Infusion of Hibiscus sabdariffa (H. sabdariffa) is a very popular drink in many parts of the world. Its phytochemical composition is associated to antioxidant, hypotensive, and antiatherosclerotic effects. However, the molecular mechanisms involved in these processes are not well known. The aim of this review was to report the scientific evidence supporting that regular use of H. sabdariffa decreases oxidative stress, atherosclerosis, lipid profile, and blood pressure.
Materials and methodsA search of recent publications was made in the following specialized electronic databases: Elsevier Journal, SciELO, FSTA, Science Direct, Springer Link, and NCBI. Results of research conducted in clinical trials in humans and in animal models and cell cultures were recorded. Keywords used included H. sabdariffa, oxidative stress, polyphenols, hypertension, atherosclerosis, and lipid profile.
ResultsResults of the different articles suggested a possible therapeutic effect of H. sabdariffa extracts on oxidative stress, lipid profile, hypertension, and atherosclerosis thanks to its composition rich in phenolic compounds. Anthocyanins significantly decrease LDL oxidation, inhibit adipogenesis by regulating adipogenic signaling pathways and transcription factors, and modulate gene expression of certain microRNAs. No adverse events or side effects were reported.
ConclusionsFurther more homogeneous, placebo-controlled studies in humans are needed to state that H. sabdariffa has therapeutic efficacy in humans.
La infusión de Hibiscus sabdariffa (H. sabdariffa) es una bebida muy popular en muchos lugares del mundo. Su composición fitoquímica se asocia a efectos antioxidantes, hipotensores y antiateroscleróticos. No obstante, no se conocen con profundidad los mecanismos moleculares implicados en estos procesos. El objetivo de la presente revisión fue describir las evidencias científicas que apoyan que el consumo regular de H. sabdariffa reduce el estrés oxidativo, la aterosclerosis, el perfil lipídico y la tensión arterial.
Material y métodosSe realizó una búsqueda de publicaciones recientes en las siguientes bases de datos electrónicas especializadas: Elsevier Journal, SciELO, FSTA, Science Direct, Springer Link y NCBI. Se describieron los resultados de trabajos llevados a cabo en ensayos clínicos en humanos, modelos animales y cultivos celulares. Las palabras clave utilizadas fueron Hibiscus sabdariffa, estrés oxidativo, polifenoles, hipertensión, aterosclerosis y perfil lipídico.
ResultadosLos resultados de los diferentes artículos evidenciaron un posible efecto terapéutico de los extractos de H. sabdariffa sobre el estrés oxidativo, el perfil lipídico, la hipertensión y la aterosclerosis, gracias a su composición rica en compuestos fenólicos. Las antocianinas reducen significativamente la oxidación de la lipoproteína LDL, inhiben la adipogénesis mediante la regulación de las vías de señalización adipogénicas y factores transcripcionales, y modulan la expresión génica de determinados microARN. No se comunicaron acontecimientos adversos ni efectos secundarios.
ConclusionesSon necesarios más estudios en humanos, estudios más homogéneos y controlados con placebo, para poder aseverar que H. sabdariffa posee eficacia terapéutica en humanos.
Highly reactive free radicals (OFRs) are atoms or atom groups with an unpaired or free electron. To achieve electrochemical stability, OFRs start a chain reaction that may damage biological macromolecules such as lipids, proteins, carbohydrates, and nucleic acids, and imbalance body homeostasis.1 Most OFRs result from normal cell metabolism.2 However, OFR production may also increase due to the metabolism of some exogenous substances, exposure to sun rays or ionizing radiation, pesticides, and heavy metals.3 Other factors affecting OFR production are associated with exposure to the action of some xenobiotics (chloroform, acetaminophen, carbon tetrachloride), tobacco smoke, or inadequate diet, either as excess harmful substances or as deficient antioxidants.3 All of these factors may cause excess OFRs in cells and increase susceptibility to the occurrence of pathological conditions such as cancer, cell aging, atherosclerosis, high blood pressure (HBP) or hyperlipidemia.1,4
HBP is a very important and common cardiovascular risk factor in modern society.5,6 In fact, HBP is one of the main risk factors for the development of cardiovascular disease together with smoking, dyslipidemia and, especially, high plasma levels of low density lipoprotein (LDL) cholesterol.7–11 To these should be added other predisposing risk factors, such as obesity and sedentary lifestyles, which exert their action through intermediate, causative or conditional risk factors.8,9 Cardiovascular diseases are the leading cause of death in Spain, and are responsible for almost 40% of all deaths.12 The mechanism starting HBP is unknown in 90% of cases, but there is evidence which appears to suggest that increased OFR production is related to its pathogenesis.13–15 In fact, it has been noted that individuals with HBP may experience an increase in blood levels of thiobarbituric acid, a marker of lipid peroxidation, and a reduction in antioxidant activities of the enzymes superoxide dysmutase, glutathione peroxidase, and catalase.16,17 Moreover, Ward et al.18 found a decrease in non-enzymatic antioxidants, such as vitamin E and reduced glutathione, in patients with HBP. The pathogenesis of HBP has also been related to metabolic abnormalities,19–21 hormonal factors,22 and genetic changes. Specifically, it has been estimated from epidemiological and familial studies that the genetic component could cause approximately 40% of interindividual variability in HBP values.23 The central role of lipid metabolism in the homeostasis of hypertension justifies the extensive analysis made of the genetic varieties of genes that encode for proteins of this system. Maximum attention has been paid to polymorphisms related to functional changes in proteins encoding, for example, polymorphisms in apolipoprotein B24 and A5,24 CD36 (cluster of differentiation 36),25 USF1 (upstream transcription factor 1),26,27 FADS3 (fatty acid desaturase 3),27 and GCKR genes (glucokinase regulatory protein).24 Ischemic heart disease and cerebrovascular disease or stroke are manifestations of atherosclerosis.28 The characteristic lesion is the atheroma plaque, consisting of lipid, fibrous tissue, and immune system cells. One of the earliest events in atherosclerosis is LDL accumulation in the arterial wall. One of the most widely accepted hypotheses to explain the development of atherosclerosis is slow oxidation of LDL trapped in the subendothelial space by the action of OFRs generated by vascular cells, demonstrating a close relationship between OFRs and LDL.29 The accumulation of lipoproteins in arterial endothelium triggers the activation of macrophage scavenger receptors, leading to the conversion of macrophages into foam cells. Progressive foam cell accumulation contributes to lesion progression.30
Plants and animals have endogenous antioxidant systems to remove excess production of OFRs, such as glutathione, vitamin D and vitamin E, catalase, superoxide dysmutase, and several peroxidases.31 Glutathione peroxidase, superoxide dysmutase, or the catalase in peroxisomes are endogenous or primary antioxidant enzymes able to metabolize OFRs generated in cellular redox processes. By contrast, alpha lipoic acid, vitamins C, E and A, and polyphenols are considered non-enzymatic or secondary antioxidants able to directly destroy OFRs.32
Polyphenols are a very numerous and heterogeneous group of molecules which share the characteristic of having several phenol groups in their structures. Many epidemiological studies support the antioxidant properties of polyphenols,33–36 although their antioxidant capacity depends on their bioavailability and absorption. In turn, this is greatly affected by several factors such as climate, type of soil, type of culture, and sun exposure, amongst others.37 Most polyphenols are metabolized by colonic microorganisms before being absorbed, and the products resulting from this fermentation are partly responsible for their systemic effects.38–42 The antioxidant capacity of polyphenols accounts for their vasodilating, antithrombotic, anti-inflammatory, and antiapoptotic actions,43 and also for their antilipidemic44,45 and antiatherogenic properties.46 The antioxidant activity of polyphenols is tenfold higher than that of vitamin A and 100-fold higher than that of vitamin E or carotenoids.47 More specifically, there are studies suggesting that phenolic compounds may attenuate the oxidation of LDL and high density lipoproteins (HDL).48–53 In healthy humans, it has been suggested that resveratrol, one of the main phenolic compounds in wine, could prevent the oxidation of LDL and decrease lipid hydroperoxide levels.54 Recently, Castaner et al.48 showed that phenolic compounds in olive oil could decrease LDL oxidation and the expression of the CD40L (CD40 ligand) gene, as well as genes related to inflammation processes in humans. The molecular processes associated with the antilipidemic and antiatherogenic properties of polyphenols result from their ability to regulate the expression of different genes associated with the immune system and energy metabolism, and/or from their epigenetic regulation capacity55 through the induction of changes in the methylation pattern of DNA CpG islands,56,57 histone acetylation,58 and modulating the expression of some miRNAs.59 In this regard, for example, quercetin, the active component of Hibiscus sabdariffa (H. sabdariffa), has been reported to inhibit the activity of histone acetyltransferase in the promoter region of genes associated with the manifestation of inflammation.58 Joven et al.60 used hyperlipidemic mice with LDL receptor deficiency to assess the role of polyphenols in the prevention of liver disease by regulating the expression of hepatic microRNAs miR103/107 and miR122. In their results, these authors emphasized that the oral administration of polyphenols reverted changes induced in non-specific microRNAs miR103/107 after chronic polyphenol intake, and the lack of response of the specific miRNA miR122, speculating about the potential implication of polyphenols in cell metabolism. The authors reported that modulation of microRNA expression may be a significant and additional intervention mechanism in chronic diseases. Crozier et al.61 have shown that polyphenolic extract-miRNA specificity may exist, given the potential variety of different structures and compositions of extracts, depending on their botanical origin. Additional studies in humans are however needed to clarify the epigenetic effects of polyphenols.
