Traditionally, calcitriol has been considered a calcium and phosphate regulating hormone, but has recently been shown to play a pivotal role in innate immunity. Many barrier and immune cells have membrane and intracellular receptors that recognize different microbial antigens. Activation of these receptors induces synthesis of 1α-hydroxylase, which acts on 25 hydroxyvitamin D to generate intracellular calcitriol. Calcitriol activates its receptor and enhances the synthesis of important human antibiotics like cathelicidin and β2-defensin while inhibiting hepcidin. These pluripotent peptides have an important role in innate immunity, and their regulation is abnormal in hypovitaminosis D. The literature on their secretion mechanisms, levels in different organic fluids, mechanism of action, and relationship with vitamin D is reviewed here.
El calcitriol ha sido considerado durante años exclusivamente como una hormona reguladora del metabolismo fosfocálcico, pero últimamente se ha demostrado que numerosas células implicadas en la inmunidad innata (epitelios de barrera, monocitos/macrófagos, etc.) son capaces de reconocer determinadas moléculas repetitivas características de diversos gérmenes patógenos mediante receptores de membrana o intranucleares. La activación de estos receptores induce la síntesis de la 1α-hidroxilasa, con lo que dichas células son capaces de sintetizar calcitriol a partir de la 25 hidroxivitamina D circulante. El calcitriol, a través del receptor la vitamina D, modula la expresión de determinados péptidos antimicrobianos, como la catelicidina, la β2-defensina o la hepcidina. Estos péptidos representan un mecanismo versátil de la lucha antibacteriana innata y su producción se ve alterada en la hipovitaminosis D. Se realiza un análisis de la literatura sobre sus mecanismos de secreción, las concentraciones en diversos líquidos orgánicos, y los mecanismos de acción y su relación con la vitamina D.
Humans obtain vitamin D or calciferol from diet (in a small proportion) and, primarily, from endogenous synthesis in the epidermis through the effect of ultraviolet radiation.1 To be active, calciferol should be hydroxylated twice, first at position 25 and then at position 1α, to be converted into 1α,25-dihydroxycalciferol or calcitriol, which behaves at systemic (endocrine) level as an essential hormone for phosphorus and calcium metabolism, and at local (autocrine or intracrine) level as a substance regulating multiple cell functions independent of calcium metabolism.
Calciferol is initially hydroxylated in the liver through the effect of 25-hydroxylase of microsomal CYP2R1.2 This enzyme is poorly regulated, and massive, direct conversion of the calciferol that reaches the liver into 25-hydroxycalciferol therefore occurs. This is bound to vitamin D binding protein and has a long half life. Thus, its plasma levels are the main indicator of the nutritional status of vitamin D.
The second hydroxylation may occur in the kidney (classical hormonal pathway) or in other cells unrelated to phosphorus and calcium metabolism (nonclassical intracrine or paracrine pathways)2 through 1α-hydroxylase (CYP27B1) which generates the active metabolite. This enzyme, unlike 25-hydroxylase, is strongly regulated, but regulation at kidney level and at other cells is different. PTH and other factors activate the enzyme in the kidney, while phosphate and fibroblast growth factor 23 (FGF23) are negative regulators.2
Calcitriol exerts its regulatory effects mainly, but not only, through activation of its receptor (VDR).3 VDR is a nuclear receptor which has both a carboxy-terminal portion that binds calcitriol, and an amino-terminal portion that binds to DNA. Calcitriol binding to VDR induces heterodimerization with the X receptor activated by retinoic acid. This is bound to specific DNA sequence elements (VDRE) in the promoter region of genes that will respond to vitamin D. Finally, a molecular complex is assembled that induces or represses gene transcription, thus modulating the synthesis of many proteins.3
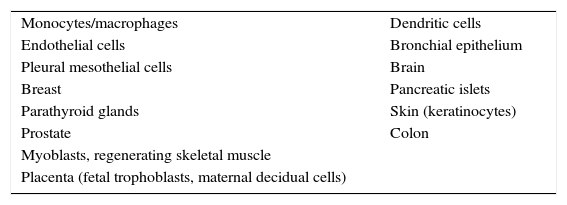
The presence of nonclassical effects (independent of calcium and phosphorus metabolism) of calcitriol is based on: (1) a demonstration of 1α-hydroxylase activity in multiple extrarenal cells, regulated by their own mechanisms (Table 1); (2) the presence of VDR in multiple cells (it is estimated that up to 3% of the human genome is modulated by calcitriol) (Table 2); and (3) the existence of specific effects mediated by VDR activation in these cells.4–6 This is a very primitive pathway from the evolutionary point of view, prior to the hormone pathway. The effect of vitamin D and its deficiency on calcitriol-modulated human antibiotics is reviewed below.
