Pseudomonas aeruginosa displays resistance to several available antibiotics. Infections caused by this pathogen are associated with a high mortality, morbidity, and considerable healthcare resource utilization and costs. This study was aimed at describing the use of ceftolozane/tazobactam (C/T) for the treatment of patients with P. aeruginosa infections.
MethodsCase series analysis of hospitalized patients treated with C/T for P. aeruginosa infections in five public Portuguese hospitals. Patients presenting with infections caused by this pathogen and receiving C/T for at least 72h during hospitalization were eligible.
ResultsSixty-four hospitalized patients with P. aeruginosa infections treated with C/T were evaluated between December 2016 and July 2019. Most patients were aged between 60 and 79 years (53.9%). Patients presented a total of 68 P. aeruginosa infections, with respiratory infections being the most common (28.1%, 18 out of 64). Most P. aeruginosa strains (85.9%, 55 out of 64) were extensively drug-resistant (XDR). C/T was mostly used as targeted therapy (98.4%, 63 out of 64 patients) and as monotherapy (72.7%, 47 out of 64 patients). Combination therapy was used in 47.4% (9 out of 19) of patients with bacteriemia. Most patients had successful microbiological (79.2%, 42 out of 53) and clinical (78.7%, 48 out of 61) outcomes. All-cause in-hospital mortality rate was 34.4%.
ConclusionThe present case series contributes to the body of evidence suggesting that C/T is an effective and safe option for treating P. aeruginosa infections, namely those caused by XDR strains, both when used as mono- or combination therapy.
Pseudomonas aeruginosa presenta mecanismos de resistencia a diversos antibióticos. Las infecciones causadas por este patógeno aumentan la morbimortalidad, el uso de recursos y los costos asociados. Este estudio tuvo como objetivo describir el uso de ceftolozano/tazobactam (C/T) para el tratamiento de pacientes con infecciones por P. aeruginosa.
MétodosSerie de casos de pacientes hospitalizados con infecciones por P. aeruginosa que fueron tratados con C/T en 5 hospitales portugueses. Fueron incluidos pacientes que presentaban infecciones por este patógeno y recibían terapéutica con C/T durante al menos 72 horas.
ResultadosEntre diciembre de 2016 y julio de 2019 fueron analizados 64 pacientes hospitalizados con infecciones por P. aeruginosa tratados con C/T. La mayoría tenían entre 60 y 79 años (53,9%). Se aislaron 68 infecciones por P. aeruginosa, siendo más frecuentes las respiratorias (28,1%; 18/64). La mayoría de las cepas de P. aeruginosa (85,9%; 55764) eran extremadamente resistentes. El C/T se utilizó principalmente como terapia dirigida (98,4%; 63/64 pacientes) y en monoterapia (72,7%; 47/64 pacientes). La terapia combinada se utilizó en el 47,4% (9/19) de los pacientes con bacteriemia. La mayoría de los pacientes tuvieron resultados microbiológicos (79,2%; 42/53) y clínicos (78,7%; 48/61) satisfactorios. La tasa de mortalidad intrahospitalaria por todas las causas fue del 34,4%.
ConclusiónLa presente serie de casos sustenta que la terapéutica con C/T es una alternativa efectiva y segura para las infecciones por P. aeruginosa, particularmente por cepas extremadamente resistentes, en monoterapia o en terapia combinada.
The growing number of infections due to multidrug resistant (MDR) Gram-negative pathogens, together with the scarcity of new and effective antibiotics in the last years, has hindered the selection of appropriate treatments, with negative consequences on patients’ morbidity and mortality.1–3
The treatment of Pseudomonas aeruginosa infections is particularly challenging, as this pathogen displays resistance to several available antibiotics, namely aminoglycosides, quinolones, and β-lactams (including carbapenem).4,5 Inappropriate empiric therapy is common within the context of these infections due to the high prevalence of resistance and restricted treatment options.6,7 Consequentially, infections caused by resistant P. aeruginosa are characterized by a high mortality, morbidity, and considerable healthcare resource utilization and costs.3,8–10 Worrisomely, the prevalence of resistant strains of P. aeruginosa has been growing in the past decades.7,11–13 In Portugal, a recent study conducted in intensive care units reported high rates of resistance among P. aeruginosa isolates (21.2% and 23.2% were MDR and extensively drug-resistant [XDR], respectively).14
The rise of antimicrobial resistance among Gram-negative bacteria has sparked the development of new therapeutic agents.15 In 2017, the World Health Organization (WHO) listed P. aeruginosa, Enterobacterales and Acinetobacter baumannii as pathogens of critical priority for the development of new antibiotics.16 In this context, novel antibiotics, such as β-lactam/β-lactamase inhibitor combinations (ceftolozane-tazobactam [C/T] and ceftazidime/avibactam), have been developed and introduced in recent years. C/T is currently indicated for the treatment of adults with complicated intra-abdominal and urinary tract infections, acute pyelonephritis, and hospital-acquired pneumonia, including ventilator-associated pneumonia.17
Various observational studies have demonstrated that C/T is an effective alternative for the treatment of MDR and XDR P. aeruginosa infections.1,18–23 Indeed, clinical success was documented in more than 70% patients treated with C/T in six of the seven referenced studies, with percentages ranging from 63.8% to 88.0%.1,18–23 Favourable safety results have also been reported – namely a low incidence (3.0%) of adverse events (AEs) (mild severity only) – regardless of infection type.18
Real-world data on the use of C/T for the treatment of P. aeruginosa infections are still scarce. We herein describe the experience of five Portuguese public hospitals with this antimicrobial for the treatment of patients with P. aeruginosa infections.
