To study the prevalence of hyperuricemia in children with overweight or obesity and analyze the relation with metabolic syndrome and the diseases that define it.
Materials and methodsThis is a cross-sectional prevalence study in 148 children recruited from pediatric endocrinology consultation, with overweight or obesity (12±3 years, 48% boys, BMI 31.8±6.1). We measured BMI, waist-height, waist circumference, blood pressure with standard instrumentation and glucose (fasting and after overload with 75g), insulin resistance, cholesterol HDL, triglycerides and uric acid.
ResultsThe prevalence of hyperuricemia was 53%. Patients with hyperuricemia had greater BMI (33.9 vs 30.6, p=0.003), plus waist circumference (101.4 vs 91.1cm, p<0.001), higher blood pressure: systolic (123.4 vs 111.9mm Hg, p<0.001), diastolic (78.2 vs 68.7mm Hg, p<0.001). They presented greater blood glucose after overload oral glucose (107.5 vs 100.7mg/dl, p=0.03), insulin was higher (29.2 vs 20.7mg/dl, p=0.001) as well as HOMA IR (6.5 vs 4.4, p<0.001) and HDL levels were lower (49.5 vs 54.4mg/dl, p=0.02).
Uric acid's level which most is the likely diagnosis of metabolic syndrome corresponds to 5.4mg/dl in our sample (sensitivity: 64% and specificity 62%).
ConclusionThe prevalence of hyperuricemia in children with overweight and obesity is high. In the group of patients with obesity and hyperuricemia, we found out that the parameters measured to diagnose with metabolic syndrome were less favorable. Uric acid's level from where there is a higher possibility to see metabolic syndrome is 5.4mg/dl.
Estudiar la prevalencia de hiperuricemia en niños con sobrepeso u obesidad y analizar la relación con el síndrome metabólico y las enfermedades que lo definen.
Materiales y métodosSe realizó un estudio de prevalencia transversal en 148 niños con sobrepeso u obesidad(12±3 años, 48% chicos, IMC 31,8±6,1) reclutados de una consulta de endocrinología pediátrica. Se determinaron el IMC, la cintura-talla, el perímetro de la cintura, la presión arterial con el equipo habitual y la glucosa (en ayunas y tras sobrecarga con 75g), la resistencia a la insulina, el colesterol HDL, los triglicéridos y el ácido úrico.
ResultadosLa prevalencia de hiperuricemia era del 53%. Los pacientes con hiperuricemia tenían valores superiores de IMC (33,9 frente a 30,6; p=0,003), perímetro de cintura (101,4 frente a 91,1cm; p<0,001) y presión arterial sistólica (123,4 frente a 111,9mmHg; p<0,001) y diastólica (78,2 frente a 68,7mmHg; p<0,001). Mostraban además una glucemia más alta después de la sobrecarga oral de glucosa (107,5 frente a 100,7mg/dl; p=0,03), valores superiores de insulina (29,2 frente a 20,7mg/dl; p=0,001) y HOMA IR (6,5 frente a 4,4; p<0,001) y concentraciones más bajas de HDL (49,5 frente a 54,4mg/dl; p=0,02).
El valor de ácido úrico correspondiente con mayor probabilidad al diagnóstico de síndrome metabólico en nuestra muestra era de 5,4mg/dl (sensibilidad del 64% y especificidad del 62%).
ConclusiónLa prevalencia de hiperuricemia en niños con sobrepeso y obesidad es alta. En el grupo de pacientes con obesidad e hiperuricemia hallamos que los parámetros determinados para diagnosticar el síndrome metabólico eran menos favorables. La concentración de ácido úrico a partir de la cual existe una mayor posibilidad de encontrar síndrome metabólico es de 5,4mg/dl.
Metabolic syndrome is the result of the occurrence of modifiable factors in the same matter associated with an increased risk of developing a future cardiovascular disease and diabetes mellitus.1,2
Obesity in children is notably increasing during the last few years in developed countries and is becoming the most common nutritional disorder.3,4 The incidence of metabolic syndrome in this population is less known but in the hope that it has increased in proportion to obesity.5 Many studies in adult population show that hyperuricemia appears as a common finding in subjects with metabolic syndrome. It is known that adult patients with hyperuricemia have a higher risk of developing metabolic syndrome and that this prevalence increases with elevation of plasma uric acid levels; even some articles suggest that this relationship may be casual.6
In other studies, the relationship between hyperuricemia and different components of metabolic syndrome such as insulin resistance7 and hypertension8 in adult population, is established. However, there is little literature on this relationship in obese pediatric population.