Tissues with extrarenal expression of 1α-hydroxylase and calcitriol production.
| Monocytes/macrophages | Dendritic cells |
| Endothelial cells | Bronchial epithelium |
| Pleural mesothelial cells | Brain |
| Breast | Pancreatic islets |
| Parathyroid glands | Skin (keratinocytes) |
| Prostate | Colon |
| Myoblasts, regenerating skeletal muscle | |
| Placenta (fetal trophoblasts, maternal decidual cells) |
Organs and tissues thought to express calcitriol receptors.
| Musculoskeletal system: osteoblasts, bone marrow, chondrocytes, striated muscle, smooth muscle |
| Circulatory system: atrial myoendocrine cells, cardiomyocytes, endothelial cells |
| Gastrointestinal tract: parotid, epithelial cells of the mouth, stomach, enterocytes, colonic cells, hepatocytes, gallbladder |
| Kidney and urinary tract: tubular epithelial cells (proximal and distal), urothelium |
| Immune system: activated T- and B-cells, monocytes, macrophages, dendritic cells, neutrophils, spleen, thymus, and lymph node cells |
| Reproductive system: amnion, chorioallantoic membrane, alveolar and ductal breast cells, ovary, oviduct, uterus, placenta, epididymis, Sertoli and Leydig cells of testis, prostate, eggshell gland (hen) |
| Skin: epidermis, fibroblasts, hair follicles, keratinocytes, melanocytes, sebocytes, sebaceous glands |
| Nervous system: encephalon (hippocampus, cerebellar Purkinje and granular cells, stria terminalis, central nucleus of the amygdala), sensory ganglia, spinal cord, choroid plexuses, lacrimal glands, retina |
| Endocrine system: adipose tissue, adrenal gland (cortex and medulla), β cells of pancreas, pituitary gland, thyroid gland (follicular cells, C cells), parathyroid glands |
| Tumor cells: melanoma, myeloid leukemia, B-cell lymphoma, breast, prostate, stomach, colon, squamous cell carcinoma, endometrial carcinoma |
| Respiratory tract: lung, respiratory tract epithelium |
The association of rickets and infection has a long history. In the 19th century, the use of cod liver oil (an excellent source of vitamins A and D) was recommended not only for treating rickets, but also for pulmonary tuberculosis, and, a little later, sanatoriums specializing in heliotherapy under natural sunlight became fashionable. In 1903, Niels Finsen was awarded the Nobel Prize for Medicine for his work showing that ultraviolet light could cure tuberculosis of the skin.
