The main objective was to determine whether ventilator-associated tracheobronchitis (VAT) is related to increased length of ICU stay. Secondary endpoints included prolongation of hospital stay, as well as, ICU and hospital mortality.
DesignA retrospective matched case-control study. Each case was matched with a control for duration of ventilation (±2 days until development of ventilator-associated tracheobronchitis), disease severity (Acute Physiology and Chronic Health Evaluation II) at admission ±3, diagnostic category and age ±10 years.
PatientsCritically ill adults admitted to a polyvalent 30-beds ICU with the diagnosis of VAT in the period 2013–2016.
Main resultsWe identified 76 cases of VAT admitted to our ICU during the study period. No adequate controls were found for 3 patients with VAT. There were no significant differences in demographic characteristics, reasons for admission and comorbidities. Patients with VAT had a longer ICU length of stay, median 22 days (14–35), compared to controls, median 15 days (8–27), p=0.02. Ventilator days were also significantly increased in VAT patients, median 18 (9–28) versus 9 days (5–16), p=0.03. There was no significant difference in total hospital length of stay 40 (28–61) vs. 35days (23–54), p=0.32; ICU mortality (20.5 vs. 31.5% p=0.13) and hospital mortality (30.1 vs. 43.8% p=0.09). We performed a subanalysis of patients with microbiologically proven VAT receiving adequate antimicrobial treatment and did not observe significant differences between cases and the corresponding controls.
ConclusionsVAT is associated with increased length of intensive care unit stay and longer duration of mechanical ventilation. This effect disappears when patients receive appropriate empirical treatment.
El objetivo primario fue determinar si la traqueobronquitis asociada a ventilación mecánica (TAV) está asociada con un aumento de estancia en UCI. Los objetivos secundarios incluyeron prolongación de estancia hospitalaria, así como mortalidad en UCI y hospitalaria.
DiseñoEstudio retrospectivo caso-control. Apareamos cada caso con un control en base a los siguientes criterios: periodo de VM al menos tan extenso, como el tiempo en que el caso desarrolla la TAV ± 2 días, gravedad evaluada por la escala APACHE II al ingreso en UCI, igual ± 3, igual motivo de ingreso del paciente, igual edad±10 años.
PacientesPacientes adultos ingresados en una UCI polivalente de 30 camas, con el diagnóstico de TAV en el periodo 2013-2016.
ResultadosIdentificamos 76 pacientes con TAV que ingresaron en UCI en el periodo de estudio. No se encontraron controles adecuados para 3 pacientes con TAV. No se encontraron diferencias significativas entre ambos grupos en cuanto a características demográficas, motivo de ingreso y comorbilidades. La estancia media en UCI de los pacientes con traqueobronquitis asociada a ventilación mecánica fue más prolongada en los casos que en los controles, mediana 22d (14-35), comparada con los controles mediana 15d (8-27), p=0,02. Los casos presentaron mayor número de días de VM respecto a los controles, mediana 18 días (9-28) vs. 9 días (5-16) p = 0,03. No encontramos diferencias significativas respecto a la estancia hospitalaria 40d (28-61) vs. 35d (23-54), p= 0,32; mortalidad en UCI (20,5 vs. 31,5% p=0,13) y mortalidad hospitalaria (30,1 vs. 43,8% p= 0,09). Realizamos un análisis del subgrupo de pacientes con TAV con documentación microbiológica y tratamiento empírico adecuado sin encontrar diferencias significativas en ninguno de los aspectos analizados.
ConclusionesLa TAV, prolonga los días de estancia en UCI y de ventilación mecánica. Este efecto desaparece cuando los pacientes reciben tratamiento empírico adecuado.