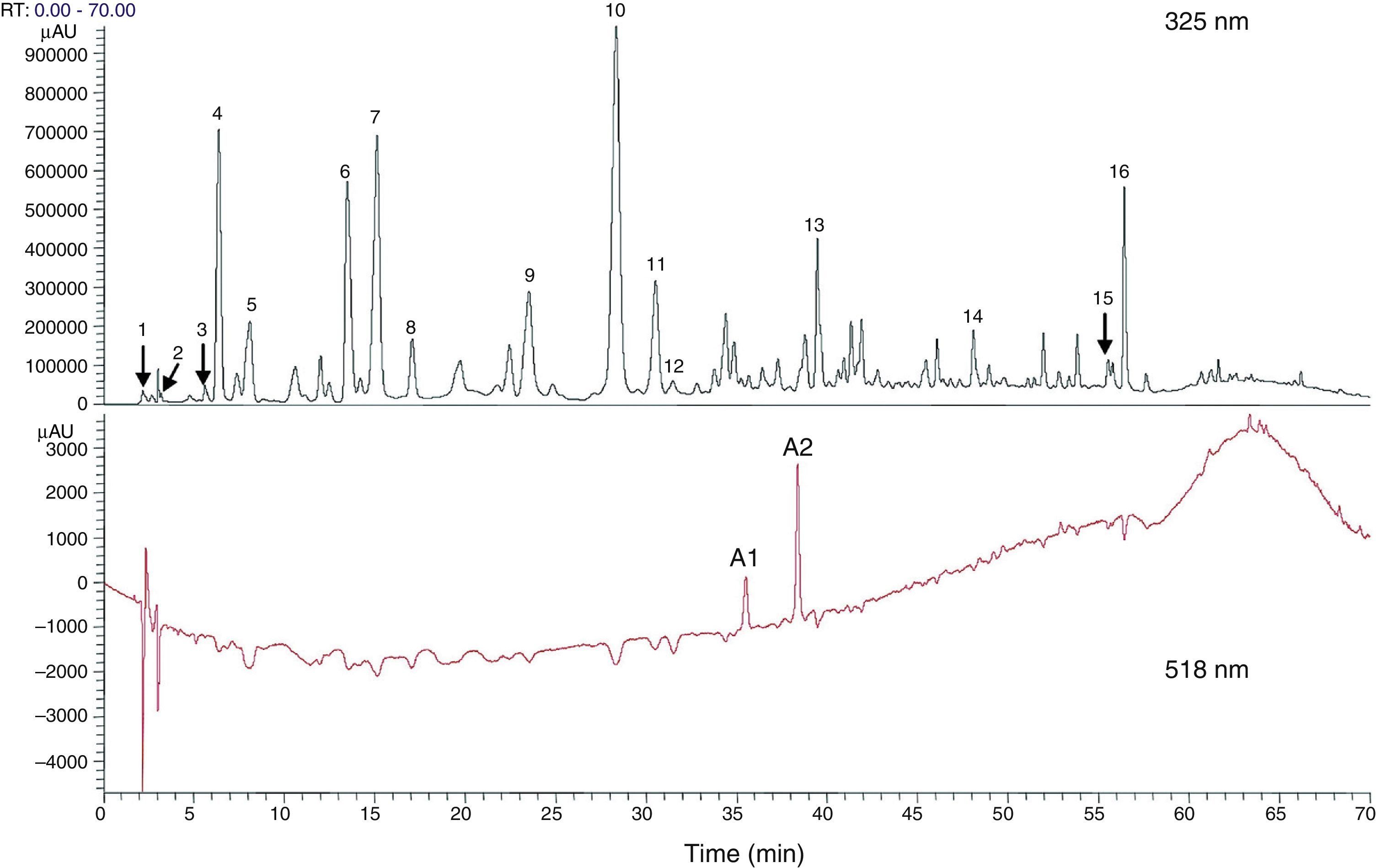
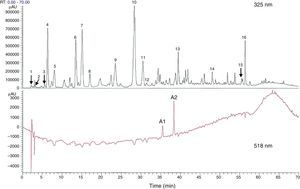
H. sabdariffa has a high polyphenol content.47 It is also called Jamaica sorrel, roselle, or karkade, and belongs to the malvaceae family native to tropical Africa, although it is cultivated in Mexico, Central America, and south-east Asia. It is an annual herbaceous plant living in dry, subtropical, mountain climates. Its calyces, fleshy and of a vivid red color, have high concentrations of l-ascorbic, arachidic, citric, stearic, and malic acids, in addition to pectins, phytosterols (e.g. β-sitosterol and ergosterol) and polyphenols.62 Peng et al.,63 in their research to assess the hypoglycemic and hypolipidemic effect of the polyphenolic extract of H. sabdariffa, found at least 18 different phenolic compounds in H. sabdariffa (Fig. 1). Commercial preparations of concentrates of calyces, and occasionally leaves, of H. sabdariffa are very commonly found as fluid or powder for the preparation of instant drinks or infusions.62 In addition, its widespread use as a herbal treatment in popular medicine has led to a high acceptance rate by the general population,64 especially in countries such as the United States,65 Mexico,62,66,67 some eastern African countries,68–70 Iran,71,72 India,73 Taiwan,63 Brazil,74 and Greece.75 The ethnobotanical studies available usually describe the origin and parts of the plant used, the properties attributed to it and the form of preparation, without specifying the dosage. No demographic studies supporting the role of the plant in disease prevention have been found.74,76 On the other hand, scientific studies have shown that the antioxidant effects of polyphenols in H. sabdariffa have an antiatherogenic action and decrease hypertension and hyperlipidemia with no reported adverse events or side effects in animals and humans.65–67,72,77–79
Total phenolic and flavonoid components estimated with high performance liquid chromatography (HPLC) from dry flower extract of Hibiscus sabdariffa. Total phenolic and flavonoid components were estimated as 58.8±1.34mg and 13.57±0.65mg/g of dry flowers respectively.63
1, hibiscus acid; 2, hibiscus acid ester 6-methyl; 3, gallic acid; 4, not identified; 5, 5 hydroxymethylfurfural; 6, protocatechuic acid; 7, 5-caffeoylquinic acid; 8, feruloyl derivative; 9, chlorogenic acid; 10, 4-caffeoylquinic acid; 11, caffeic acid; 12, galloyl ester; 13, feruloylquinic derivative; 14, kaempferol-3-glucoside; 15, quercetin derivative; 16, tiliroside; Al, delphinidin-3-sambubioside; A2, cyanidin-3-sambubioside.
The purpose of this paper was therefore to collect and unify the evidence showing that regular consumption of H. sabdariffa may have a beneficial effect on human health because of the antioxidant, antihypertensive, and lipid-lowering effects of its phenolic components.
Materials and methodsIn this review, a search for recent publications was made in the following specialized electronic databases: NCBI, Elsevier Journal Finder, SciELO, ScienceDirect and SpringerLink. Results from studies conducted in vitro, in animal models and in humans were collected. Reviews collecting and analyzing the effectiveness of H. sabdariffa in given treatments, such as antihypertensive and lipid-lowering therapies, amongst others, and articles referring to the phytochemical, pharmacological, and toxicological aspects of H. sabdariffa were also included. The concepts of oxidative stress, antioxidant capacity, hyperlipidemia, high blood pressure, and atherosclerosis were also analyzed to describe in more detail the molecular mechanisms of H. sabdariffa. The keywords used included: H. sabdariffa, oxidative stress, polyphenols, high blood pressure, atherosclerosis, and lipid profile.