In 1981, Barbour et al. showed extrarenal calcitriol production in sarcoidotic granulomas in an anephric patient with hypercalcemia.7 Calcitriol was later shown to promote the fusion of alveolar mouse macrophages.8 This was the first evidence of the effect of calcitriol on immune system cells. In 1983, Proveddini et al. found VDR in human WBCs.9 In 1986, vitamin D and interferon-γ were shown to be able to control the proliferation of Mycobacterium tuberculosis in human monocytes.10
Wang et al. showed that calcitriol induced the expression of two human antimicrobial peptides, cathelicidin and β2-defensin.11 In 2006, it was demonstrated that the recognition of Mycobacterium tuberculosis antigens by toll-like receptors (TLRs) in the membrane of monocytes, or cells related to innate immunity (skin, respiratory and urinary epithelium, etc.), induced the activation of 1α-hydroxylase and VDR genes,12 which led in turn to the activation of cathelicidin and β2-defensin genes. Liu et al. also showed that adequate release of the latter required normal 25-hydroxyvitamin D levels in the medium,12 which suggested that hypovitaminosis D restricted the production of such substances. The gene promoter of both molecules contains at least one VDRE which, interestingly, in the case of cathelicidin is located in a small nuclear sequence that only occurs in humans and higher primates. This suggests that regulation by vitamin D of this aspect