ObjectiveThe objective of our study was to estimate the prevalence of hyperuricemia in children with overweight and obesity and correlate the presence of hyperuricemia with metabolic syndrome, waist size, body mass index (BMI), fasting glucose, glucose after 75g overload oral of glucose, insulin resistance, dyslipidemia with low HDL, hypertriglyceridemia and hypertension.
We established the levels of uric acid in blood which will most likely be a diagnosis of metabolic syndrome in this population.
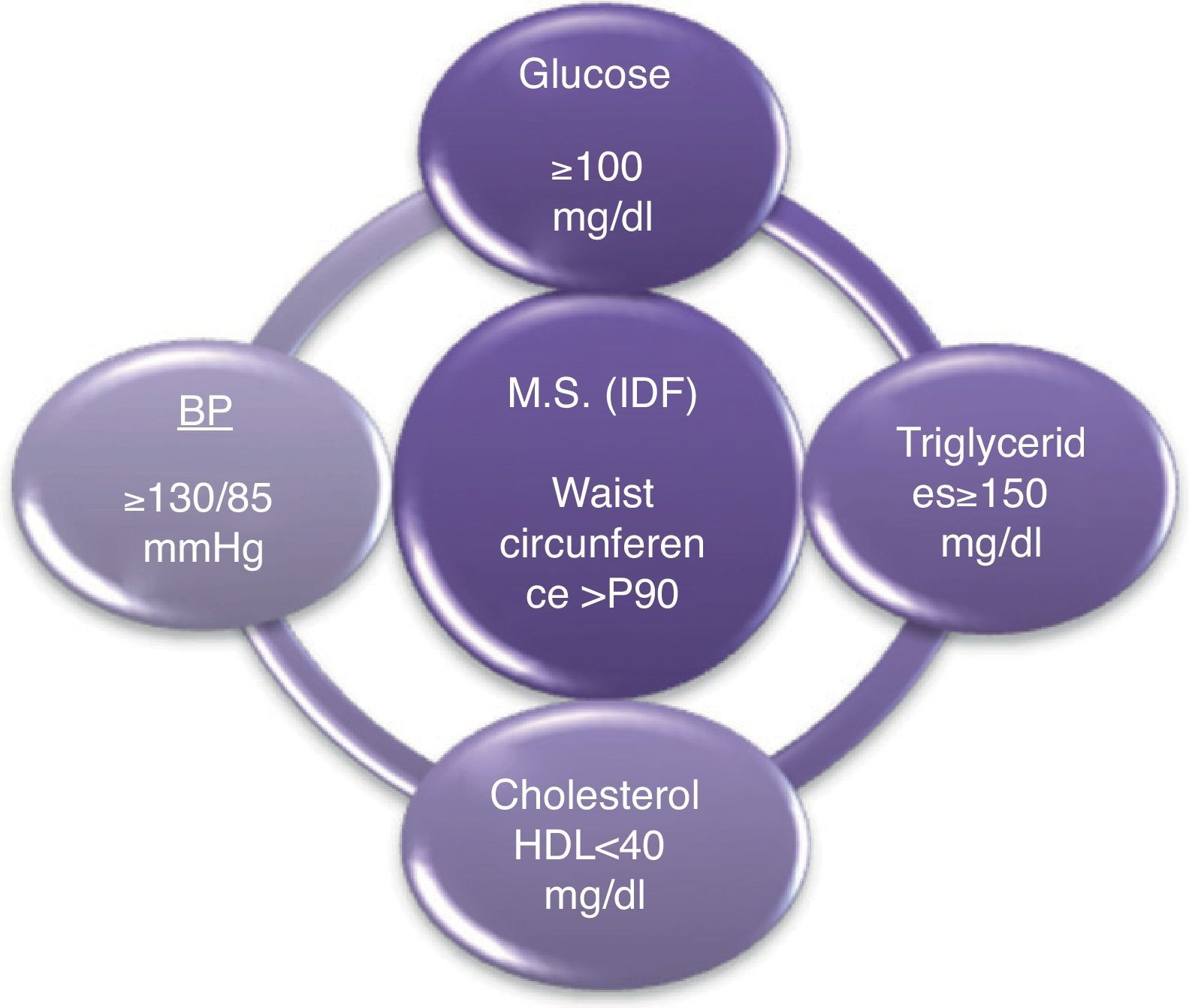
Materials and methodsIn children between 10 and <16 years old, according to the criteria of the IDF (International Diabetes Federation), the metabolic syndrome is diagnosed whether the waist circumference is above 90th percentile with the presence of at least 2 other clinical features: triglycerides≥150mg/dl, HDL cholesterol<40mg/dl, blood pressure≥130/85 mmHg, fasting glucose≥100mg/dl9 (Fig. 1).
