pegvisomant at the end of follow-up had IGF-1 levels within the normal range. Pegvisomant is highly effective for normalizing IGF-1 levels,33 and these data therefore suggest problems of therapeutic inertia in its stepped dosage, although other factors beyond the scope of analysis in this study, inherent to the patient, the tumor itself, and other treatments used may affect drug response.34,35
Finally, it should be stressed that this study, despite having strengths, including prospective data collection, intention-to-treat analysis of the study variables, and multicentricity, that support the information obtained from it, is not devoid of some weaknesses that should be taken into account when analyzing the results. These include the lack of accurate data of two-dimensional or volumetric tumor size, the lack of data on tumor invasiveness, and the lack of central measurements of GH or IGF-1 levels, all of which would have allowed for a greater standardization of the results.
In conclusion, surgery is the treatment modality for acromegaly initially preferred by a representative sample of specialists in endocrinology and nutrition, irrespective of tumor size, but only achieves disease control in 27% of patients, and results are especially poor in macroadenomas. In patients in whom initial surgery has failed, the treatment most commonly used is the administration of SAs. First line medical treatment achieves biochemical control in approximately 20% of patients, and in 50% of the patients in our series was followed by surgery because of inadequate disease control. These results in real clinical practice agree with data from the REA and national registries in our field from other countries, and emphasize the significance of the creation and maintenance of multicentric databases, which allow for the collection of highly valuable practical information and support the creation of reference centers, as they do with other so-called rare diseases, along with systematized study and treatment protocols to try and improve results in terms of therapeutic success.
FundingThis study was made possible thanks to the logistic support from IMS Health Barcelona, and to the support and funding from NOVARTIS FARMACÉUTICA, S.A.
Conflicts of interestThe authors have no conflict of interest to declare.
The authors acknowledge the work and commitment of all centers, investigators, and patients participating in the study.
Albero R., Acha J., Hospital Universitario Miguel Servet, Saragossa; Ballesteros M., Hospital de León, León; Bernal C., Hospital Universitario 12 de Octubre, Madrid; Blanco C., Hospital Universitario Príncipe de Asturias, Madrid; Boronat M., Hospital Universitario Insular de Gran Canaria, Las Palmas; Catalá M., Hospital Clínico Universitario de Valencia, Valencia; Díez A., Hospital del Bierzo, Ponferrada, León; Donnay S., Hospital Universitario Fundación de Alcorcón, Alcorcón, Madrid; Fernández P., Hospital de Montecelo, Pontevedra; Ferrer J.C., Hospital General Universitario de Valencia, Valencia; García A., Hospital Universitario Puerta del Mar, Cádiz; García H., Hospital Universitari Son Dureta, Palma, Majorca, Balearic Islands; Gaztambide S., Hospital de Cruces, Baracaldo, Bizkaia; López J.F., Hospital Rafael Méndez, Lorca, Murcia; López P., Hospital General Universitario de Elche, Elche, Alicante; de Luis D., Hospital Universitario Río Hortega, Valladolid; Martínez P., Complejo Hospitalario de Jaén, Jaén; Montreal M., Hospital Universitario Miguel Servet, Saragossa; Moreno A., Complejo Hospitalario de Jaén, Jaén; Pardo C., Hospital Virgen de los Lirios, Alcoi, Alicante; Pavón I., Hospital Universitario de Getafe, Getafe, Madrid; Pazos F., Hospital Universitario Marqués de Valdecilla, Santander, Cantabria; Pérez J., Hospital Universitario de Canarias, La Laguna, Tenerife; Pinedo R., Hospital General de Elda, Elda, Alicante; Rodríguez P., Hospital General Universitario Gregorio Marañón, Madrid; Salinas I., Hospital Universitari Germans Trias i Pujol, Badalona, Barcelona; Sanabria C., Hospital Clínico San Carlos, Madrid; Sillero A., Hospital de Mérida, Mérida, Badajoz; Soto A., Hospital Universitario Virgen del Rocío, Seville; Tarroba C., Hospital Universitario Río Hortega, Valladolid; Villabona C., Hospital Universitari de Bellvitge, L’Hospitalet de Llobregat, Barcelona.
On behalf of Spanish Group of the OASIS Study (Appendix 1).
Please cite this article as: Luque-Ramírez M, et al. Estudio OASIS: manejo terapéutico de la acromegalia en un escenario de práctica clínica habitual. Evaluación de la eficacia de las diversas estrategias de tratamiento aplicadas. Endocrinol Nutr. 2011;58:478–86.