of innate immunity is evolutionarily recent and occurs almost only in humans, so that the results of some animal studies cannot necessarily be extrapolated to our species.13 The intracellular mediators through which the activation of TLRs 2/1 stimulates the synthesis of calcitriol and its receptor are beginning to be known.14 On the other hand, there are both stimulating cytokines (interleukin-1, interleukin-15, interferon-γ) and inhibitory cytokines (interleukin-4, interleukin-10).14
Obviously, vitamin D not only has the above mentioned immune and antimicrobial effects, but also has an impact on many other areas of both innate and adaptive immunity.3–5
Calcitriol-modulated human antibioticsThese include cathelicidin or LL-37, β2-defensin and hepcidin.
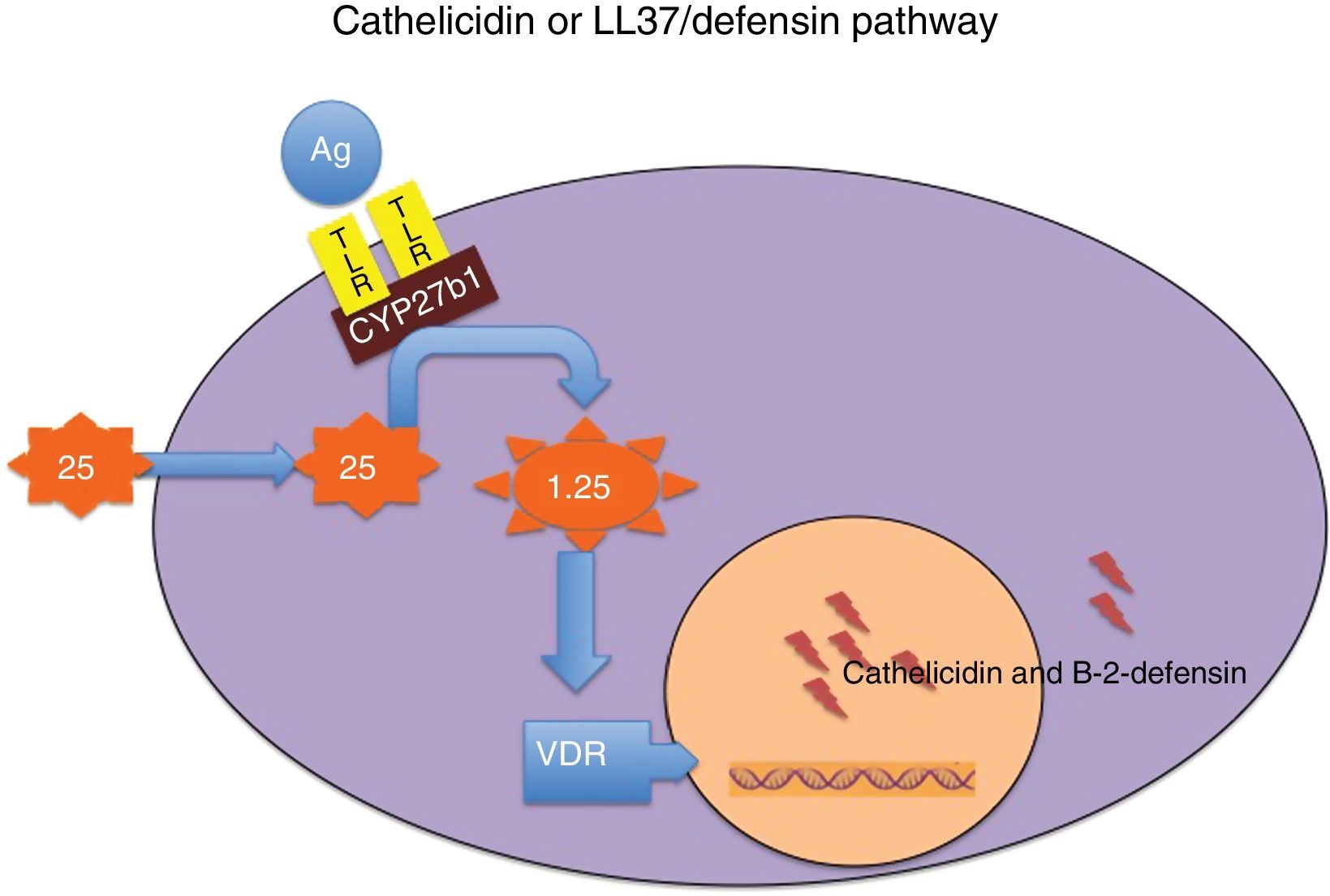
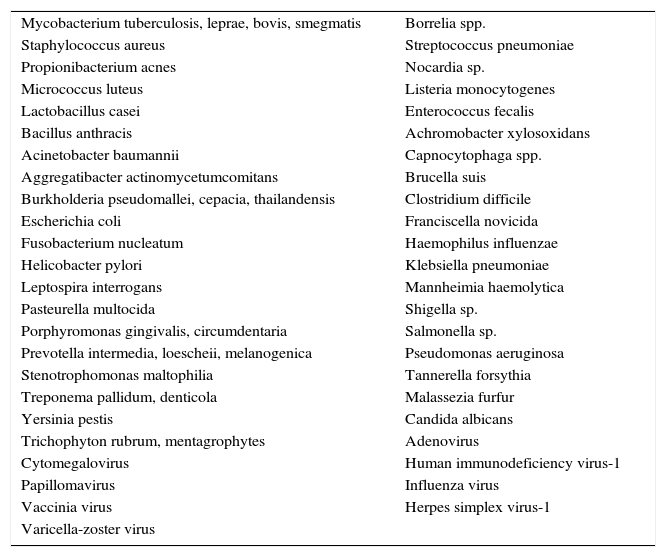
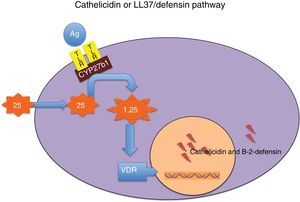
Cathelicidin and defensins are part of a group of small, multifunctional cationic polypeptides, evolutionarily very primitive, with potent antimicrobial effects (Table 3)14–16 and resistant to proteolysis, so that germs do not develop resistance to them. Their production mechanism is shown in Fig. 1. They also have other functions, as they modulate the initiation and activation of inflammatory response (“alarmins”) and the subsequent repair of damaged tissue.14–16
Biological agents sensitive to the effect of the vitamin D-vitamin D-dependent antibiotics.