A total of 104 articles were reviewed in preparing this paper. The articles selected were divided into the following categories: (1) generic articles on the pharmacological, chemical, and ethnobotanical properties of H. sabdariffa; (2) articles on the relationship between the consumption of H. sabdariffa and oxidative stress, and its antioxidant, antihypertensive, hypolipidemic, and antiatherosclerotic potential.
Results and discussionStudies analyzing the therapeutic effects of H. sabdariffa used in this review are grouped into three tables. In each table, the part of H. sabdariffa used and the procedure to obtain the extract of H. sabdariffa are given, as well as a summary of the most relevant results and conclusions. Tables 1 and 2 show the results of studies conducted on different cell lines and animal models respectively. Table 3 shows the results of studies conducted in humans.
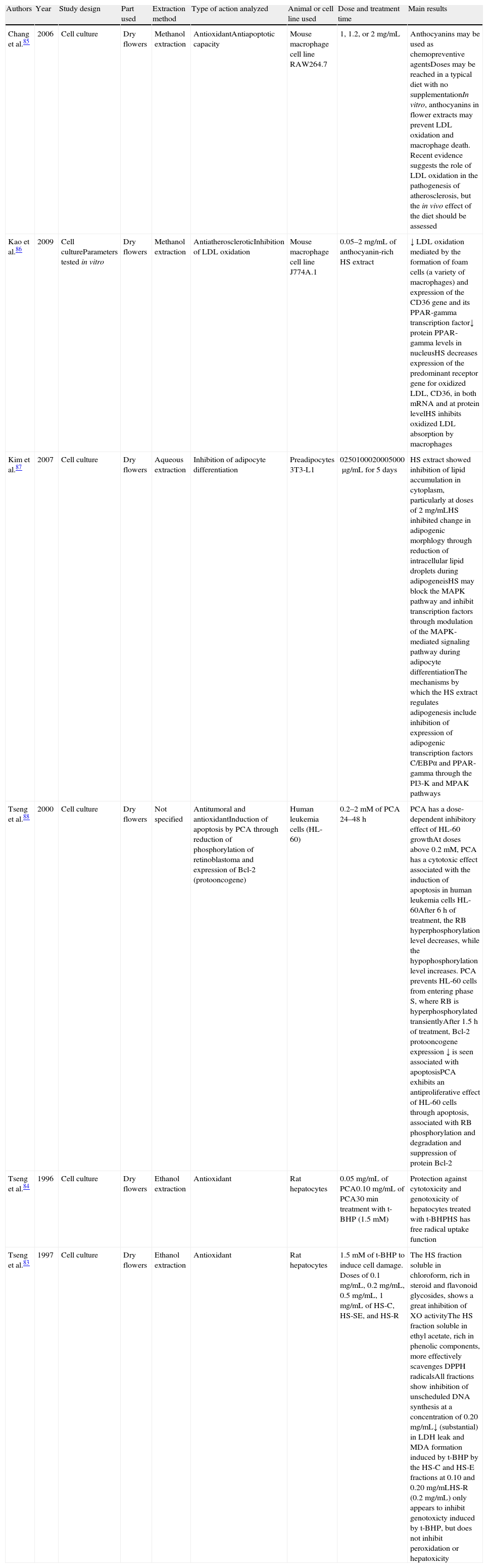
Cell culture studies showing the different effects of Hibiscus sabdariffa.
| Authors | Year | Study design | Part used | Extraction method | Type of action analyzed | Animal or cell line used | Dose and treatment time | Main results |
| Chang et al.85 | 2006 | Cell culture | Dry flowers | Methanol extraction | AntioxidantAntiapoptotic capacity | Mouse macrophage cell line RAW264.7 | 1, 1.2, or 2mg/mL | Anthocyanins may be used as chemopreventive agentsDoses may be reached in a typical diet with no supplementationIn vitro, anthocyanins in flower extracts may prevent LDL oxidation and macrophage death. Recent evidence suggests the role of LDL oxidation in the pathogenesis of atherosclerosis, but the in vivo effect of the diet should be assessed |
| Kao et al.86 | 2009 | Cell cultureParameters tested in vitro | Dry flowers | Methanol extraction | AntiatheroscleroticInhibition of LDL oxidation | Mouse macrophage cell line J774A.1 | 0.05–2mg/mL of anthocyanin-rich HS extract | ↓ LDL oxidation mediated by the formation of foam cells (a variety of macrophages) and expression of the CD36 gene and its PPAR-gamma transcription factor↓ protein PPAR-gamma levels in nucleusHS decreases expression of the predominant receptor gene for oxidized LDL, CD36, in both mRNA and at protein levelHS inhibits oxidized LDL absorption by macrophages |
| Kim et al.87 | 2007 | Cell culture | Dry flowers | Aqueous extraction | Inhibition of adipocyte differentiation | Preadipocytes 3T3-L1 | 0250100020005000μg/mL for 5 days | HS extract showed inhibition of lipid accumulation in cytoplasm, particularly at doses of 2mg/mLHS inhibited change in adipogenic morphlogy through reduction of intracellular lipid droplets during adipogeneisHS may block the MAPK pathway and inhibit transcription factors through modulation of the MAPK-mediated signaling pathway during adipocyte differentiationThe mechanisms by which the HS extract regulates adipogenesis include inhibition of expression of adipogenic transcription factors C/EBPα and PPAR-gamma through the PI3-K and MPAK pathways |
| Tseng et al.88 | 2000 | Cell culture | Dry flowers | Not specified | Antitumoral and antioxidantInduction of apoptosis by PCA through reduction of phosphorylation of retinoblastoma and expression of Bcl-2 (protooncogene) | Human leukemia cells (HL-60) | 0.2–2mM of PCA 24–48h | PCA has a dose-dependent inhibitory effect of HL-60 growthAt doses above 0.2mM, PCA has a cytotoxic effect associated with the induction of apoptosis in human leukemia cells HL-60After 6h of treatment, the RB hyperphosphorylation level decreases, while the hypophosphorylation level increases. PCA prevents HL-60 cells from entering phase S, where RB is hyperphosphorylated transientlyAfter 1.5h of treatment, Bcl-2 protooncogene expression ↓ is seen associated with apoptosisPCA exhibits an antiproliferative effect of HL-60 cells through apoptosis, associated with RB phosphorylation and degradation and suppression of protein Bcl-2 |
| Tseng et al.84 | 1996 | Cell culture | Dry flowers | Ethanol extraction | Antioxidant | Rat hepatocytes | 0.05mg/mL of PCA0.10mg/mL of PCA30min treatment with t-BHP (1.5mM) | Protection against cytotoxicity and genotoxicity of hepatocytes treated with t-BHPHS has free radical uptake function |
| Tseng et al.83 | 1997 | Cell culture | Dry flowers | Ethanol extraction | Antioxidant | Rat hepatocytes | 1.5mM of t-BHP to induce cell damage. Doses of 0.1mg/mL, 0.2mg/mL, 0.5mg/mL, 1mg/mL of HS-C, HS-SE, and HS-R | The HS fraction soluble in chloroform, rich in steroid and flavonoid glycosides, shows a great inhibition of XO activityThe HS fraction soluble in ethyl acetate, rich in phenolic components, more effectively scavenges DPPH radicalsAll fractions show inhibition of unscheduled DNA synthesis at a concentration of 0.20mg/mL↓ (substantial) in LDH leak and MDA formation induced by t-BHP by the HS-C and HS-E fractions at 0.10 and 0.20mg/mLHS-R (0.2mg/mL) only appears to inhibit genotoxicty induced by t-BHP, but does not inhibit peroxidation or hepatoxicity |
CD36: protein encoding for the CD36 gene; DPPH: radical 1,1-diphenyl-2-picrylhydrazyl; HS: Hibiscus sabdariffa; HS-C: HS fraction soluble in chloroform; HS-E: HS fraction soluble in ethyl acetate; HS-R: residual HS fraction; LDH: lactate dehydrogenase; LDL: low density lipoprotein; MAPK: mitogen-activated protein kinase; MDA: malondialdehyde; PCA: protocatechuic acid; RB: retinoblastoma; t-BHP: tert-butyl hydroperoxide; XO: xanthine oxidase activity.