MethodsStudy designCase series analysis of hospitalized patients treated with C/T for P. aeruginosa infections in five public Portuguese hospitals. Four participating hospitals belong to tertiary hospital centers – Santa Maria Hospital and Pulido Valente Hospital are part of the Centro Hospitalar Universitário Lisboa Norte, and the Curry Cabral Hospital and Santo António dos Capuchos Hospital are part of the Centro Hospitalar Universitário Lisboa Central. One (Centro Hospitalar Barreiro-Montijo) is a district hospital. Patients presenting with infections caused by P. aeruginosa and receiving C/T for at least 72h during hospitalization were eligible for inclusion in this case series.
This project was approved by the independent ethics committees of the participating hospitals, all of which provided a waiver of informed consent.
Data collectedData were retrospectively collected from the clinical records of eligible patients using a pre-defined form. The following variables were collected: patients’ demographic (age, sex), anthropometric (weight), and clinical characteristics (neutropenia <500cells/mm3, other immunosuppression), and comorbidities; site and type of infection; microbiological data, including susceptibility test results and resistance profile of P. aeruginosa isolated strains; presence of bacteremia, septic shock, coinfection with other pathogens, and ventilator-dependent respiratory failure during C/T courses; C/T treatment-related characteristics, namely empiric/targeted, monotherapy/combination therapy, duration, and dose, minimum inhibitory concentration (MIC), creatinine clearance (CrCl), and adverse reactions; outcomes, including microbiological and clinical success after C/T treatment, development of resistance, and all cause in-hospital mortality.
Definitions and microbiological methodsA successful clinical outcome was defined as a positive evolution of clinical signs/symptoms and an improvement in inflammation laboratory parameters. Independently of this evolution, though usually in its sequence, hospital discharge was documented as a “success” while in-hospital death due to the infectious process was considered a “failure”. A successful microbiological outcome was defined as the occurrence of negative microbiological test(s), or tests with isolation of pathogen(s) other than the one which motivated antimicrobial therapy with C/T, in previously positive product samples and after suspension of antibiotic therapy. The definitions of MDR and XDR bacteria published by Magiorakos A.P. et al. were followed.24
Cultures, identification of microorganisms, and susceptibility testing were performed at each participating hospital according to their own practice. The minimum inhibitory concentrations for C/T were determined using E-test or Vitek 2 antibiotic susceptibility testing cards and interpreted according to the European Committee on Antimicrobial Susceptibility Testing breakpoints.
Ceftolozane/tazobactam dose in patients with pneumoniaThough C/T was not yet indicated for the treatment of pneumonia during the period encompassed by this case series, we explored whether patients with these infections received the dose currently recommended in the most recent summary of product characteristics.17 C/T dose should be adjusted based on the patients’ renal function, with progressively lower doses being recommended for patients with CrCl between 30 and 50mL/min, 15–29mL/min, and those with end stage renal disease on hemodialysis. No dose adjustment is necessary for patients with mild renal impairment (CrCl>50mL/min). Generally, the dose recommended for the treatment of pneumonia is double of that recommended for other indications of C/T (complicated intra-abdominal infections, complicated urinary tract infections, and acute pyelonephritis), except for patients with end stage renal disease on hemodialysis. For these patients, the recommended maintenance dose is three times higher than that recommended for the remaining indications.
For this assessment, we first determined the number of patients who received one of the recommended doses for the treatment of pneumonia infections, regardless of whether it was correctly adjusted to their renal function. For instance, a patient with CrCl between 30 and 50mL/min treated with 3g of C/T every 8h was considered to have received a dose recommended for the treatment of pneumonia, despite this dose not being correctly adjusted to renal function. We then assessed, among these patients, how many had their dose correctly adjusted to renal function. Lastly, for those who did not, we determined the number of patients who received doses that were higher/lower than those recommended for their renal function.