| Mycobacterium tuberculosis, leprae, bovis, smegmatis | Borrelia spp. |
| Staphylococcus aureus | Streptococcus pneumoniae |
| Propionibacterium acnes | Nocardia sp. |
| Micrococcus luteus | Listeria monocytogenes |
| Lactobacillus casei | Enterococcus fecalis |
| Bacillus anthracis | Achromobacter xylosoxidans |
| Acinetobacter baumannii | Capnocytophaga spp. |
| Aggregatibacter actinomycetumcomitans | Brucella suis |
| Burkholderia pseudomallei, cepacia, thailandensis | Clostridium difficile |
| Escherichia coli | Franciscella novicida |
| Fusobacterium nucleatum | Haemophilus influenzae |
| Helicobacter pylori | Klebsiella pneumoniae |
| Leptospira interrogans | Mannheimia haemolytica |
| Pasteurella multocida | Shigella sp. |
| Porphyromonas gingivalis, circumdentaria | Salmonella sp. |
| Prevotella intermedia, loescheii, melanogenica | Pseudomonas aeruginosa |
| Stenotrophomonas maltophilia | Tannerella forsythia |
| Treponema pallidum, denticola | Malassezia furfur |
| Yersinia pestis | Candida albicans |
| Trichophyton rubrum, mentagrophytes | Adenovirus |
| Cytomegalovirus | Human immunodeficiency virus-1 |
| Papillomavirus | Influenza virus |
| Vaccinia virus | Herpes simplex virus-1 |
| Varicella-zoster virus |
Mechanism through which vitamin D modulates the secretion of cathelicidin and beta-2-defensin. Binding of some microbial antigens (Ag) to toll-like receptors (TLRs) activates 25-hydroxyvitamin D–1α-hydroxylase (CYP27b1), so that, depending on intracellular concentrations of 25-hydroxyvitamin D, local synthesis of 1,25-dihydroxyvitamin D is enhanced. The latter in turn binds to its receptor (VDR), and the intranuclear hormone-receptor complex activates the transcription of the cathelicidin and beta-2-defensin genes. Intracellular concentrations of 25-hydroxyvitamin D depend on plasma concentrations of this molecule, which is the most reliable marker of the nutritional status of this vitamin.
Human cathelicidin, or LL-37, a secretory protein generated enzymatically from human preprocathelicidine,14 has a signal peptide, followed by a highly conserved cathelin domain (hence the name of the molecule) and a structurally very variable, cationic carboxy-terminal peptide with broad antimicrobial effects. In humans, the molecule is encoded by a single gene, called cathelicidin antimicrobial peptide. Human cathelicidin is a 37-amino acid peptide that starts with the amino acids leucine-leucine, hence its name of LL-37. It is produced in many tissues (Table 4), especially in cells involved in innate immunity,15,16 and may be measured in almost all human biological fluids.
Cells and tissues producing cathelicidin.
| Bone marrow |
| Granulocytes (storage) |
| Monocytes/macrophages |
| Mast cells |
| Placenta |
| Skin (keratinocytes, sweat gland cells) |
| Epithelial cells: eye, lacrimal glands, nose, oral cavity, gingival cells, salivary glands, gastrointestinal tract, Paneth cells, genitourinary tract, respiratory tract |
| Breast |
| Cancer cells (melanoma, myeloid leukemia, B-cell lymphoma, breast, prostate, stomach, colon, squamous cell carcinoma, endometrium, ovary, breast, lung) (varies depending on cell lines) |
Cathelicidin levels in the biological fluids of a healthy person are assumed to express the basal, constitutive secretion of the peptide in the bone marrow, neutrophils, and other cells. In patients with infection, trauma, and other conditions, however, levels are increased by the secretion induced through activation of the TLR 2/1-VDR system in the innate immunity cells.17 It is generally accepted that, assuming an equal antigen challenge, in vivo response to cathelicidin is lower in patients with hypovitaminosis D, although this has only been confirmed to date in in vitro studies. However, this is a simplification, because cathelicidin secretion is influenced by multiple positive and negative factors18 which do not all depend on calcitriol-VDR signaling.
