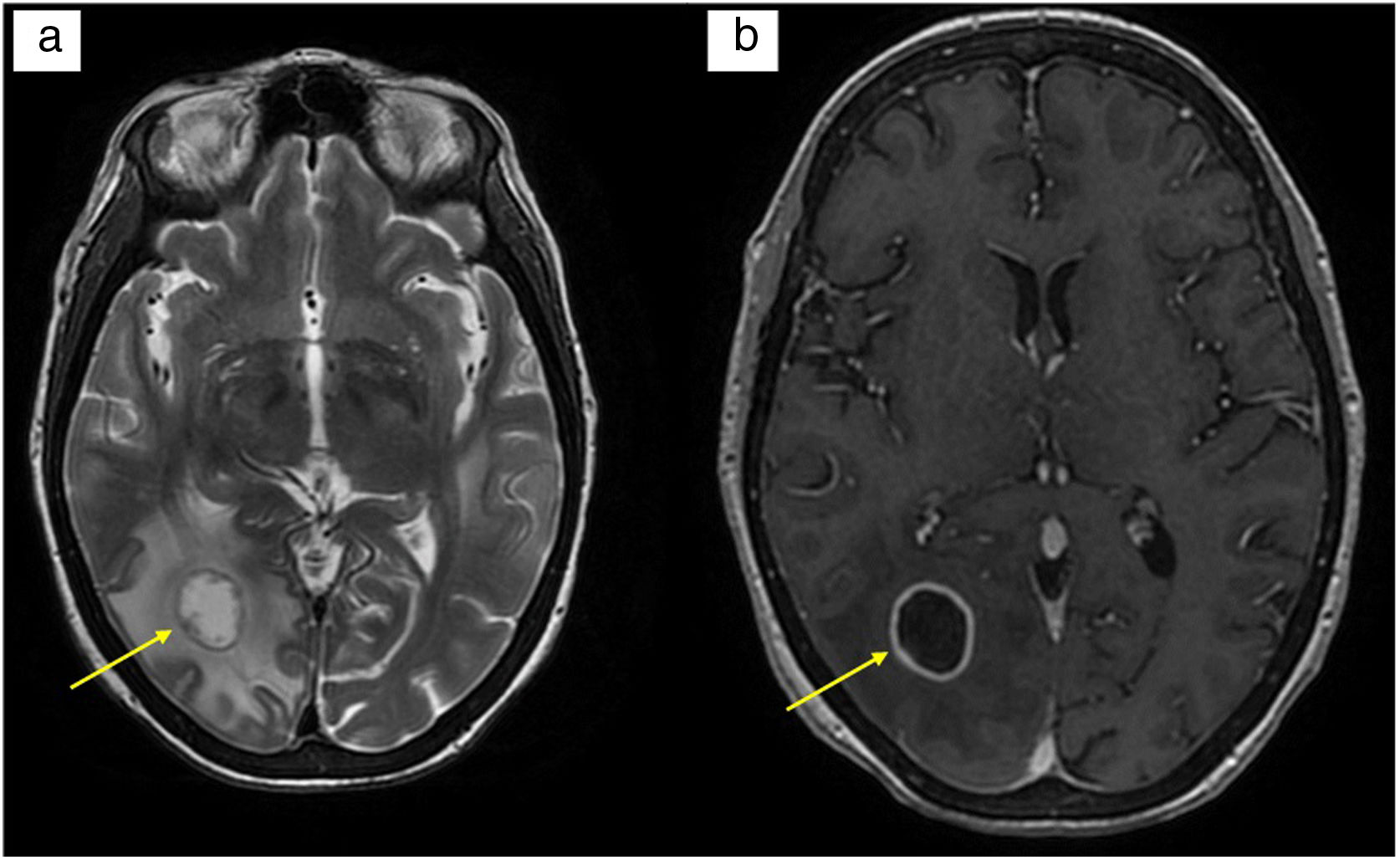
We present the case of a 56-year-old woman with portal hypertension (PHT) secondary to echinococcosis of the liver, who underwent surgery, and with post-operative Budd-Chiari syndrome. She underwent a liver transplant (LT), suffered primary graft dysfunction and required liver retransplantation three days later. Her clinical course during resuscitation was unfavourable, with a multitude of complications: acute renal failure requiring renal replacement therapy, pneumothorax, metabolic uraemic encephalopathy with neurological repercussions (normal CT), septic shock, critical illness myopathy and hyperbilirubinaemia. Treatment was started with mycophenolate, methylprednisolone, basiliximab, tacrolimus and ganciclovir (until the receptor serology was known). After several days on the liver disease ward, the patient developed neurological symptoms in the form of tetraparesis, oculocephalic reflex to the right and seizures. A brain scan was performed, which showed a right parieto-occipital hypodensity suggestive of acute/subacute ischaemia, with suspected reversible posterior leukoencephalopathy syndrome associated with tacrolimus. The brain MRI revealed three intra-axial lesions of probable infectious aetiology, the largest suggestive