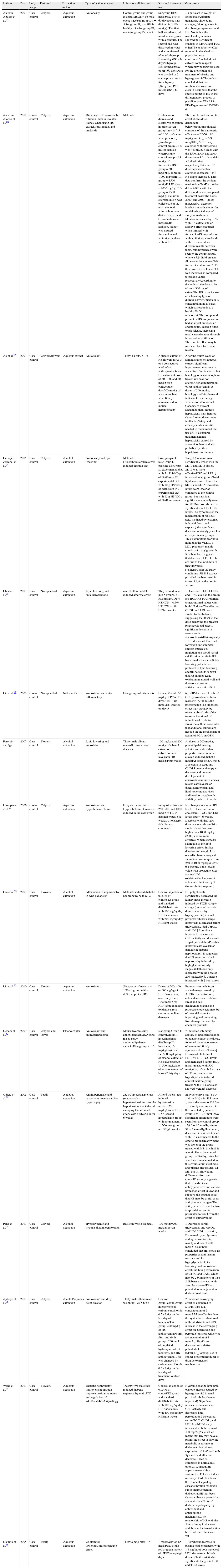
Studies in animal models showing the different effects of administration of Hibiscus sabdariffa.
| Authors | Year | Study design | Part used | Extraction method | Type of action analyzed | Animal or cell line used | Dose and treatment time | Main results |
| Alarcon-Aguilar et al.95 | 2007 | Case–control | Calyces | Aqueous extraction | Antiobesity | Control group and group injected MSGn=16 male obese miceSubgroup I, n=8Subgroup II, n=8Eight healthy miceSubgroup III, n=4Subgroup IV, n=4 | Subgroup I:120mg/kg/day of HS; 60 daysDose was divided in 2 (60mg/kg). The first half was dissolved in saline and given with a cannula. The second half was dissolved in water and administered ad libitumSubgroup II:4mL/kg (ISS); 60 daysSubgroup III:120mg/kg/day of HS; 60 daysDose was divided in 2 (same procedure as for subgroup I)Subgroup IV:4mL/kg (ISS); 60 days | ↓ significant in weight of obese miceAspartate transferase showed no changes↓ blood glucose in the obese group treated with HS. Not in healthy miceHealthy animals showed no significant changes in CHOL and TGC eitherThe antiobesity effect reported in the Mexican population was confirmedConcluded that calyces contain agents which may possibly be used for the prevention and treatment of obesity and hyperglycemiaThe authors concluded that the mechanisms were not clearThis suggests that the specific target of HS in the differentiation process of preadipocytes 3T3-L1 is PPAR-gamma and C/EBP-α |
| Alarcon-Alonso et al.105 | 2012 | Case–control | Calyces | Aqueous extraction | Diuretic effectTo assess the filtration index in isolated kidney when using HS-extract, furosemide, and amiloride | Male rats | Evaluation of diuresis and electrolyte excretion in urine:Seven groups, n=6. 7.5mL/100g of saline were previously givenNegative control group=1.5mL of distilled waterPositive control group=13mg/kg of furosemideHS I group=500mg/kgHS II group=1000mg/kgHS III group=1500mg/kgHS IV group=2000mg/kgHS V group=2500mg/kgTotal urine excreted in 5h was collected. For the tests, the total volume/hour was dividedNa, K, and Cl contents were measuredIn addition, kidney was infused furosamide and amiloride, with or without HS | The diuretic and natriuretic effect shows dose-dependent behaviorPharmacological constants of the natriuretic effect were ED50=86mg/kg and Emax=0.9mEq/100g/5hUrinary excretion with furosemide was 4.8mL/h. Values with the 1500, 2000, and 2500 doses were 3.0, 4.3, and 4.4mL/h of urine respectivelyEvidence of dose dependenceNa excretion increased ↑ as ↑ HS doses increased. This data confirms the evident natriuretic effectK excretion did not differ with the different doses as compared to control dosesThe 1500, 2000, and 2500 ↑ doses increased Cl excretion levelsAs regards the in situ model using kidneys of study animals, renal filtration increased by 48% with HS extract and an additive effect occurred when infused with furosamideKidney infusion with amiloride or amiloride with HS showed no different results between them, but differences were seen to the control group, where a 3.9-↑fold greater filtration ratio was seenWith furosemide alone and ↑HS there were 2.4-fold and 3.4-fold increases as compared to basline values respectivelyAccording to the authors, the dose to be taken is 300mg of extractThe HS extract show an interesting type of diuretic activity, maintain K concentration in all cases, which corresponds to a healthy Na/K relationshipThe compound present in HS, as quercetin, had an effect on vascular endothelium, causing nitric oxide release, increasing renal vasorelaxation through increased renal filtration. The diuretic effect may be mediated by nitric oxide release |
| Ali et al.89 | 2003 | Case–control | Calyces/flowers | Aqueous extract | Antioxidant | Thirty-six rats, n=6 | Aqueous extract of HS flowers for 2, 3, or 4 consecutive weeksOral anthocyanins from HS calyces at doses of 50, 100, and 200mg/kg for 5 consecutive days700mg/kg of acetaminophen were finally administered to induce hepatotoxicity | After the fourth week of administration of aqueous extract, significant improvement was seen in some liver function tests, but histology of acetaminophen-treated rats was not alteredAfter administration of HS anthocyanins: at doses of 200mg/kg, histology and biiochemical indices of liver damage were restored to normal. Capacity to prevent acetaminophen-induced hepatoxicity was therefoe shownLower doses were ineffectiveSafety and efficacy studies are still needed to recommend the use of HS as natural treatment against hepatoxicity caused by acetominophen, and also probably by other hepatotoxic substances |
| Carvajal-Zarrabal et al.94 | 2005 | Case–control | Calyces | Alcohol extraction | Antiobesity and lipid lowering | Male rats. Hypercholesterolemia was induced through diet | Five groups of rats:Group I, baseline dietGroup II. experimental diet with 5g HS/100g of dietGroup III, experimental diet with 10g HS/100g of dietGroup IV. experimental diet with 15g HS/100g of dietFour weeks | Weight ↑increase was significantly lower with the SD10 and SD15 doses. SD15 was more effectiveTGC and LDL ↓ increased in all groupsTotal lipid levels were lower for SD10 and SD15Cholesterol levels were lower as compared to the control group, but statistical significance was only seen for SD5No dose showed a significant result for HDL levels.The hypothesis is that racemization of hibiscus acid, mediated by enzymes in bowel flora, could explain ↓ the significant decrease in triacylglycerol in all experimental groups. This is important bearing in mind that the VLDL, a LDL precursor, mainly consists of triacylglycerols. It is therefore↓ suggested that decreased LDL levels are due to the inhibition of triacylglycerol synthesisUnder the study conditions, 5% HS extract provided the best result in terms of lipid reduction in serum |
| Chen et al.79 | 2003 | Case–control | Not specified | Aqueous extraction | Lipid lowering and antiatherosclerotic | n=30 albino rabbits induced atherosclerosis | They were divided into 5 groups, n=5ControlHCD1% HSHCD+0.5% HSHCD+1% HSTen weeks | ↓ Decreased TGC, CHOL, and LDL levels in the group fed HCD HSTGC returned to near normal values with both HS dosesThe effect on CHOL and LDL was similar for both doses, suggesting that 0.5% is the dose achieving the greatest pharmacolocial effect↓ significant decrease in severe aortic atherosclerosisHistologically ↓, HS decreased foam cell formation and inhibited smooth muscle cell migration and blood vessel calcification in rabbitsHS has virtually the same lipid-lowering potential as probucol (a lipid-lowering agent)The results suggest that HS inhibits LDL oxidation in arterial wall and therefore exerts an antiatherosclerotic effect |
| Liu et al.91 | 2002 | Case–control | Not specified | Not specified | Antioxidant and anti-inflammatory | Five groups of rats, n=6 | Doses, 50 and 100mg/kg of PCA. Five days. t-BHP (0.1mmol/kg) injected on day 5 | t-↓BHP decreased levels of GSH peroxidase, a stress markerPCA inhibits the phenomenonThe inhibitory effect may partially be related to blockade of the transduction signal of induction of oxidative stressThe authors concluded that additional studies are needed on the mechanism of action of PCA on GSH |
| Farombi and Ige | 2007 | Case–control | Flowers | Alcohol extraction | Lipid lowering and antioxidant | Thirty male albino miceAlloxan-induced diabetes | 100mg/kg and 200mg/kg of ethanol extract of HS calyces versus lovastatin (10mg/kg)Four weeks | At doses of 200mg/kg, potent lipid-lowering activity and antioxidant properties are seen in the alloxan-induced diabetic modelAt doses of 200mg/g, ↓ decrease in LDL and CHOLPotential therapy to decrease and prevent development of atherosclerosis and diabetes-related cardiovascular diseaseAntioxidant and lipid-lowering activities attributed to polyphenols and dihydrobenzoic acids |