Cathelicidin secretion is stimulated by TLR receptor agonists (bacterial, fungal, or viral antigens),18 vitamin D receptor agonists (e.g. paricalcitol),18 interferon-γ,18 interleukin-15,18 some bile acids (lithocholic, ursodeoxycholic),18 butyrate and its derivative 4-phenylbutyrate,18 agents causing endoplasmic reticulum stress that release sphingosine-1-phosphate,19 and some components of the diet (curcumin, resveratrol, genistein, etc.).20–22 Curcumin20 and resveratrol21 act through mechanisms independent of VDR and enhance the effects of calcitriol. The activation of β-estrogen receptors releases cathelicidin by a mechanism independent of VDR, mediated by sphfingosine-1-phosphate.22 By contrast, the release of LL-37 is inhibited by some endotoxins,18 Shigella,18 and FGF23,23 amongst others.18 The response is tissue-specific (it is not the same in the vaginal epithelium, sensitive to estrogens, as is it in the urothelium, the bronchial epithelium, or skin keratinocytes).
Tiosano et al. found that monocytes from patients with rickets due to inactivating mutation in VDR,24 in whom signaling through VDR cannot occur, expressed little (but not zero) basal cathelicidin, and their response capacity in the presence of 25-OH vitamin D was much lower than that of monocytes from healthy subjects (but not zero either). This data emphasizes the role of VDR, but also suggests that other stimuli may release cathelicidin, because in the absence of signaling through VDR, monocytes are able to produce it, which suggests that while calcitriol is a potent positive modulator of cathelicidin secretion, it is not the only one.
Cathelicidin concentrations in human fluidsIn 1997, Sorensen et al. published the first study that measured LL-37 in plasma.25 No sex or age differences were found in healthy individuals between plasma and serum. Other authors found higher concentrations in patients with cystic fibrosis and bronchial infection than in patients with no infections.26 The results reported in severe sepsis are conflicting: levels lower27,28 than or similar29 to those in control subjects have been reported, but the procedures used to measure LL-37 have differed.
Yamshchikov et al. found hypovitaminosis D in 85% of patients with tuberculosis. The highest LL-37 levels were reported in those with positive sputum, but no correlation was seen between LL-37 and 25 hydroxycalciferol levels.30 Another study also found no correlation between cathelicidin and 25-OH-vitamin D concentrations in healthy women before delivery or in their babies, but a potent correlation existed between cathelicidin levels in maternal and umbilical cord blood.31
Bhan et al. reported that after administering vitamin D to healthy subjects, those in the tertile with the highest 25-OH-vitamin D levels had a greater elevation in LL-37 values than those in the tertile with the lowest vitamin D levels.32
Dixon et al. showed a positive correlation between 25-OH vitamin D and cathelicidin concentrations in subjects with low levels of the nutritional marker of vitamin D, but such a correlation was not seen in subjects with normal levels.33 Two previous studies by the same authors found no correlation between the levels of the vitamin D nutritional marker and cathelicidin in patients on dialysis,34 or in patients who attended a clinic specializing in bone metabolic diseases.35 Our group found higher cathelicidin levels in healthy elderly subjects with higher 25-OH vitamin D concentrations.36 Lehouck et al. found no correlation between nutritional vitamin D status and LL-37 levels before or after high-dose supplementation with vitamin D in patients with chronic obstructive pulmonary disease.37 Similar subsequent studies in healthy subjects are available.38
To sum up, cathelicidin levels in blood vary depending on the method used, show a significant dispersion, and have little or no correlation with concurrent 25-OH vitamin D levels in healthy subjects, except possibly in those with hypovitaminosis D. To interpret the results in patients, the development stage of the process and treatment should also be taken into account, because levels vary as the disease evolves.