of fungal abscess (Fig. 1). Other alternatives, including pyogenic abscess, primary neoplasia and toxoplasmosis, were considered. Empirical antifungal and antibiotic therapy with meropenem, linezolid and liposomal amphotericin B was started.
(a) T2-weighted brain MRI: rounded lesion of layered appearance in the white matter of the right occipital lobe measuring 1.7 cm in diameter. Moderate surrounding vasogenic oedema; (b) T1-weighted brain MRI with contrast: homogeneous, smooth and fine annular peripheral enhancement following administration of intravenous contrast.
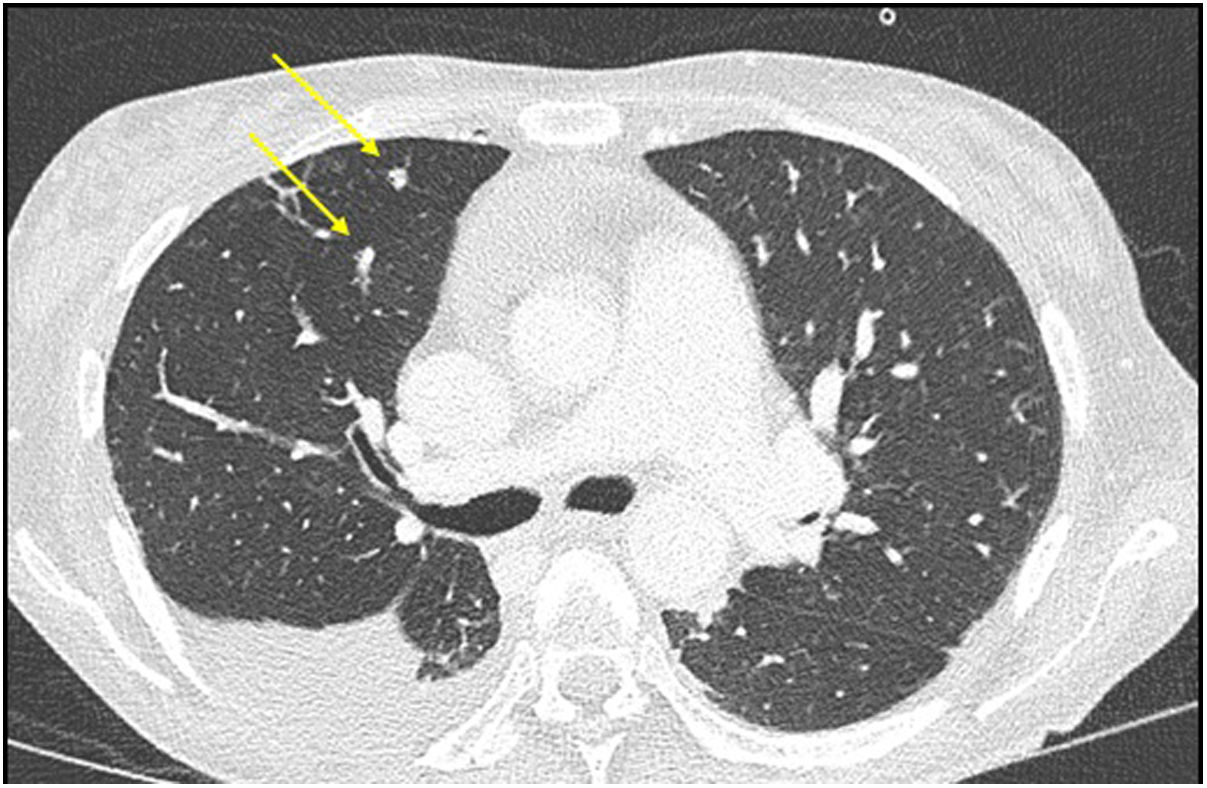
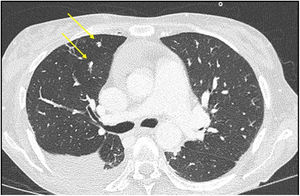
The study was concluded with a full-body scan, which revealed large hepatic collections and multiple lung micronodules of probable infectious aetiology (Fig. 2). A transoesophageal echocardiogram was performed, which identified a linear mass in the aortic valve consistent with endocarditic vegetation.
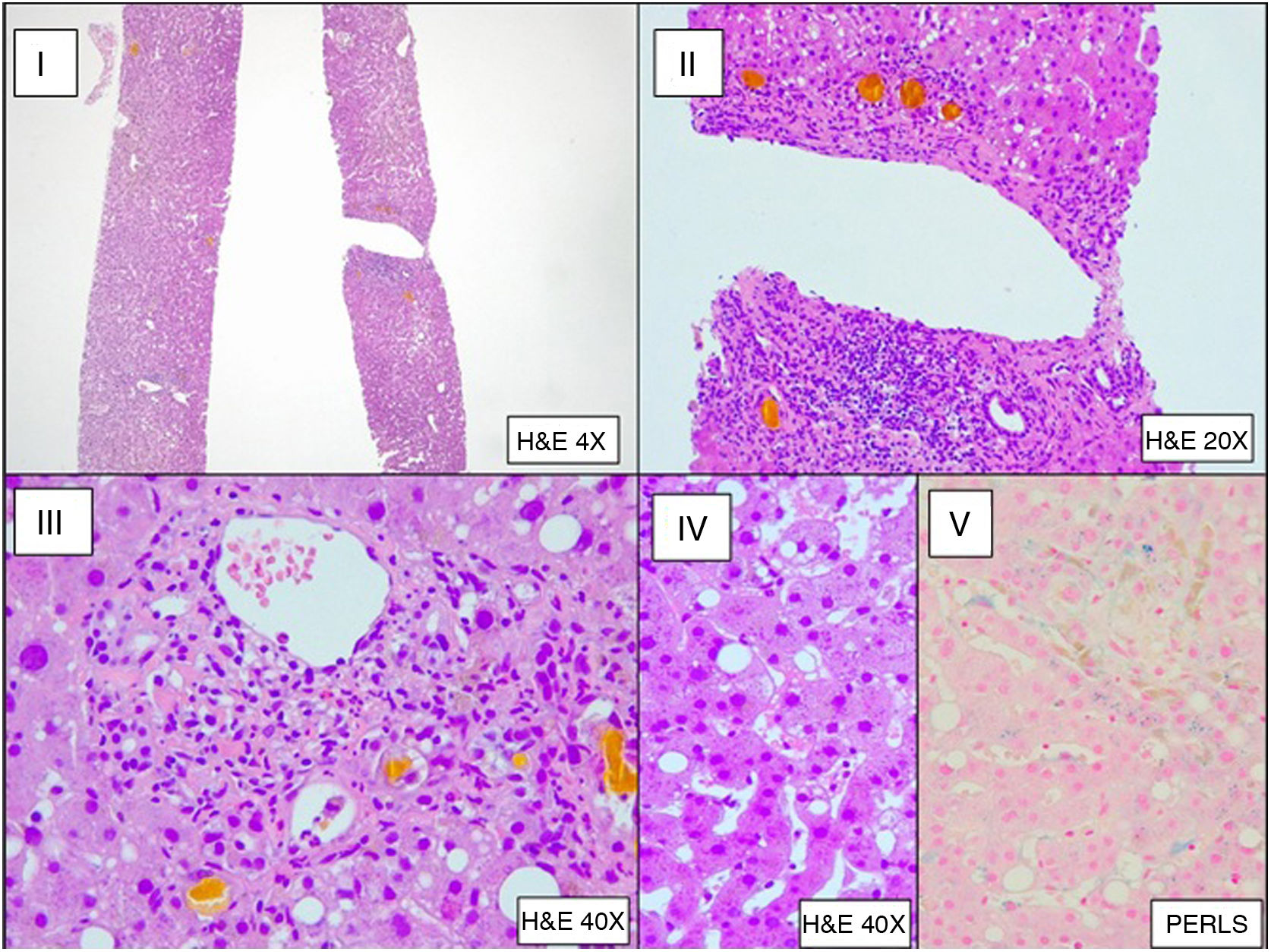
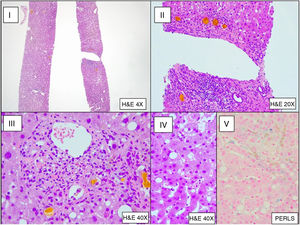
Bronchoalveolar lavage (BAL) was performed, which upon direct examination revealed fungal structures. A subsequent culture was positive for Aspergillus fumigatus (800 CFU/mL). Galactomannan testing in CSF and BAL was positive (index of 1.117 in CSF and >3.000 in BAL) and negative in serum. The liver biopsy showed histological changes secondary to sepsis (Fig. 3). The patient was diagnosed with invasive aspergillosis.