Mechanical ventilation (MV) is associated with lower respiratory tract infections which are a significant cause of morbidity and mortality in critically ill patients.1 In recent years, ventilator-associated tracheobronchitis (VAT) has roused the interest of clinicians and investigators alike due to being a relatively common entity that affects 1 in 10 patients receiving MV.2
However, although ventilator-associated pneumonia (VAP) is a nosocomial infection that has been subject to multiple studies which have analysed the impact thereof on morbidity and mortality3 in critically ill patients (attributable mortality of 13% according to a meta-analysis), there are few studies with controversial results in relation to the impact of VAT on the prognosis of these types of patients.4–6 Two of these studies were carried out in adult patients5,6 and the other in the paediatric population,4 but only one took into account the impact of adequate antibiotic treatment.6 Both studies in adult patients—one with a case-control design and the other a prospective cohort study—showed increased durations of ICU stay and mechanical ventilation among patients who developed VAT, with no significant differences among the patients who received adequate treatment in the study analysed.6 Neither of these two studies found an increase in mortality attributable to VAT. However, in the study carried out in the paediatric population, they were unable to show that patients who developed VAT presented significant differences with regard to mortality and mean stay, but they did demonstrate an increase in the duration of mechanical ventilation.4 An improved understanding of VAT may help us with prevention, early diagnosis and adequate treatment, although there are disputes regarding the need for the latter due to the absence of a uniform recommendation across all of the clinical practice guidelines.7,8
The crude mortality rate of VAT or the hospital stay generated as a result of the infection may be influenced by various factors such as previous diseases or a severe underlying condition. It is therefore necessary to employ a methodological approach that enables us to discern the effect of VAT on mortality and hospital stay. Better knowledge of the infection's impact on prognosis will enable us to define optimal endpoints and calculate sample sizes when designing studies on the prevention or treatment of VAT.9
In light of the above, we aimed to carry out this study with the primary endpoint of determining whether VAT leads to an increased duration of ICU stay which is attributable to the infection. The secondary endpoints included determining whether VAT prolongs the number of days on MV, the hospital stay and whether mortality may be attributed to the infection.
Material and methodsDesign: the study was carried out in a multipurpose, 30-bed adult ICU with no coronary admissions, at a university hospital. We conducted a retrospective case-control study (one case to one control), using a database where the details of all patients admitted to our unit are entered prospectively. The variables analysed in this study have thus been gathered prospectively. The study received the favourable opinion of the Independent Ethics Committee of the Hospital Universitario Virgen Macarena, exempting us from obtaining informed consent (Code 1132-N-17).
All patients diagnosed with VAT from January 2013 to December 2016 were potentially eligible. Diagnosis was performed by the patient's usual clinician. Two independent clinicians (MLCB and AAS) review all cases of nosocomial infection before inputting them in the ENVIN-HELICS database, where they are entered according to the definitions described in the user manual.10
A single investigator who had been blinded to the results (MLCB) matched each of the cases to another ICU patient with no VAT diagnosis and the following characteristics:
- •
A mechanical ventilation period of at least the same duration as the patient who developed VAT, ±2 days.
- •
Same severity on admission to the ICU, assessed according to the APACHE II scale, ±3 points.
- •
Same reason for admission, differentiated into medical patients, scheduled surgery patients and emergency surgery patients.
- •
Same age, with a variability range of ±10 years.
In order to calculate the APACHE II scale score, which was performed for all patients admitted onto the unit, the worst value of each variable analysed within the first 24h of admission was taken into account.
The comorbidities analysed were those entered in the ENVIN-HELICS database.
Patients who developed nosocomial pneumonia in the ICU, either before or after VAT, were excluded, regardless of whether it was related to MV or not. Likewise, control patients with pneumonia who presented the same characteristics were also excluded.6
In order to choose the controls, any patients entered in the database during the case selection period were evaluated first. However, if no controls were found in the study period, we conducted our search among patients included in the database between 2009 and 2012.
DefinitionsCase: VAT was defined as any patient with increased purulent discharge and at least two of the following criteria (a) Temp >38.5°C or <36.5°C not attributable to any other cause, (b) leucocyte count >12,000 or <4000 per mm3 and (c) absence of new infiltrates on the chest X-ray. All patients were exposed to the risk factor (MV) for at least 48h.11 At least one bronchoaspirate sample was obtained from each patient.
Microbiologically confirmed case: one which also presented quantitative bronchoaspirate with 106cfu/ml.6
Adequate antibiotic treatment: an active in vitro treatment against the isolated microorganism, administered within the first 24h of the VAT diagnosis. Antibiotics (ATB) were always given via the intravenous route, with no patients receiving nebulised treatment.12
This analysis is written in accordance with the recommendations from the STROBE guidelines.13
Statistical analysis: the descriptive statistical analysis was conducted using the SPSS program, version 22. Continuous variables are expressed as medians (25th–75th percentile) and the categorical variables as percentages. Statistical significance was determined using the Student's t-test and Mann–Whitney U test to compare continuous variables and the chi-square or Fisher's exact test when necessary for categorical variables. Statistical significance was set at p<0.05, and the results are expressed with their 95% confidence intervals.