LL-37 has been shown to occur in sperm, vaginal fluid, sweat, saliva, tears, nasal mucus, sputum, milk, pleural fluid, feces, and urine.25 LL-37 is thought to exert in all these places a broad spectrum antibacterial effect, acting as an early response when the barrier zones of the body detect pathogenic antigens. In our research, the highest cathelicidin concentrations were found in infected pleural fluid, as compared to tumor transudates or exudates.39 In these patients, a positive correlation was seen between serum cathelicidin and serum calcitriol, but not 25-hydroxyvitamin D.39
Antibacterial mechanisms of action of cathelicidinMost of the direct antimicrobial effects of LL-37 can be attributed to its α-helical structure and its cationic and hydrophobic properties.40 The N-terminal helix is related to chemotaxis and resistance to proteolysis, while the C-terminal helix is responsible for antimicrobial effects. LL-37 reaches the microbial membrane, covers its surface, and perforates it, forming in the membrane pores that eventually destroy the germ. LL-37 essentially binds to cell membranes that contain lipopolysaccharides (Gram-negative) or teichoic acid (Gram-positive) with a negative charge, different from the zwitterionic membranes of eukaryotes. The antiviral action is also due to interaction with the membrane envelope and protein capsid.
The induction of autophagy in human monocytes/macrophages is the mechanism which accounts for the capacity of this peptide to kill intracellular pathogens. Autophagy is a very primitive biological process that serves to ensure cell cytoplasm homeostasis.41 When autophagy is activated, some cytoplasm materials become trapped inside a dual membrane vacuole called an autophagosome which, after merging with lysosomes, becomes an autophagolysosome, in which lysosomal enzymes degrade all trapped materials. Autophagy was formerly thought to serve mainly for the recycling of nutrients from damaged molecules, but it is now known that it is involved in a wide variety of processes, including antimicrobial activity.42,43 Autophagy activates in response to cathelicidin production, cell fasting, or rapamycin treatment, and is inhibited in response to nutrients, hormones, and some cytokines. This mechanism makes possible normal cell function in changing metabolic conditions, and activates the destruction of some intracellular germs such as the various mycobacteria, which would otherwise remain alive indefinitely inside the cells.41–43
Cathelicidin is an essential mediator in the formation and function of autophagosomes and autophagolysosomes in human monocytes,41–43 but it is not the only one, as many other mechanisms inducing autophagy independent of the calcitriol-cathelicidin system have been discovered. LL-37 induces the expression of two key factors for the development of autophagy, beclin-1 and autophagin (Atg)-5.41–44 These are essential for the maturation process of autophagy and for the merging of autophagosomes and lysosomes. Autophagy mediated by curcumin, cell fasting, or rapamycin is not regulated by the calcitriol-cathelicidin system.44
Cathelicidin has additional effects, which may vary depending on the local concentration.44 It has been shown in vitro, for example, that at doses less than 1μmol, cathelicidin induces neutrophil chemotaxis and survival, and stimulates angiogenesis and fibroblast migration and proliferation (beneficial effects on wound healing), while very high concentrations, usually beyond those reached in the usual response, have cytotoxic and pro-inflammatory effects.44
Defensins: β2-defensin or HBD2Defensins are other very primitive antimicrobial agents from the evolutionary point of view.45,46 They contain six cysteine residues that form disulfide bonds. Variations in the alignment of these bonds and molecular structures result in different families.
Humans have eight α-defensins, which are not modulated by vitamin D. At least four different human HBDs are known, of which HBD2 is modulated by VDR. HBDs are expressed in various immune (monocytes, macrophages) and epithelial cells. HBD1 is constitutively expressed, while the secretion of HBD2 is stimulated by some bacterial products (lipopolysaccharides, lipoteichoic acid) and pro-inflammatory cytokines (tumor necrosis factor-α, interleukin-1α).46 HBD2 is a small cationic peptide (38 amino acids; 4.1kDa)47 expressed in the lung, thymus, skin, bowel, WBCs, liver, and trachea.