Clinical courseAfter several days being treated in hospital, the patient was discharged under the supervision of the home hospitalisation unit (HHU) with amphotericin 180 mg/24 h/for six weeks and voriconazole 200 mg/12 h/for 12 weeks, as well as immunosuppressive therapy with mycophenolate and tacrolimus. The patient responded satisfactorily to treatment and exhibited no infection sequelae.
Final comment/discussionLT entails an immunocompromised state that can predispose patients to opportunistic infections. Aspergillus fumigatus may be uncommon, but it is still a significant cause of morbidity and mortality (33–100%) in this context1. The case study presented above concerns Aspergillus fumigatus infection in the form of invasive aspergillosis in a female liver transplant patient. Clinical signs and symptoms usually manifest between week 2 and week 6 post-LT. The infection is nosocomial and normally affects patients with associated risk factors2. These include kidney failure requiring dialysis or continuous ultrafiltration, retransplantation (except if performed in the first seven days) and severe initial graft dysfunction (ALT > 2.000 U/l and Quick <40%) that does not normalise by day 5. Our patient required liver retransplantation and experienced post-transplant acute renal failure. The suspected diagnosis was clinical and radiological, which had to be confirmed using diagnostic biomarkers. Non-invasive biomarkers include galactomannan and beta-d-glucan in serum or sputum culture. Galactomannan tends to be negative in LT but positive in neutropaenic patients. Invasive tests comprise bronchoscopy with bronchoalveolar lavage (BAL) or lung biopsy3. Azoles, such as voriconazole or isavuconazole, are one of the first-line treatments. In severe cases, combination therapy with echinocandin is recommended for the first two weeks4. This is a rare but very serious complication that should be ruled out at the slightest suspicion due to its potentially high morbidity and mortality rates. Although there is a lack of consensus regarding the administration of universal prophylactic treatment5, it could certainly be beneficial in patients with the aforementioned risk factors6.
Please cite this article as: Mínguez Sabater A, Vinaixa Aunes C, Ladrón Abia P, Prieto Castillo M. Abscesos cerebrales en el postrasplante hepático precoz. Enferm Infecc Microbiol Clin. 2021;39:518–520.