| Hirunpanich et al.97 | 2006 | Case–control | Calyces | Aqueous extraction | Antioxidant and hypocholesterolemic | Forty-two male mice. Hypercholesterolemia was induced in the case group | Intragastric doses of 250, 500, and 1000mg/kg of HS in distilled water. Six weeks. Cholesterol-rich diet was continued | No changes in serum HDL levels↓ Decreased serum cholesterol, TGC, and LDL levels after 4–6 weeks. Decrease with the↓ 250 dose was not relevantPrior studies show that doses higher than 1000mg/kg (2000) are not more effective, which suggests saturation of the lipid-lowering effect. In fact, diarrhea and weight loss occurIts pharmacological saturation dose ranges from 250 to 1000mg/kgIn vitro, 0.1mg/mL is the lowest value with protective effect against LDL oxidationMechanisms of action not elucidated yet (future studies required) |
| Lee et al.92 | 2009 | Case–control | Flowers | Alcohol extraction | Attenuation of nephropathy in type 1 diabetes | Male rats induced diabetic nephropathy with STZ | Control: injection of 0.05M of citrateSTZ group and standard dietDiabetic rats with 100mg/kg/day HPDiabetic rats with 200mg/kg/day HPEight weeks | HS polyphenols significantly decreased the kidney mass increase induced by STZHydropic change (impaired osmotic diuresis caused by hyperglycemia) in renal proximal tubular change improved↓ Decreased serum triglycerides, total CHOL, and LDL↑ Significant increase in catalase and GSH activity and decreased ↓ lipid peroxidationPossibly improves cardiovascular damage in diabetic neprhopathyIt is suggested that HP reverses diabetic nephropathy induced by high glucose in early stagesGlutathione only increased with the dose of 200mg/kg/day↑. Catalase increased with ↑ both doses |
| Liu et al.90 | 2010 | Case–control | Flowers | Aqueous extraction | Antioxidant | Six groups of mice. n=10Each group with a different protocolRT | Doses of 200, 400, or 600mg/kg of HS. Two weeks, once dailyThen, 1000mg/kg of: APP (drug inducing oxidative stress, causes acute liver damage) | Protects liver cells from acute damage caused by APPIts mechanism of ↓ action decreases oxidative stress and cell deathAnthocyanins and protocatechouc acid may be of potential value for improving and preventing liver damage induced by chemical products |
| Ochani et al.82 | 2009 | Case–control | Calyces and leaves | Ethanol/water | Antioxidant and antihyperlipidemic | Mouse liver to study antioxidant activityAlbino rats to study antihyperlipidemic capacityFive group, n=6 | Rat group:Group I: controlGroup II: hyperlipidemic dietGroup III: lovastatin, 10mg/kg/dayGroup IV: 500mg/kg/day of ethanol extract of HS calycesGroup V: 500mg/kg/day of ethanol extract of leavesThirty days | ↑ Increased inhibitory activity of lipid peroxidation of ethanol extract of calyces, followed by ethanol extract of leaves and finally, aqueous extract of leaves↓ Decreased cholesterol, LDL, VLDL, TGC levels and increased ↑ serum HDL in rats treated with 500mg/kg/day of alcohol extract of HS as compared to hyperlipidemia induced control ratsThe group treated with HS alone also showed weight↓ decrease |
| Odigie et al.70 | 2003 | Case–control | Petals | Aqueous extraction | Antihypertensive and capacity to reverse cardiac hypertrophy | 2K-1C hypertensive rats (renovascular hypertension)Renovascular hypertension was induced clamping the left renal artery with a silver clip for 6 weeks | After 6 weeks, rats induced hypertension received250mg/kg/day of HS, n=5A second hypertensive group with no treatment, n=5Control group, n=5Eight weeks | In hypertensive rats (BP>140mmHg) with HS there ↓ was a decrease to 139.6±1.6mmHg as compared to the untreated hypertensive group, 174±2.4mmHgNo significant differences were seen from the control group, 139.6±1.6mmHg versus 32±3.4mmHgHeart rate ↓ decreased in animals treated with HS as compared to the other 2 groupsHeart weight was lower in the group treated with HS, in which it was similar to the control group; cardiac hypertrophy was therefore attenuated in this groupSerum creatinine and plasma electrolytes, Cl, Mg, Na, K, showed no differences from the controlThe study suggests that HS exhibits an antihypertensive and cardiac protection effect in vivo and supports the popular belief that HS may be useful as an antihypertensive agentThe antihypertensive mechanism is speculative, and is postulated to result from the effect of anthocyanins |
| Peng et al.63 | 2011 | Case–control | Calyces | Alcohol extraction | Hypoglycemic and hypoinsulinemicAntioxidant | Rats con type 2 diabetes | 100mg/day200mg/daySeven weeks | ↓ Decreased serum triglycerides and CHOL, and LDL/HDL risk ratio.↓ Decreased hyperglycemia and hyperinsulinemia, mainly at doses of 200mg/kgThe authors concluded that HS shows its properties as anti-insulin-resistant and its hypoglycemic, lipid-lowering, and antioxidant effect, inhibiting expression of CTFG and RAG, which may be 2 biomarkers of type 2 diabetes associated with vascular diseaseHS has potential as an adjuvant in diabetic treatment |
| Ajiboye et al.96 | 2011 | Case–control | Calyces | Alcohol/aqueous extraction | Antioxidant and drug detoxification | Thirty male albino mice weighing 175±6.6g | Control groupSecond group: interperitoneal carbon tetrachloride 0.5mL/kg on the last day of treatmentThird group: 200mg/kg of HS anthocyaninsFourth, fifth, and sixth groups: 200mg/kg of butylated hydroxyanisole, α-tocoferol, and HS anthocyanins. This was changed by carbon tetrachloride 0.5mL/kg on the last day of treatmentFourteen days | ↑ Increased scavenging effect as compared to DPPH, 92% at a concentration of 2mg/mLMore effective than the syntheticc oxidant used in the study69% and 90% increase in the scavenging effect on superoxide and peroxide ions respectively at a concentration of 1mg/mL↓ Significant decrease in oxidative potential of k3Fe(CN)6Potential use in cancer preventionInducer of drug detoxification mechanisms |
| Wang et al.93 | 2011 | Case–control | Flowers | Aqueous extraction | Diabetic nephropathy improvement through improved oxidative status and regulation of Akt/Bad/14-3-3 signalingγ | Twenty-five male rats induced diabetic nephropathy with STZ | Control: injection of 0.05M of citrateSTZ group and standard dietDiabetic rats with 100mg/kg/day HPDiabetic rats with 400mg/kg/day HPEight weeks | Hydropic change (impaired osmotic diuresis caused by hyperglycemia) in renal proximal tubular change improved↑ Significant increase in catalase and GSH activity and ↓ decreased lipid peroxidation↓ Decreased serum TGC, CHOL, and LDL levelsHDL only increased with the dose of 400mg/↑kg/day, which means that HS may have a promising effect in slowing metabolic syndrome in diabetesAt both doses, expression of Akt/Bad/14-3-3γ recovered after the decrease ↓ seen as compared to normal rats upon STZ injectionIt appears reasonable to assume that HS may induce recovery of Akt levels and the resultant signaling cascade through oxidative stress improvement in diabetic ratsHS has been shown to have a potential to attenuate the effects of diabetic neprhopathy by antioxidant and antiapoptotic mechanisms.The relationship of HS with the Akt parhway in diabetes and the mechanism of action have not been elucidated yet |
| Olatunji et al.68 | 2005 | Case–control | Petals | Aqueous extraction | Cholesterol-loweringCardioprotective effect | Thirty albino ratsn=6 | 1mg/kg/day or 1.5mg/kg/day of the red or green variety of 1HSTwenty-eight days | ↓ Significant decrease in plasma total cholesterol with 1.5mg/kg of both varieties↓ LDL decrease with both doses of both varietiesNo significant changes in HDL and TGC levels |
APP: acetaminophen; COL: high-fat diet; CTGF: connective tissue growth factor; DPPH: αdiphenyl-β-picrylhydrazyl; GSH: glutathione; HCD: high cholesterol diet; HDL: high density lipoprotein; HS: Hibiscus sabdariffa; ISS: saline solution; LDL: low density lipoprotein: MSG: monosodium glutamate; PCA: protocatechuic acid; BP: blood pressure; RAGE: receptor for advanced glycation end-products; SD: supplemental diet; STZ: streptozotocin injection; t-BHP: tert-butyl hydroperoxide; TGC: triglycerides; VLDL: very low density lipoprotein.