Statistical analysisContinuous variables were summarized by descriptive statistics, namely mean, median, standard deviation, minimum and maximum, while categorical variables were summarized by absolute and relative frequencies. Comparison of categorical variables between subgroups was performed with the Chi-Square test and Fisher Exact test.
All hypothesis tests were two-sided and a p value ≤0.05 was considered statistically significant. All statistical procedures were performed using SAS 9.4 software.
ResultsA total of 64 hospitalized patients treated for at least three days with C/T for P. aeruginosa infections were evaluated. The cases herein reported occurred between December 2016 and July 2019.
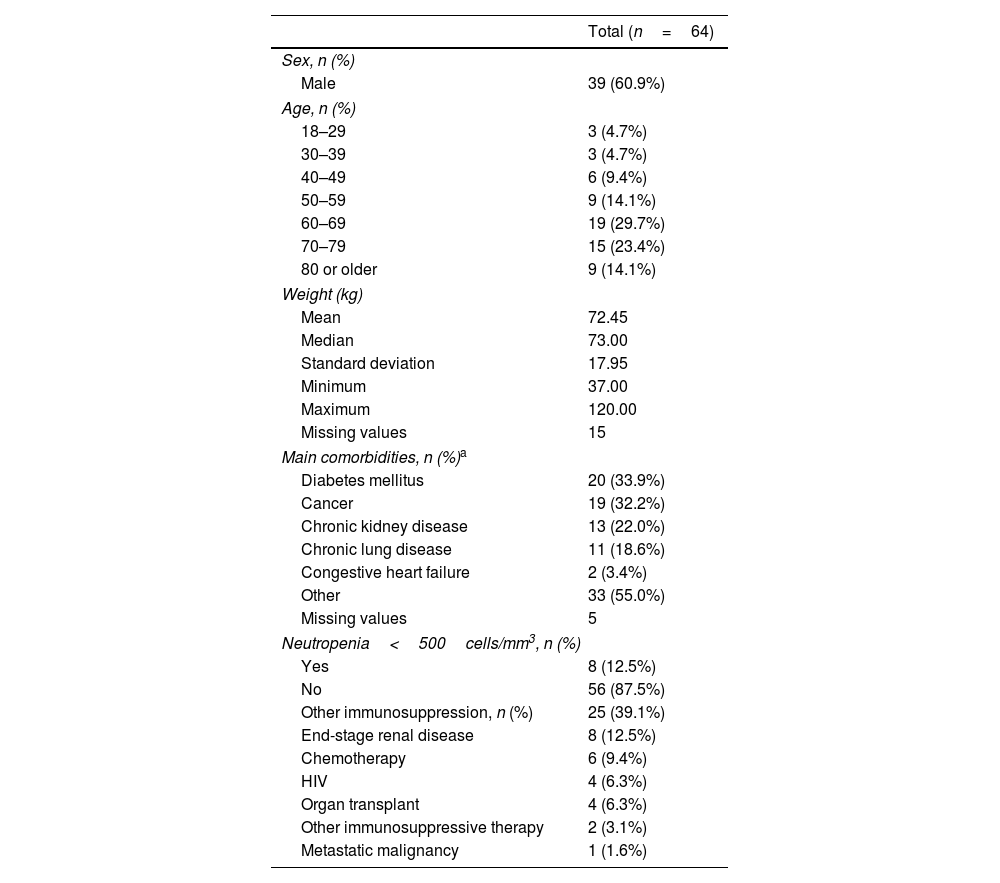
Patient characteristicsMost patients were male (60.9%) and between 60 and 79 years (53.1%) – Table 1. Median weight was 73kg (range: 37–120kg). Diabetes mellitus was the most common comorbidity (33.9% of patients), followed by cancer (32.2%). Twenty-five patients (39.1%) were immunosuppressed and eight (12.5%) had neutropenia <500cells/mm3.
Patient characteristics.