This cross-sectional prevalence study was conducted on children and adolescents with overweight and obesity (BMI>85th percentile adjusted for age and sex), plus age 5–19 years, who were recruited from the Endocrinology and Nutrition consultations of Getafe University Hospital in Madrid, Spain.
We studied 148 children and adolescents between 5 and 19 years old with overweight (BMI>85th percentile) and obesity (BMI>95th percentile) who were referred to endocrinology clinic from primary care mainly for this reason.
Patients were evaluated at the Endocrinology consultation by a detailed medical history (personal history, family and current diseases). We collected anthropometric data (height, weight, BMI, waist circumference [WC], and waist-height [WH]) and biochemical data (uric acid, glucose, insulin, insulin resistance index measured by HOMA IR, HDL cholesterol and triglycerides). Overload oral glucose was also conducted with 75g.
The weight was taken with the patient at the center of the scale without moving, equally distributed weight on both legs, wearing light clothes and without shoes. We took the size with the patient standing barefoot and vertical measuring rod.
BMI is calculated with the Quetelet formula (weight in kg divided by height in meters squared). To determine the degree of overweight or obesity in each patient, we used tables recommended by the National Center for Health Statistics (NCSH)10 formed in 2000 and updated to include records of BMI for age and gender specific. Patients with BMI>85th percentile were those who participated in the study. The waist circumference was measured with the patient standing with arms raised horizontally at the midpoint between the lower edge of the last rib and the upper edge of the iliac crest. Disease was considered if it was greater than 90 percentile for age. The measurement of systolic and diastolic blood pressure was performed according to the recommendations of the Spanish Society of Hypertension with mercury sphygmomanometer.
Analytical tests were done on patients who previously had been fasting for 12h. The analytical parameters were uric acid, glucose, insulin, insulin resistance index measured by HOMA IR, HDL cholesterol and triglycerides. The following analytical values were considered normal: uric acid<5.5mg/dl (taking into account criteria of normality in our reference laboratory), glucose 60–100mg/dl, cholesterol<200mg/dl (HDL>40mg/dl), triglycerides<150mg/dl. Hyperinsulinemia was considered insulin values>15IU (90th percentile) and insulin resistance was HOMA>3.5 (90th percentile). In the oral glucose tolerance test with 75g glucose was considered normal at 60min<140mg/dl, carbohydrate intolerance between 140 and 199 and diabetes mellitus>200mg/dl.
As for the statistical analysis, quantitative variables were expressed as mean±standard deviation and the quantitative variables as frequencies. Since the sample corresponds to a normal distribution, the student test was used to analyze the association between uric acid levels above 5.5mg/dl and BMI, WC, waist size, blood pressure and diastolic baseline and after glucose load, insulin, HOMA IR, HDL cholesterol and triglycerides. Chi-squared test was conducted to investigate the relationship between metabolic syndrome and hyperuricemia.
ROC curve was constructed to determine the level of uric acid with more sensitivity and specificity for association with metabolic syndrome diagnosis. The statistical program used was SPSS 15.0. A P value<0.05 was considered significant.