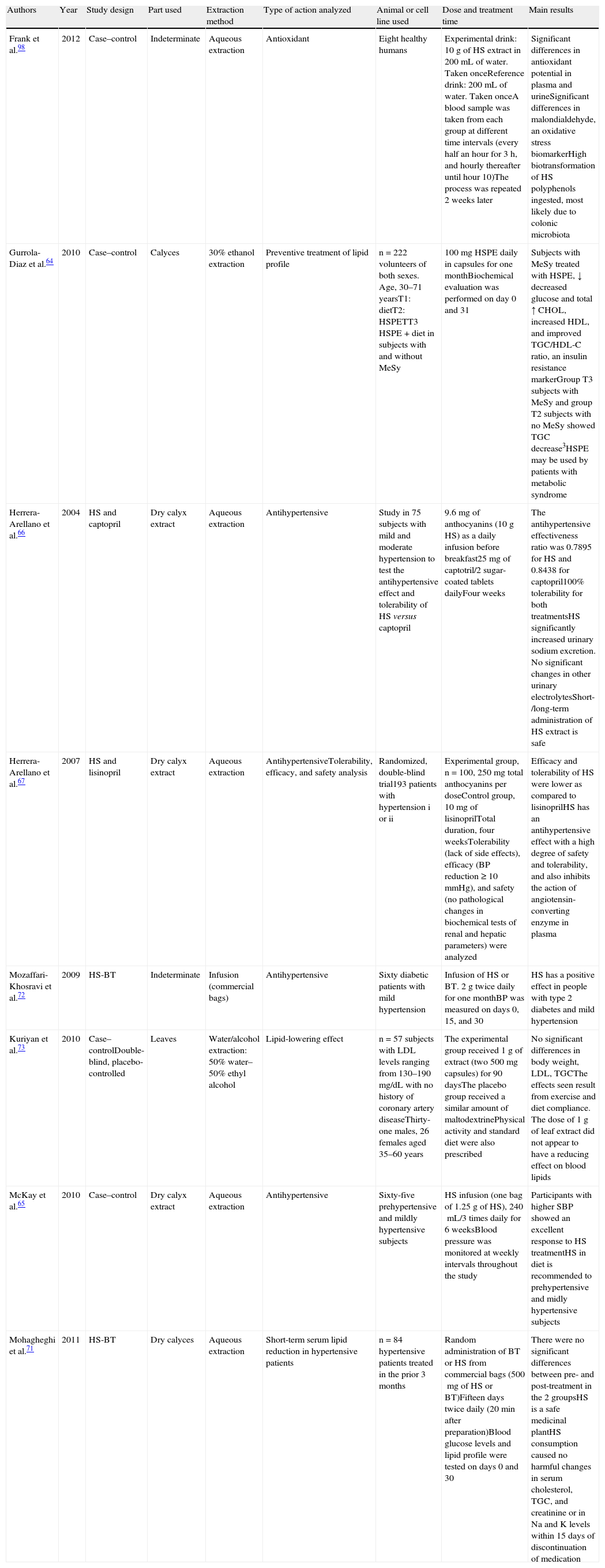
Studies in humans showing the different effects of intake of Hibiscus sabdariffa.
| Authors | Year | Study design | Part used | Extraction method | Type of action analyzed | Animal or cell line used | Dose and treatment time | Main results |
| Frank et al.98 | 2012 | Case–control | Indeterminate | Aqueous extraction | Antioxidant | Eight healthy humans | Experimental drink: 10g of HS extract in 200mL of water. Taken onceReference drink: 200mL of water. Taken onceA blood sample was taken from each group at different time intervals (every half an hour for 3h, and hourly thereafter until hour 10)The process was repeated 2 weeks later | Significant differences in antioxidant potential in plasma and urineSignificant differences in malondialdehyde, an oxidative stress biomarkerHigh biotransformation of HS polyphenols ingested, most likely due to colonic microbiota |
| Gurrola-Diaz et al.64 | 2010 | Case–control | Calyces | 30% ethanol extraction | Preventive treatment of lipid profile | n=222 volunteers of both sexes. Age, 30–71 yearsT1: dietT2: HSPETT3 HSPE+diet in subjects with and without MeSy | 100mg HSPE daily in capsules for one monthBiochemical evaluation was performed on day 0 and 31 | Subjects with MeSy treated with HSPE, ↓ decreased glucose and total ↑ CHOL, increased HDL, and improved TGC/HDL-C ratio, an insulin resistance markerGroup T3 subjects with MeSy and group T2 subjects with no MeSy showed TGC decrease3HSPE may be used by patients with metabolic syndrome |
| Herrera-Arellano et al.66 | 2004 | HS and captopril | Dry calyx extract | Aqueous extraction | Antihypertensive | Study in 75 subjects with mild and moderate hypertension to test the antihypertensive effect and tolerability of HS versus captopril | 9.6mg of anthocyanins (10g HS) as a daily infusion before breakfast25mg of captotril/2 sugar-coated tablets dailyFour weeks | The antihypertensive effectiveness ratio was 0.7895 for HS and 0.8438 for captopril100% tolerability for both treatmentsHS significantly increased urinary sodium excretion. No significant changes in other urinary electrolytesShort-/long-term administration of HS extract is safe |
| Herrera-Arellano et al.67 | 2007 | HS and lisinopril | Dry calyx extract | Aqueous extraction | AntihypertensiveTolerability, efficacy, and safety analysis | Randomized, double-blind trial193 patients with hypertension i or ii | Experimental group, n=100, 250mg total anthocyanins per doseControl group, 10mg of lisinoprilTotal duration, four weeksTolerability (lack of side effects), efficacy (BP reduction≥10mmHg), and safety (no pathological changes in biochemical tests of renal and hepatic parameters) were analyzed | Efficacy and tolerability of HS were lower as compared to lisinoprilHS has an antihypertensive effect with a high degree of safety and tolerability, and also inhibits the action of angiotensin-converting enzyme in plasma |
| Mozaffari-Khosravi et al.72 | 2009 | HS-BT | Indeterminate | Infusion (commercial bags) | Antihypertensive | Sixty diabetic patients with mild hypertension | Infusion of HS or BT. 2g twice daily for one monthBP was measured on days 0, 15, and 30 | HS has a positive effect in people with type 2 diabetes and mild hypertension |
| Kuriyan et al.73 | 2010 | Case–controlDouble-blind, placebo-controlled | Leaves | Water/alcohol extraction: 50% water–50% ethyl alcohol | Lipid-lowering effect | n=57 subjects with LDL levels ranging from 130–190mg/dL with no history of coronary artery diseaseThirty-one males, 26 females aged 35–60 years | The experimental group received 1g of extract (two 500mg capsules) for 90 daysThe placebo group received a similar amount of maltodextrinePhysical activity and standard diet were also prescribed | No significant differences in body weight, LDL, TGCThe effects seen result from exercise and diet compliance. The dose of 1g of leaf extract did not appear to have a reducing effect on blood lipids |
| McKay et al.65 | 2010 | Case–control | Dry calyx extract | Aqueous extraction | Antihypertensive | Sixty-five prehypertensive and mildly hypertensive subjects | HS infusion (one bag of 1.25g of HS), 240mL/3 times daily for 6 weeksBlood pressure was monitored at weekly intervals throughout the study | Participants with higher SBP showed an excellent response to HS treatmentHS in diet is recommended to prehypertensive and midly hypertensive subjects |
| Mohagheghi et al.71 | 2011 | HS-BT | Dry calyces | Aqueous extraction | Short-term serum lipid reduction in hypertensive patients | n=84 hypertensive patients treated in the prior 3 months | Random administration of BT or HS from commercial bags (500mg of HS or BT)Fifteen days twice daily (20min after preparation)Blood glucose levels and lipid profile were tested on days 0 and 30 | There were no significant differences between pre- and post-treatment in the 2 groupsHS is a safe medicinal plantHS consumption caused no harmful changes in serum cholesterol, TGC, and creatinine or in Na and K levels within 15 days of discontinuation of medication |
CHOL: cholesterol; HDL: high density lipoprotein; HS: Hibiscus sabdariffa; HSPE: powder extract; LDL: low density lipoprotein; MeSy: metabolic syndrome; BP: blood pressure; DBP: diastolic blood pressure; SBP: systolic blood pressure; TGC: triglycerides; BT: black tea; T1, T2, T3: treatment.