ResultsDuring the study period, 5329 patients were admitted to the ICU, of which 67.29% (n=3586) received MV, lasting a median of two days (1–5). 76 episodes of VAT were identified, implying an incidence density of 5.79 VAT cases per 1000 days of MV. 73 cases were included in the study, since no compatible controls were found for three episodes. Of these, 67 (91.8%) were matched with controls from the study period, while the other six controls were matched with cases from the same database but which had been entered prior to the study (2009–2012). Most of the patients were post-surgical.
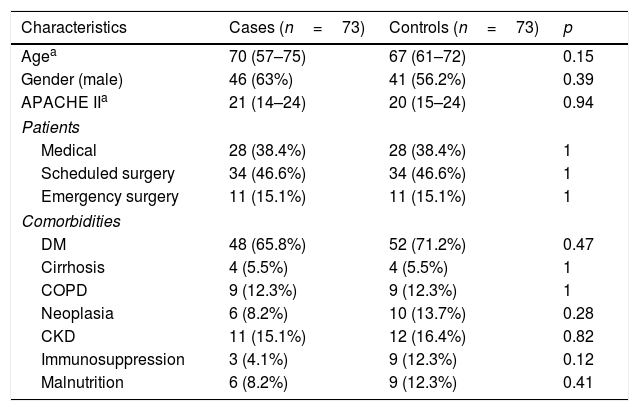
The demographic data of the cases and controls are shown in Table 1. No significant differences were found between the two groups as regards demographic characteristics, the reason for admission and comorbidities. All of the cases received antibiotic treatment. The median number of days using ATB was seven days (5–8).
Demographic characteristics and comorbidities of patients with VAT and their respective controls.
| Characteristics | Cases (n=73) | Controls (n=73) | p |
|---|---|---|---|
| Agea | 70 (57–75) | 67 (61–72) | 0.15 |
| Gender (male) | 46 (63%) | 41 (56.2%) | 0.39 |
| APACHE IIa | 21 (14–24) | 20 (15–24) | 0.94 |
| Patients | |||
| Medical | 28 (38.4%) | 28 (38.4%) | 1 |
| Scheduled surgery | 34 (46.6%) | 34 (46.6%) | 1 |
| Emergency surgery | 11 (15.1%) | 11 (15.1%) | 1 |
| Comorbidities | |||
| DM | 48 (65.8%) | 52 (71.2%) | 0.47 |
| Cirrhosis | 4 (5.5%) | 4 (5.5%) | 1 |
| COPD | 9 (12.3%) | 9 (12.3%) | 1 |
| Neoplasia | 6 (8.2%) | 10 (13.7%) | 0.28 |
| CKD | 11 (15.1%) | 12 (16.4%) | 0.82 |
| Immunosuppression | 3 (4.1%) | 9 (12.3%) | 0.12 |
| Malnutrition | 6 (8.2%) | 9 (12.3%) | 0.41 |
CKD: chronic kidney disease; COPD: chronic obstructive pulmonary disease; DM: diabetes mellitus.
The interval between admission to the ICU and the onset of VAT was a median of five days (3–9).
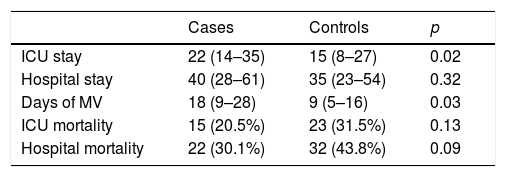
Of the 73 cases, 15 died in the ICU, corresponding to a crude mortality rate of 20.5% (95% CI 11–30). Among the controls, meanwhile, 23 died, resulting in a crude mortality of 31.5% (95% CI 21–42) (p=0.13). Crude hospital mortality was 30.1% (95% CI 19–41) for the cases and 43.8% (95% CI 32–55) for the controls (p=0.09). The mean ICU stay among the survivors was longer in the cases than in the controls, with no significant differences found in the length of hospital stay (Table 2). The cases presented a greater number of days of MV in relation to the controls, with a median of 18 days (9–28) vs. 9 days (5–16), p=0.03.
Mean stay, crude mortality and days of MV in patients with VAT and their respective controls.
| Cases | Controls | p | |
|---|---|---|---|
| ICU stay | 22 (14–35) | 15 (8–27) | 0.02 |
| Hospital stay | 40 (28–61) | 35 (23–54) | 0.32 |
| Days of MV | 18 (9–28) | 9 (5–16) | 0.03 |
| ICU mortality | 15 (20.5%) | 23 (31.5%) | 0.13 |
| Hospital mortality | 22 (30.1%) | 32 (43.8%) | 0.09 |
ICU: intensive care unit; MV: mechanical ventilation.