ResultsWe recruited 148 children and adolescents between 5 and 19 years old (78 boys and 70 girls) with an average age of 12±3 years old and an average BMI of 31.8±6.1. 4.8% were overweight (BMI between 85 and 95 percentile for age and sex) and 94% were obese (percentile>95). From the last ones, 96.9% had morbid obesity (percentile>97). 19.6% had metabolic syndrome criteria according to the IDF. The averages of the data studied are: WC: 95.1±15.6cm, WH: 62.4±8.3cm, systolic blood pressure: 116±14.3mm Hg, diastolic blood pressure: 72.5±11.5mm Hg, triglycerides: 102.1±49.8mg/dl, HDL 52.5±11.4mg/dl, insulin 23.9±14.1mg/dl, HOMA IR: 5.1±3.2. 7.4% had an impaired fasting glucose and 3.4% were diagnosed with carbohydrate intolerance on oral glucose 75g with a prevalence of prediabetes 10.8%. None met criteria of diabetes mellitus. Average plasma uric acid was 5.2±1.2mg/dl. 53% of the sample had hyperuricemia considering this uric acid levels>5.5mg/dl. (Table 1). According to clinical parameters, we found that patients with hyperuricemia had greater BMI than those with normal levels of uric acid (BMI 33.9 vs 30.6, p=0.003); they also had more waist circumference (101.4 vs 91.1cm, p<0.001) and higher blood pressure both systolic (123.4 vs 111.9mm Hg, p<0.001) and diastolic (78.2 vs 68.7mm Hg, p<0.001). Regarding laboratory parameters, patients with hyperuricemia had a greater number of blood glucose after oral glucose (107.5 vs 100.7mg/dl, p=0.03). Regarding insulin resistance, insulin in patients with hyperuricemia was significantly higher (29.2 vs 20.7mg/dl, p=0.001) as well as HOMA IR (6.5 vs 4.4, p<0.001). HDL levels were significantly lower in patients with hyperuricemia (49.5 vs 54.4mg/dl, p=0.02) and triglyceride levels were higher (110.7 vs 97.6mg/dl, p=0.1. There were no statistically significant differences in the levels of cholesterol or LDL (Table 2). It was found that patients diagnosed with metabolic syndrome had higher levels of uric acid, 5.8 vs 5.1mg/dl, p=0.006. Among the patients with hyperuricemia, there were more cases of diagnosis of metabolic syndrome (43% vs 57%, p=0.04) (Table 3). Uric acid's level, from which a diagnosis of metabolic syndrome was most likely, corresponded to 5.4mg/dl in our sample (area under the curve 0.66, sensitivity: 64% and specificity 62%). There were not statistically significant differences between hyperuricemia and waist size, triglycerides, fasting glucose or any association with prediabetes (impaired fasting glucose and/or carbohydrate intolerance).
Sample frills.
| Age (years) | 12±3 |
| BMI (kg/m2) | 31.8±6.1 |
| Overweight (85–95 percentile) (%) | 4.8 |
| Obesity (>95 percentile) (%) | 94 |
| Morbid obesity (>97 percentile) (%) | 81.8 |
| Metabolic syndrome (IDF) (%) | 19.6 |
| Waist circumference (cm) | 95.1±15.6 |
| Waist-height (cm) | 62.4±8.3 |
| Systolic blood pressure (mm Hg) | 116±14.3 |
| Diastolic blood pressure (mm Hg) | 72.5±11.5 |
| Triglyceridemia (mg/dl) | 102.1±49.8 |
| HDL cholesterol (mg/dl) | 52.5±11.4 |
| Insulin (mg/dl) | 23.9±14.1 |
| HOMA RI | 5.1±3.2 |
| Impaired fasting glucose (%) | 7.4 |
| Carbohydrate intolerance (%) | 3.4 |
| Uric acid (mg/dl) | 5.2±1.2 |
| Hyperuricemia (A.U.≥5.5mg/dl) (%) | 53 |
Differences between groups with or without hyperuricemia.
| Uric acid group<5.5mg/dl | Uric acid group≥5.5mg/dl | Statistical significance | |
| Metabolic syndrome (%) | 43 | 57 | 0.04 |
| BMI (kg/m2) | 30.6 | 33.9 | 0.003 |
| Waist circumference (cm) | 91.1 | 101.4 | <0.001 |
| Systolic blood pressure (mm Hg) | 111.9 | 123.4 | <0.001 |
| Diastolic blood pressure (mm Hg) | 68.7 | 78.2 | <0.001 |
| Glycemia after oral glucose overload (g) | 100.7 | 107.5 | 0.03 |
| Insulin (mg/dl) | 20.7 | 29.2 | 0.001 |
| HOMA IR | 4.4 | 6.5 | <0.001 |
| HDL cholesterol (mg/dl) | 54.4 | 49.5 | 0.02 |
| Waist-height | 61.7 | 63.7 | 0.07 |
| Fasting glucose (mg/dl) | 88.9 | 90.5 | 0.8 |
| Impaired fasting glucose±carbohydrate intolerance (%) | 9.2 | 13.7 | 0.4 |
| Triglycerides (mg/dl) | 97.6 | 110.7 | 0.1 |
There were no statistically significant linear correlation between levels of uric acid and metabolic syndrome parameters.