The part of the plant used and the extraction method are important because the different parts of H. sabdariffa, their color, the harvesting procedure, and the method used to extract its phytochemical components appear to have a great influence on the volatile composition of the extract, which has an influence on dosage.80 It should be noted, for instance, that a higher antioxidant capacity has been found in flowers harvested 35 days after maturation as compared to more immature flowers.81 The extraction of calyx components with ethanol promotes the antioxidant capacity of H. sabdariffa as compared to ethanol extraction from leaves or aqueous extraction from both parts.82 Aqueous extraction is, however, of the greatest interest when testing the effectiveness of the different parts of the plant because it is the traditional preparation procedure and is therefore of greater significance with regard to its implications for public health.66,67,71
Specifically, Table 1 shows studies conducted on different cell lines. Various cell lines were selected to conduct the experiments, ranging from rat hepatocytes,83,84 mouse macrophage cell lines RAW264.785 and J774A.1,86 and preadipocyte cell line 3T3-L187 to human leukemia cells HL-60.88 Most of these studies focused on the evaluation of the antioxidant capacity of H. sabdariffa derived from either the effect of its anthocyanin content,85,86,88 the action of protocatechuic acid,84,88 or the biological action of other H. sabdariffa molecules.83 Using cell lines of rat hepatocytes pretreated with the cytotoxic compound t-BHP, Tseng et al.83 showed the antioxidant effect (especially cytotoxic and genotoxic) of the different soluble fractions of H. sabdariffa through free radical removal and the inhibition of unscheduled DNA synthesis. The antioxidant effect of H. sabdariffa was mainly attributed to anthocyanins. The antiatherosclerotic capacity of anthocyanins in H. sabdariffa through the inhibition of LDL oxidation was analyzed by Kao et al.86 using mouse macrophages. Their objective was to assess anthocyanin action in foam cell formation and in the expression of the predominant receptor gene for oxidized LDL, CD36, and its transcription factor PPAR-gamma (peroxisome proliferator-activated receptor gamma). The authors showed that H. sabdariffa inhibits the absorption of oxidized LDL by macrophages by decreasing the expression of the CD36 receptor gene. Chang et al.85 similarly reported the in vitro role of anthocyanins in H. sabdariffa in the inhibition of LDL oxidation and, thus, in the prevention of atherosclerosis. Kim et al.87 analyzed the capacity of H. sabdariffa extract for the inhibition of adipocyte differentiation and its potential benefit in obesity prevention. They suggested that H. sabdariffa may inhibit adipogenesis through the inhibition of three different pathways: (1) the inhibition of adipogenic transcription factors C/EBPα (enhancer binding αprotein) and PPAR-gamma (peroxisome proliferator-activated receptor gamma), (2) the inhibition of PI3K (phosphoinositide 3-kinase) pathways, and (3) the inhibition of metabolic pathways associated with MAPK (map kinase). Treatment with H. sabdariffa extract during the adipogenic differentiation process showed a significant reduction in protein and mRNA expression of factors C/EBPα and PPAR-gamma. The authors also reported a decrease in the mRNA of leptin, a hormone regulating intake and energy expenditure and which is partially activated by C/EBPα at the transcriptional level. It should also be noted that the H. sabdariffa extract inhibited PI3K phosphorylation and expression during adipogenesis. Because of these results, the authors stated that H. sabdariffa is able to block the PI3K and MAPK signaling pathways and inhibit transcriptional factors during the early differentiation phases of adipocytes. Based on all of the foregoing, the authors postulated that H. sabdariffa extracts are beneficial for obesity prevention and may lead to body fat loss in vivo. To sum up, Table 1 clearly states the antioxidant effect of H. sabdariffa, mainly attributed to anthocyanins, which leads one to hypothesize about its capacity to take up OFRs and, among other properties, the inhibition of unscheduled DNA synthesis and the epigenetic changes which could be derived from them. These results open the way to continuing research in animal models and humans to validate the postulated hypotheses.
In the animal models in Table 2, healthy animals68,89–91 or animals previously manipulated to induce diabetes or atherosclerosis,69,92,93 hypercholesterolemia or hyperlipidemia,82,94 obesity,94,95 or high blood pressure70 to test the effects of H. sabdariffa consumption were used. Ajiboye et al.96 reported the potential of H. sabdariffa to take up the radicals di-phenyl-2,4,6-trinitrophenyl iminoazanium and 2,2-diphenyl-1-picrylhydrazyl at a dose of 2mg/mL of anthocyanin extract from H. sabdariffa, and to take up superoxide ion and hydrogen peroxide at a concentration of 1mg/mL. The antioxidant potential of H. sabdariffa was analyzed in blood and liver of albino rats. Liu et al.90 showed that doses of 200, 400, and 600mg/kg of aqueous extract of H. sabdariffa flowers decreased lipid peroxidation and increased catalase activity and GSH levels in plasma and liver tissue from mice. Ali et al.89 reported that doses of 200mg/kg of aqueous extract of calyces restored to normal the biochemical indices of liver damage caused by acetaminophen in mice, showing the capacity of H. sabdariffa to prevent liver damage. Olatunji et al.68 showed that chronic intake for 28 days of 1–1.5mg/kg/day of aqueous extract of H. sabdariffa petals significantly decreased plasma LDL levels in experimental rats. Only the 1.5mg/kg/day doses of aqueous extract of H. sabdariffa petals showed a significant decrease in plasma total cholesterol levels, with no significant changes in HDL or total triglyceride levels.68 No relevant changes were seen either in serum HDL levels after the use of H. sabdariffa in experiments conducted in models of hypercholesterolemia, hyperlipidemia, or induced obesity,63,69,94,97 but significant improvements occurred in total cholesterol, LDL and/or total triglyceride levels. In parallel, Hirunpanich et al.97 also showed its effectiveness at doses higher than 250mg/kg/day after 6 weeks of intake of aqueous extract of calyces. These same authors noted that doses higher than 1000mg/kg were not associated with greater effectiveness, which suggested saturation of the lipid-lowering effect. The authors showed the pharmacological dose of aqueous extract of calyces to range from 250–1000mg/kg/day. By contrast, Ochani and D’Mello82 and Wang et al.93 showed that doses of 500mg/kg/day of alcoholic extract of leaves and calyces and 400mg/kg/day of aqueous extract of flowers, respectively, not only decreased total cholesterol, LDL and total triglyceride levels in serum, but also increased HDL levels. In rat models with induced diabetes, alcoholic extracts of H. sabdariffa flowers or calyces caused a reduction of serum levels of total cholesterol, LDL and/or total triglycerides.69,92 So far, there have been no articles relating the use of H. sabdariffa to epigenetic changes. Further studies are therefore needed to understand the molecular mechanisms associated with the use of H. sabdariffa and disease pathogenesis, particularly in humans. Continuing with the study of the effects of H. sabdariffa on the regulation of energy and cell metabolism, Chen et al.79 worked with an atherogenic rabbit model to assess the antiatherosclerotic and lipid-lowering effect of H. sabdariffa extracts. Histologically, they showed that exposure for 10 weeks to doses of a 0.5–1%/diet of H. sabdariffa aqueous extract resulted in decreased foam cell formation and the inhibition of blood vessel calcification, as well as serum total cholesterol, LDL, and triglyceride levels. The authors concluded that the antiatherosclerotic activity of H. sabdariffa is related to the prevention of LDL oxidation in the arterial wall and that its consumption may be beneficial for decreasing disease incidence. Wang et al.93 emphasized antioxidative and antiapoptotic mechanisms as a potential explanation for decreases in total cholesterol, LDL, and total triglyceride levels. Lee et al.92 reported increased catalase and glutathione activities at doses of 200mg/kg and decreased lipid peroxidation as mechanisms for regulating plasma lipid levels. The results of animal studies show the antihypertensive and antioxidant effects of H. sabdariffa. However, the results on the effect of H. sabdariffa intake on cholesterol metabolism, and thus on the parameters of the associated diseases, are more variable. In this regard, the heterogeneity of the results in terms of HDL levels after the consumption of H. sabdariffa should be noted, because although HDL levels significantly increased with high doses of H. sabdariffa extract in a few studies, they did not usually change. A single conclusion is difficult to reach because of the high diversity in the methods used, the number of subjects used in each group, and the doses used in the different experimental protocols.