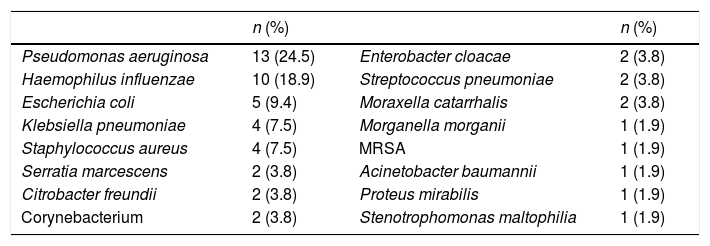
Fifty-three of the VAT episodes were confirmed microbiologically. All of the VAT infections were monomicrobial, with the most commonly isolated bacteria being Pseudomonas aeruginosa (P. aeruginosa; 24.5%) and Haemophilus influenzae (18.9%), as shown in Table 3. None were associated with bacteraemia and antibiotic treatment was adequate in 69.8% of the episodes.
Most common bacteria in VAT.
| n (%) | n (%) | ||
|---|---|---|---|
| Pseudomonas aeruginosa | 13 (24.5) | Enterobacter cloacae | 2 (3.8) |
| Haemophilus influenzae | 10 (18.9) | Streptococcus pneumoniae | 2 (3.8) |
| Escherichia coli | 5 (9.4) | Moraxella catarrhalis | 2 (3.8) |
| Klebsiella pneumoniae | 4 (7.5) | Morganella morganii | 1 (1.9) |
| Staphylococcus aureus | 4 (7.5) | MRSA | 1 (1.9) |
| Serratia marcescens | 2 (3.8) | Acinetobacter baumannii | 1 (1.9) |
| Citrobacter freundii | 2 (3.8) | Proteus mirabilis | 1 (1.9) |
| Corynebacterium | 2 (3.8) | Stenotrophomonas maltophilia | 1 (1.9) |
MRSA: methicillin-resistant Staphylococcus aureus.
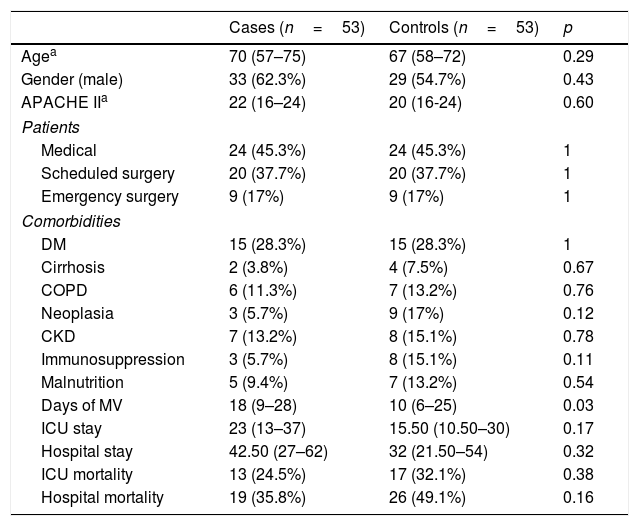
We performed a subgroup analysis on patients with VAT and microbiological documentation as well as on those with VAT, microbiological documentation and adequate treatment within the first 24h. The results are shown in Tables 4 and 5.
Patients with VAT and microbiological documentation.