Wang et al. showed that calcitriol causes the release of HBD2, although in smaller amounts than cathelicidine.17 Full induction of the HBD2 gene requires the convergence of the interleukin-1β and calcitriol pathways.48 On the other hand, it has also been shown that the activation of nucleotide-binding oligomerization domain protein 2, an intracellular receptor, by its ligand, muramyl dipeptide, a derivative of lysosomal catabolism of bacterial peptidoglycans, induces HBD2 gene expression.49 Calcitriol strongly induces the expression of the nucleotide-binding oligomerization domain protein 2 receptor in human barrier cells, thus enhancing HBD2 expression.50 Other known mechanisms activating HBD2 include butyrate, ceramide-1-phosphate released by endoplasmic reticulum stress, or sulforaphane from cruciferous plants, while retinoic acid inhibits HBD2 expression in normal keratinocytes, and cigarette smoke blocks its production in the respiratory epithelium.15,16,45,46
Few studies measuring β2-defensin levels are available. Leow et al. found that plasma HBD2 concentrations in patients with pneumonia did not correlate to 25-OH vitamin D levels or a 30-day mortality risk.51 In another model, Lippross et al. assessed changes in serum HBD2 levels over time in a group of patients with multiple trauma and compared them to other markers of inflammation and innate immunity.52 HBD2 levels at admission were seven-fold greater than normal and progressively decreased until they normalized at eight days, while cathelicidin levels were 15 times higher at baseline and remained elevated during the 14 days of follow-up. These data show that when tissue damage occurs, there is an earlier and stronger response of cathelicidin as compared to β2-defensin.
HepcidinIn 2001, a small antimicrobial peptide produced in the liver was identified and called hepcidin (from hep- “liver”, and -cidin from “microbicidal”).53 Hepcidin is cationic, amphipathic, and rich in cysteines, which generate four intramolecular disulfide bonds and stabilize the molecule in a folding structure β. Hepcidin is active against Gram-positive bacteria, but also inhibits the development of some fungi and Gram-negative bacteria with an antibacterial spectrum similar to that of β1-defensin.54 Nemeth et al. showed hepcidin production in response to interleukin-6. Other inflammatory cytokines also induced its synthesis, but to a lesser extent.55 Hepcidin is the main regulator of iron homeostasis, because it binds to ferroportin, the only known iron transporter to the outside of the cell. Hepcidin binding to ferroportin induces the internalization and degradation of ferroportin, which blocks the exit of iron from the cells. The cells mainly affected by this interaction include enterocytes (which block iron absorption in the bowel) and macrophages (which prevent iron release into blood). The consequence is the occurrence of the so-called “anemia of chronic diseases”, due to decreased circulating iron concentrations. Low serum iron levels restrict iron availability to extracellular bacteria, which are deprived of an essential nutrient and are therefore easier to kill. Paradoxically, however, intracellular pathogens (Salmonella, Mycobacteria, Candida, etc.) have greater amounts of iron available and may thus more easily develop inside macrophages, which therefore increase the local production of hepcidin to kill the germs.
Calcitriol has also been shown to modulate the iron–hepcidin–ferroportin axis. Bachetta et al. showed that the calcitriol-VDR complex may directly inhibit hepcidin expression by binding to a VDRE at the hepcidin promoter.56 On the other hand, calcitriol decreases interleukin-6 production in response to the lipopolysaccharide, and thus indirectly decreases IL-6 secretion. In healthy subjects and patients with kidney failure, it has been shown that when 25-OH vitamin D levels increase, hepcidin decreases and anemia improves.56 Interestingly, a Spanish group had already shown that the intravenous administration of calcitriol to patients on hemodialysis improves anemia and reduces the need for erythropoietin.57
Final discussionCalcitriol is significantly involved in the metabolism of different antimicrobial peptides which act upon external microbiological agents through different pathways. The optimization of vitamin D levels may promote the formation of these molecules, thus improving the immune status of patients and their resistance to infection.
Conflicts of interestThe authors state that they have no conflicts of interest.
Please cite this article as: Amado Diago CA, García-Unzueta MT, Fariñas MC, Amado JA. Antibióticos humanos modulados por calcitriol: nuevos aspectos fisiopatológicos de la hipovitaminosis D. Endocrinol Nutr. 2016;63:87–94.