Table 3 shows the results of studies in humans. Observational studies with65 and without placebo,98 epidemiological, interventional, randomized case–control studies with73 and without placebo,66,67,72 and randomized studies are shown.64,71
The different studies assessed the antioxidant, lipid-lowering, and antihypertensive effects of H. sabdariffa in healthy subjects,98 patients with metabolic syndrome,64 diabetic patients with mild hypertension,72 prehypertensive and mildly hypertensive subjects,65,66,72 hypertensive patients previously given standard antihypertensive medication,66,67 and subjects with hyperlipidemia.73 Frank et al.,98 in a study on the antioxidant capacity of H. sabdariffa, administered 10g of H. sabdariffa as an infusion to an experimental group of eight healthy subjects. After intake, blood and urine were collected from the participants for 24h. The authors showed a significant decrease in malondialdehyde, an oxidative stress biomarker, and a marked increase in the antioxidant potential of human plasma and urine. They also found a significant increase in urinary excretion of hippuric acid, which showed a high biotransformation of the H. Sabdariffa polyphenols ingested and pointed to the role of colon microbiota in this transformation. Gurrola-Diaz et al.64 focused their study on the preventive and improving effects of H. sabdariffa on the lipid profile in subjects with or without metabolic syndrome. The most significant data from this study showed that subjects with evidence of metabolic syndrome and who had received experimental treatment with dry extracts of H. sabdariffa calyces had significant decreases in blood glucose and total cholesterol levels, increased HDL levels, and an improvement in the total triglycerides/HDL ratio, an insulin resistance marker. The authors showed that anthocyanins in H. sabdariffa could regulate adipocyte function, as postulated by Tsuda.99 Similarly, Mohagheghi et al.71 subsequently analyzed the short-term efficacy of extract from H. sabdariffa calyces on the reduction of serum glucose and lipid levels in hypertensive patients with a history of conventional antihypertensive treatment versus intake of the same amount of black tea. Increased levels of total cholesterol and lipoproteins bound to cholesterol (LDL and HDL) were seen with both treatments. Increases in total cholesterol and HDL levels as compared to baseline were significant in both cases. No changes harmful for health were seen in cholesterol, total triglycerides, serum creatinin, Na+, or K+ levels within 15 days of discontinuation of the medication. They, therefore, concluded that H. sabdariffa is a safe medicinal plant. Kuriyan et al.73 could not show the lipid-lowering effect of H. sabdariffa leaves in patients on a standard diet and physical exercise. The authors reported decreased body weight and LDL and total triglyceride levels in control and treated patients, and suggested that the results seen were secondary to exercise and diet.
As regards the antihypertensive effect of H. sabdariffa, McKay et al.65 showed that daily intake of three cups of tea of H. sabdariffa significantly decreased systolic pressure in prehypertensive and moderately hypertensive subjects. Ajay et al.100 showed that the antihypertensive effect with vasodilating effects and/or decreased heart rate of H. sabdariffa could be related to its anthocyanin-rich composition. Wahabi et al.,78 in a meta-analysis of the quality of studies conducted on the effectiveness of H. sabdariffa in the treatment of hypertension in prehypertensive or mildly hypertensive subjects, reported that no adequate scientific evidence was available to validate the antihypertensive properties of H. sabdariffa. In fact, these authors stated that they were studies of inadequate quality, short duration, and high methodological diversity, which did not allow them to adequately assess the potential adverse effects of continued H. sabdariffa intake.
The results given in the different tables suggest that H. sabdariffa has antioxidant potential and the capacity to attenuate high blood pressure and the diseases associated with obesity and diabetes such as nephropathy, atherosclerosis, and cardiovascular diseases. The diversity of the effects of H. sabdariffa seen in humans may be attributed to the different polyphenol concentrations in H. sabdariffa and other concomitant nutrients, to the solvents used for extraction, to the time and form of administration, as well as to the small number of studies available, methodological differences (e.g. the use of black tea as control instead of placebo), different population sizes, and inter-individual heterogeneity.
Finally, some authors suggest that H. sabdariffa extracts have a low grade of acute toxicity with a mean lethal dose (LD50) ranging from 2000 to more than 5000mg/kg/day.77,101 However, a possible adverse effect has been seen in the liver at high doses77 (Table 4). Akindahunsi and Olaleye102 reported the occurrence of liver toxicity with chronic intake of doses higher than 3000mg/kg of extract of H. sabdariffa calyces. Fakeye et al.103 pointed out that long-term consumption of high doses of flower extract may cause toxic reactions which may even be confused with episodes of chronic hepatitis. In fact, doses of 2000mg/kg/day for 90 days induced severe weight losses and diarrhea in their experimental animals. By contrast, experiments using doses ranging from 50 to 100mg/kg led to the hypothesis that ethanolic and aqueous fractions of extracts from H. sabdariffa flowers could have the potential to become pharmacological entities for stimulating immunity.104
Studies on HS toxicity and safety.
| Authors | Year | Study design | Part used | Extraction method | Type of action analyzed | Animal or cell line used | Dose and treatment time | Main results |
| Akindahunsi et al.102 | 2003 | Case–control | Calyces | Methanol–water (4:1) | Toxicity | Six groups, n=4, of Wistar albino rats | Group I: 0, physiological saline aloneG II: a dose of 250mg/gG III: 3 dosesG IV: 5 dosesG V: 10 dosesG VI: 15 dosesTreatment duration was not specified | AST and ↑ ALT significantly increased in all groups as compared to control groupHistopathological studies showed no pathological damage in liver and heart in any groupsLong-term administration of dose 15 may cause liver damageIt was concluded that although mean daily consumption of 150–180mg/kg appears safe, extract should be taken with caution because higher doses may induce liver damage |
| Fakeye104 | 2008 | Case–control | Flowers | Water–ethanol (50:50) | Study of the immunomodulatory properties and subacute toxicity profile of 2 fractions of alcohol-water extract of HS calyces | Male albino mice | Three groups, n=4, administered:50mg/kg, 100mg/kg of fraction soluble in ethyl acetate100mg/kg of the residual partGroup 4: controlSeven days | No toxicity was found with doses used, which showed immunostimulating activitiesFractions have an impressive immunostimulating activityThe residual water-soluble fraction caused a marked weight ↓ decrease as compared to the control groupBoth fractions caused a marked basophil ↓ decreaseThe residual aqueous fraction caused ↓ neutrophil decreaseBoth fractions were found to be susceptible to becoming drug entities |
| Fakeye et al.103 | 2009 | Case–control | Flowers | Water–ethanol (50:50) | Study of hematological, biochemical, and histopathological toxicity from oral intake of HS | Thirty-five albino rats. Divided into 7 groups, n=5 | Administration– 50:50 water/ethanol– 100% ethanol300mg/kg and 2000mg/kg/day of both extracts were administered. Ninety daysA seventh, control group. 2mL of water/day | Death of animals was preceded by severe weight loss, associated with diarrhea in animals with doses of 2000mg/kgAST activity improved with the administration of aqueous extract and 50% of ethanol, with a significant ↑ AST increase with higher dosesALT and creatinine levels were significantly affected by both extract types at different doses. A significant increase in creatinine levels was seen ↑ with the aqueous extract at higher dosesCholesterol levels were not significantly affected by extractsNo significant histopathological changes were seen↓ Decreased erythrocyte count. No leukocyte decreaseLong-term use of high doses causes ↑ increased liver enzyme levels, and their effects may be confused with chronic hepatitis, with no clear damage seen in hepatocytes |
AST: aspartate aminotransferase; ALT: alanine aminotransferase; HS: Hibiscus sabdariffa.
The traditional consumption of H. sabdariffa as an infusion has been related to different therapeutic properties. Specifically, the results of this review suggest that H. sabdariffa is able to take up free radicals inhibiting, for example, LDL oxidation. In addition, it appears that daily consumption of H. sabdariffa extract can significantly improve blood pressure in prehypertensive and mildly hypertensive patients and patients with type 2 diabetes. On the other hand, the use of H. sabdariffa can improve lipid profile, reducing serum levels of total cholesterol, LDL, and total triglycerides. Anthocyanins in H. sabdariffa have been shown to be able to inhibit LDL oxidation and possibly to decrease the risk of atherosclerosis. Although different studies have attempted to show the antioxidant, antihypertensive, and lipid-lowering effects of regular consumption of H. sabdariffa in humans and animal and cell models, the cellular, biological, and epigenetic mechanisms of the specific effects of H. sabdariffa have yet to be elucidated. The results published to date do not allow us to define any dose of H. sabdariffa with regard to its therapeutic properties. The lack of homogeneity in the experimental design of the different studies, as well as the low number of subjects and their heterogeneity, could be some of the causes of the lack of consistency in the results. However, the results represent interesting findings which should be thoroughly analyzed, because understanding of the distribution and function of polyphenols from H. sabdariffa in patients with different conditions may be helpful for achieving effective treatment. Continued research in the field of alternative non-drug treatments, such as functional foods, is also required. Only a limited number of H. sabdariffa extracts have been tested to date, and since the effects of different components are not equivalent, the results cannot be generalized. Future large scale studies with control of doses, active components, bioavailability, and other critical variables will therefore be crucial to provide the scientific evidence required to ascertain the efficacy of any therapeutic approach with H. sabdariffa and its doses.
Conflict of interestThe authors state that they have no conflicts of interest.
Please cite this article as: Guardiola S, Mach N. Potencial terapéutico del Hibiscus sabdariffa: una revisión de las evidencias científicas. Endocrinol Nutr. 2014;61:274–295.