| Cases (n=53) | Controls (n=53) | p | |
|---|---|---|---|
| Agea | 70 (57–75) | 67 (58–72) | 0.29 |
| Gender (male) | 33 (62.3%) | 29 (54.7%) | 0.43 |
| APACHE IIa | 22 (16–24) | 20 (16-24) | 0.60 |
| Patients | |||
| Medical | 24 (45.3%) | 24 (45.3%) | 1 |
| Scheduled surgery | 20 (37.7%) | 20 (37.7%) | 1 |
| Emergency surgery | 9 (17%) | 9 (17%) | 1 |
| Comorbidities | |||
| DM | 15 (28.3%) | 15 (28.3%) | 1 |
| Cirrhosis | 2 (3.8%) | 4 (7.5%) | 0.67 |
| COPD | 6 (11.3%) | 7 (13.2%) | 0.76 |
| Neoplasia | 3 (5.7%) | 9 (17%) | 0.12 |
| CKD | 7 (13.2%) | 8 (15.1%) | 0.78 |
| Immunosuppression | 3 (5.7%) | 8 (15.1%) | 0.11 |
| Malnutrition | 5 (9.4%) | 7 (13.2%) | 0.54 |
| Days of MV | 18 (9–28) | 10 (6–25) | 0.03 |
| ICU stay | 23 (13–37) | 15.50 (10.50–30) | 0.17 |
| Hospital stay | 42.50 (27–62) | 32 (21.50–54) | 0.32 |
| ICU mortality | 13 (24.5%) | 17 (32.1%) | 0.38 |
| Hospital mortality | 19 (35.8%) | 26 (49.1%) | 0.16 |
CKD: chronic kidney disease; COPD: chronic obstructive pulmonary disease; DM: diabetes mellitus; ICU: Intensive Care Unit; MV: mechanical ventilation.
VAT with microbiological documentation and adequate treatment.
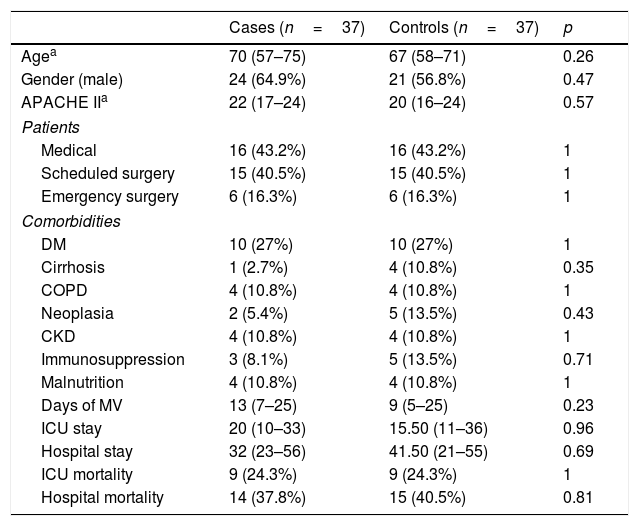
| Cases (n=37) | Controls (n=37) | p | |
|---|---|---|---|
| Agea | 70 (57–75) | 67 (58–71) | 0.26 |
| Gender (male) | 24 (64.9%) | 21 (56.8%) | 0.47 |
| APACHE IIa | 22 (17–24) | 20 (16–24) | 0.57 |
| Patients | |||
| Medical | 16 (43.2%) | 16 (43.2%) | 1 |
| Scheduled surgery | 15 (40.5%) | 15 (40.5%) | 1 |
| Emergency surgery | 6 (16.3%) | 6 (16.3%) | 1 |
| Comorbidities | |||
| DM | 10 (27%) | 10 (27%) | 1 |
| Cirrhosis | 1 (2.7%) | 4 (10.8%) | 0.35 |
| COPD | 4 (10.8%) | 4 (10.8%) | 1 |
| Neoplasia | 2 (5.4%) | 5 (13.5%) | 0.43 |
| CKD | 4 (10.8%) | 4 (10.8%) | 1 |
| Immunosuppression | 3 (8.1%) | 5 (13.5%) | 0.71 |
| Malnutrition | 4 (10.8%) | 4 (10.8%) | 1 |
| Days of MV | 13 (7–25) | 9 (5–25) | 0.23 |
| ICU stay | 20 (10–33) | 15.50 (11–36) | 0.96 |
| Hospital stay | 32 (23–56) | 41.50 (21–55) | 0.69 |
| ICU mortality | 9 (24.3%) | 9 (24.3%) | 1 |
| Hospital mortality | 14 (37.8%) | 15 (40.5%) | 0.81 |
CKD: chronic kidney disease; COPD: chronic obstructive pulmonary disease; DM: diabetes mellitus; ICU: intensive care unit; MV: mechanical ventilation.
In the first of the sub-groups analysed (Table 4), a greater number of days of MV was maintained in the cases compared to the controls, with a median of 18 days (9–28) vs. 10 days (6–25); p=0.03. Conversely, there were no significant findings with respect to the ICU or hospital stay, or mortality.
In the sub-group of patients with microbiological documentation and adequate empirical treatment (Table 5), no differences were found in any of the endpoints analysed. In other words, we observed no increase in stay or days of MV, and mortality was similar in both the cases and controls.