DiscussionChildhood obesity is a problem that, in recent decades, is reaching important dimensions. Several national work objectively show this fact.11,12
In our study we found similar results to those reported by other studies in children13 although there are few papers published in the obese child population to investigate the relationship between hyperuricemia and metabolic syndrome. Other studies have established relationship between increased plasma uric acid levels and the various items that define the syndrome.14 Thus, the study Jones et al. performed in 104 children and young adults, suggests a relationship between hyperuricemia and primary hypertension measured by ABPM (ambulatory blood pressure monitoring).8 In other study by Clausen, the association of hyperuricemia and hyperinsulinemia from a sample of 380 adults aged between 18 and 32 years old is indicated.7 The literature on this association in the adult population is more extensive.15 The pathophysiology of the relationship between hyperuricemia and metabolic syndrome was initially attributed to insulin resistance situation but may be the result of a combination of factors.
- 1.
In patients with metabolic syndrome, renal excretion of uric acid is decreased by the hyper-sodium reabsorption at the proximal tubule, as in obese and hypertensive patients.16,17
- 2.
Another mechanism that may contribute to hyperuricemia is the increased synthesis that occurs in patients with metabolic syndrome and ischemic organ damage much less common in children than in adults. Under physiological conditions the enzyme xanthine oxidase is converted into xanthine dehydrogenase that has more affinity for nicotinamide adenine dinucleotide oxidase (NAD+) than oxygen. In situations of ischemia, the degradation of ATP increases, which in turn increases the synthesis of uric acid plus xanthine dehydrogenase is converted into xanthine oxidase, that uses molecular oxygen instead of NAD+, resulting in the formation of superoxide anion and hydrogen that stimulates the making of oxygen free radicals and uric acid.18
- 3.
The third postulated mechanism is the increased intake of fructose that would determine an increased synthesis of urate. Foods, such as soft drinks and bakery products recently joined the usual diet of children. They have corn syrup as a component rich in fructose that is more stable, cheaper and has higher sweetening power than common sugar. The mechanism by which the intake of fructose increases levels of uric acid is as follows: In phosphorylation of the fructose is consumed ATP in the liver. The AMP formed can enter the degradative pathway of purine nucleotides and then result in uric acid.19,20 This mechanism could be the largest contributor to hyperuricemia in children due to increased intake of fructose-rich products in recent years. In fact, many articles relate intake of fructose with hyperuricemia and the development of metabolic syndrome.21–27
Such is the evidence of association between metabolic syndrome and hyperuricemia that some of the studies have suggested increased uric acid as predictor of different factors related to the syndrome. Thus, Tohoku et al. in their work in 1729 adolescents provided an association between obesity and hyperuricemia and suggested that uric acid levels could be used as an indicator of obesity in early adolescence.24 Zoppni et al. in a prospective study with 2726 patients with type 2 diabetes suggested that high concentrations of uric acid were predictive of cardiovascular mortality in patients with type 2 diabetes mellitus.28,29 In another study on 462 subjects who had been free of disease for 10 years, Dehghan et al. found that the incidence of type 2 diabetes mellitus was higher in subjects with high uric acid levels and concluded that hyperuricemia was a risk factor for the development of such disease.30–32
In our study, we cannot infer a causal relationship between hyperuricemia and the various parameters that make up the metabolic syndrome. However, taking into account the data obtained with a very strong statistical significance, we can say that in our sample, the finding of hyperuricemia is strongly associated with metabolic syndrome and pathological situations that make it up, as hyperinsulinemia and insulin resistance, abnormal cholesterol HDL, elevated blood pressure levels and abdominal obesity with increased waist circumference. The level of uric acid from which you most likely find the metabolic syndrome in overweight and obese children is 5.4mg/dl. In conclusion, we can say that it is important to determine and assess levels of uric acid in children with overweight or obesity because they seem to be related to the diagnosis of metabolic syndrome and the set of parameters that comprise it.
Conflicts of interestThe authors state that they have no conflicts of interest.