Healthcare associated infections (HAIs) in neonatal units are increasing due to advances in invasive therapeutic and diagnostic procedures and increased survival of preterm babies being bloodstream infections (BSIs) one of the most common type of HAIs.1,2 The drawing of paired blood samples from the catheter and a peripheral vein is considered the best approach to reduce suspicion of colonization of the device, contamination of the sample or true bloodstream infection caused by common commensal microorganisms.3
Current guidelines for the diagnosis of catheter-related bloodstream infections (CRBSI) recommend culture of catheters only when clinical suspicion are present to avoid unnecessary antimicrobial treatment.4 Catheter colonization can be diagnosed either by rolling the catheter tip on the agar surface (Maki's roll-plate technique, MT) or by sonicating the distal 4–5cm of the catheter.5 However, MT is not fully reliable in the case of silicone neonatal peripherally inserted central catheter (SN-PICC) tips based on the study of Martín-Rabadan et al. due to the low capacity of this method for microbial detection inside catheters. Culture results may improve after catheter fragmentation.6 In fact, the new protocol of ‘Microbiological diagnosis of infections associated with intravascular catheters’ elaborated by the Spanish Society of Infectious Diseases and Clinical Microbiology (SEIMC) includes roll-plate after slicing (RPS) as a good option in CRBSI diagnosis.7
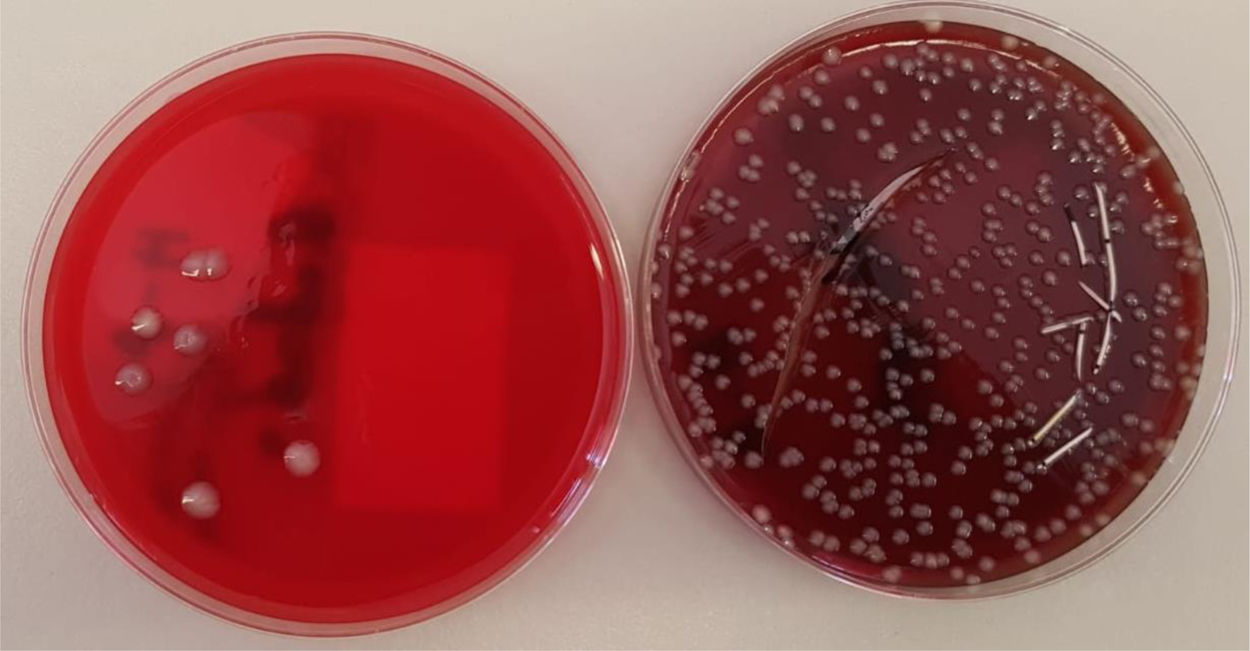
Our objective was to compare the yield of MT and RPS for the detection of colonization and CRBSI. For this purpose, tips of neonatal catheters, all of them SN-PICCs, were prospectively cultured in the Hospital Miguel Servet of Zaragoza for 2 years (January 1, 2017 to December 31, 2018). Once the MT was performed, the catheter was opened longitudinally and the contents were extended with swab, leaving the catheter in the culture plate. They were incubated 4 days, issuing a preliminary report at 48h. In all cases, culture was carried out for Malassezia furfur, adding a drop of olive oil in the initial area of the seed preventing it from spreading.
It was considered that the catheter was colonized when either of the two methods found ≥15 colony forming units (CFU). CRBSI was defined when the same microorganism was isolated in the catheter culture and peripheral blood cultures, without another source of infection.