DiscussionThe primary endpoint of our study was to determine whether VAT leads to an increased duration of ICU stay which is attributable to the infection, and the main finding is that VAT is indeed associated with a longer ICU stay. However, it should be noted that there are no significant differences regarding stay in patients who received adequate empirical antibiotic treatment. Similarly, it has been observed that patients who develop an episode of VAT spend more days on MV, and this was maintained in the sub-group of patients with microbiological documentation, but not in those who had microbiological documentation and adequate antibiotic treatment. VAT has no impact on ICU or hospital mortality.
Our data on the lack of VAT-attributable mortality conflict with the perception of intensive care physicians. Thus, in an international survey, half of the 288 participants believe that VAT increases the risk of death. Likewise, almost all of the survey respondents felt that VAT prolongs ICU stay and the duration of MV.14
These data have been demonstrated in a number of previous studies. For example, Karvouniaris et al.,5 in a prospective observational study, showed that patients with VAT spent longer in the ICU, with no impact on mortality. Moreover, in a case-control study, Nseir et al.6 discovered that VAT was associated with increased durations of MV and ICU stay, but no differences were found in the results of patients receiving adequate antibiotic treatment.
There are no differences with regard to other studies15 concerning the most commonly isolated microorganism: P. aeruginosa. However, we did find a greater proportion of adequate treatments, amounting to 69.8% in our case versus the 47% described by Nseir et al.16 in a prospective multicentre observational study evaluating the impact of appropriate antibiotic treatment on the transition from VAT to VAP.
Other data found in the study show that the onset of VAT occurs somewhat earlier (median of five days [3–9] from admission to the ICU) in relation to the timings described by Karvouniaris et al.5 and Martín-Loeches et al.,15 which show that VAT appears 6–8 days after admission to the ICU.
When we analysed the sub-group of patients with microbiological documentation and adequate empirical treatment, we found no differences in any of the endpoints analysed. This could be related to the effects of adequate antibiotic treatment on the patients’ stay and days of MV. However, if we carry out a literature review, we will encounter several disputes, including the recommendation against antibiotic treatment in cases of VAT, as published in the IDSA guidelines7 in 2016. The authors’ reasons are based on the fact that, after analysing the results of a randomised, controlled study17 and various observational studies,6,18,19 they only found one favourable effect—fewer days of MV—versus the possibility of multiple adverse effects such as increased costs, the development of antibiotic resistance and an increased number of Clostridium difficile infections. This contrasts somewhat with the reality of standard practice, where the high prevalence of antibiotic treatment in this entity is highlighted in various publications, reaching 50–100%, according to the series.14–16 Our data indicate that we should treat episodes of VAT in an early and adequate manner, since this means that developing VAT will not prolong the ICU stay or days of MV.
Our study has various limitations: firstly, it is a retrospective study over a relatively long number of years in which we cannot rule out the presence of bias. Despite this, 8.2% of the controls had to be chosen from previous years. Secondly, the sample is small, which could affect the statistical significance of some variables. Thirdly, a high percentage of VAT cases progress to VAP. Since our endpoint was to analyse the clinical impact of VAT on patients who did not develop VAP, we ruled out all cases of VAT that progressed to VAP. This enabled us to specifically assess VAT, whilst also taking into account that the transition between one and another infectious process is a continuum.15,16
In contrast, we also want to highlight the study's strengths. All of the variables were collected prospectively and the strict matching criteria used—including severity on admission, the main determinant of mortality—ensured that the cases represented the same population as the control patients.
Finally, we managed to find suitable controls for a high number of VAT cases, using strict matching criteria which enabled us to assert that both populations are similar and to determine the exact effect developing VAT has on prognosis.
VAT is a common infectious complication associated with mechanical ventilation. This study enables us to show its impact on ICU patients, which is related to increased durations of mechanical ventilation and stay, implying further increases in hospital resources and costs. Our data indicate that adequate empirical treatment could be beneficial since episodes treated adequately within the first 24h are not associated with a significantly longer ICU stay or MV duration. Future well-designed clinical trials are completely justified in order to confirm this hypothesis.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Cantón-Bulnes ML, González-García MA, García-Sánchez M, Arenzana-Seisdedos Á, Garnacho-Montero J. Estudio caso-control del impacto clínico de la traqueobronquitis asociada a la ventilación mecánica en pacientes adultos, que no desarrollan neumonía asociada a ventilación mecánica. Enferm Infecc Microbiol Clin. 2019;37:31–35.