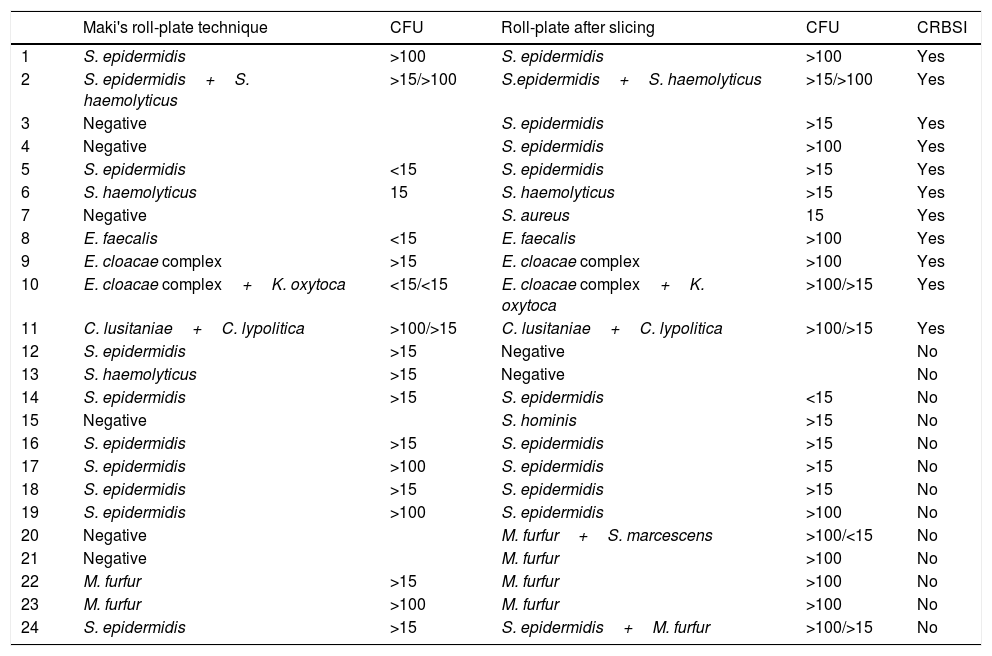
During the study period, 79 sPICCS were processed. The number of colonized catheters was 24 (30.4%), with TM detected 15/24 and with RPS 21/24 (p=0.146). The microorganisms isolated in colonized catheters were 18 Gram positive, 4 Gram-negative and 7 yeast, 5 of them M. furfur. BRC was diagnosed in 11 patients (13.9%), in 6 of them the diagnosis was made only with RPS (p=0.031). Isolated microorganisms are shown in Table 1, in which the CFUs are expressed as: negative, >15 when the CFUs are between 15–100 and >100.
Isolated microorganisms using MT and RPS.
| Maki's roll-plate technique | CFU | Roll-plate after slicing | CFU | CRBSI | |
|---|---|---|---|---|---|
| 1 | S. epidermidis | >100 | S. epidermidis | >100 | Yes |
| 2 | S. epidermidis+S. haemolyticus | >15/>100 | S.epidermidis+S. haemolyticus | >15/>100 | Yes |
| 3 | Negative | S. epidermidis | >15 | Yes | |
| 4 | Negative | S. epidermidis | >100 | Yes | |
| 5 | S. epidermidis | <15 | S. epidermidis | >15 | Yes |
| 6 | S. haemolyticus | 15 | S. haemolyticus | >15 | Yes |
| 7 | Negative | S. aureus | 15 | Yes | |
| 8 | E. faecalis | <15 | E. faecalis | >100 | Yes |
| 9 | E. cloacae complex | >15 | E. cloacae complex | >100 | Yes |
| 10 | E. cloacae complex+K. oxytoca | <15/<15 | E. cloacae complex+K. oxytoca | >100/>15 | Yes |
| 11 | C. lusitaniae+C. lypolitica | >100/>15 | C. lusitaniae+C. lypolitica | >100/>15 | Yes |
| 12 | S. epidermidis | >15 | Negative | No | |
| 13 | S. haemolyticus | >15 | Negative | No | |
| 14 | S. epidermidis | >15 | S. epidermidis | <15 | No |
| 15 | Negative | S. hominis | >15 | No | |
| 16 | S. epidermidis | >15 | S. epidermidis | >15 | No |
| 17 | S. epidermidis | >100 | S. epidermidis | >15 | No |
| 18 | S. epidermidis | >15 | S. epidermidis | >15 | No |
| 19 | S. epidermidis | >100 | S. epidermidis | >100 | No |
| 20 | Negative | M. furfur+S. marcescens | >100/<15 | No | |
| 21 | Negative | M. furfur | >100 | No | |
| 22 | M. furfur | >15 | M. furfur | >100 | No |
| 23 | M. furfur | >100 | M. furfur | >100 | No |
| 24 | S. epidermidis | >15 | S. epidermidis+M. furfur | >100/>15 | No |
In our hospital, CRBSI is an important cause of nosocomial infection. For the diagnosis of colonization and CRBSI, RPS presented significantly higher returns than MT, so the data suggests that the diagnosis of CRBSI would be improved with this new technique being interesting to use RPS routinely. Currently, in our laboratory the two techniques are being carried out in parallel to increase the sample size and obtain stronger conclusions (Fig. 1).
The authors have no conflict of interest or source of funding.