| Total (n=64) | |
|---|---|
| Sex, n (%) | |
| Male | 39 (60.9%) |
| Age, n (%) | |
| 18–29 | 3 (4.7%) |
| 30–39 | 3 (4.7%) |
| 40–49 | 6 (9.4%) |
| 50–59 | 9 (14.1%) |
| 60–69 | 19 (29.7%) |
| 70–79 | 15 (23.4%) |
| 80 or older | 9 (14.1%) |
| Weight (kg) | |
| Mean | 72.45 |
| Median | 73.00 |
| Standard deviation | 17.95 |
| Minimum | 37.00 |
| Maximum | 120.00 |
| Missing values | 15 |
| Main comorbidities, n (%)a | |
| Diabetes mellitus | 20 (33.9%) |
| Cancer | 19 (32.2%) |
| Chronic kidney disease | 13 (22.0%) |
| Chronic lung disease | 11 (18.6%) |
| Congestive heart failure | 2 (3.4%) |
| Other | 33 (55.0%) |
| Missing values | 5 |
| Neutropenia<500cells/mm3, n (%) | |
| Yes | 8 (12.5%) |
| No | 56 (87.5%) |
| Other immunosuppression, n (%) | 25 (39.1%) |
| End-stage renal disease | 8 (12.5%) |
| Chemotherapy | 6 (9.4%) |
| HIV | 4 (6.3%) |
| Organ transplant | 4 (6.3%) |
| Other immunosuppressive therapy | 2 (3.1%) |
| Metastatic malignancy | 1 (1.6%) |
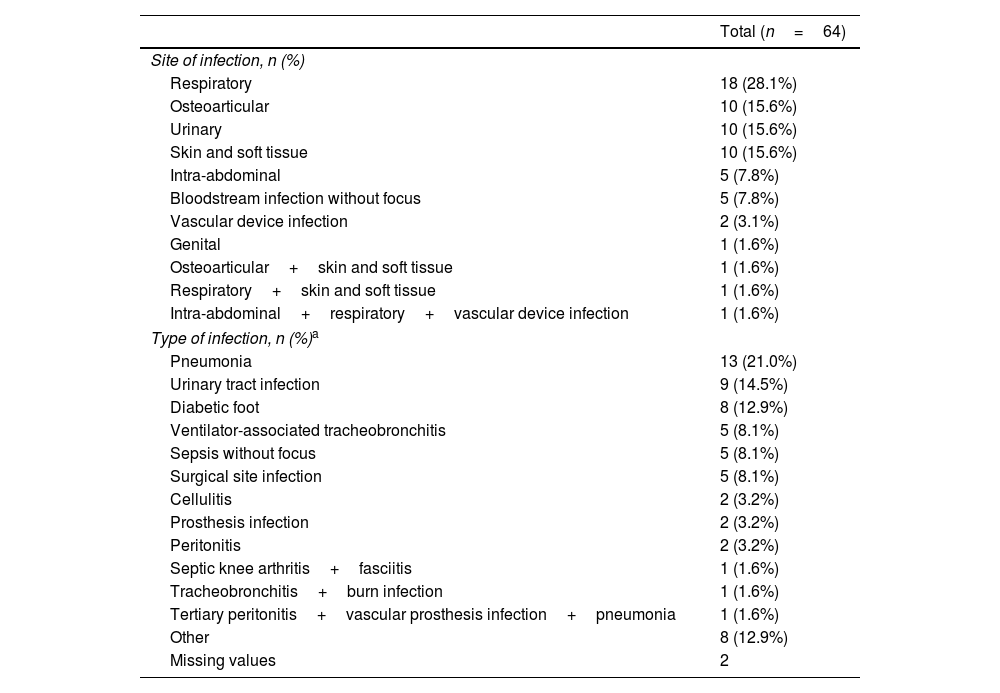
The 64 patients included in this case series presented a total of 68 P. aeruginosa infections. The great majority of patients (95.3%) had single-site P. aeruginosa infections. Multiple-site infections were observed in three patients (4.7%) – two patients had two infection sites and one patient had three. Various sites of infections were observed (Table 2), with respiratory infections being the most common – recorded in 28.1% of patients. Regarding infection types, the most frequently observed were pneumonia (21.0%), urinary tract infections (14.5%), and diabetic foot (12.9%).
Site and type of Pseudomonas aeruginosa infections treated with ceftolozane/tazobactam.
| Total (n=64) | |
|---|---|
| Site of infection, n (%) | |
| Respiratory | 18 (28.1%) |
| Osteoarticular | 10 (15.6%) |
| Urinary | 10 (15.6%) |
| Skin and soft tissue | 10 (15.6%) |
| Intra-abdominal | 5 (7.8%) |
| Bloodstream infection without focus | 5 (7.8%) |
| Vascular device infection | 2 (3.1%) |
| Genital | 1 (1.6%) |
| Osteoarticular+skin and soft tissue | 1 (1.6%) |
| Respiratory+skin and soft tissue | 1 (1.6%) |
| Intra-abdominal+respiratory+vascular device infection | 1 (1.6%) |
| Type of infection, n (%)a | |
| Pneumonia | 13 (21.0%) |
| Urinary tract infection | 9 (14.5%) |
| Diabetic foot | 8 (12.9%) |
| Ventilator-associated tracheobronchitis | 5 (8.1%) |
| Sepsis without focus | 5 (8.1%) |
| Surgical site infection | 5 (8.1%) |
| Cellulitis | 2 (3.2%) |
| Prosthesis infection | 2 (3.2%) |
| Peritonitis | 2 (3.2%) |
| Septic knee arthritis+fasciitis | 1 (1.6%) |
| Tracheobronchitis+burn infection | 1 (1.6%) |
| Tertiary peritonitis+vascular prosthesis infection+pneumonia | 1 (1.6%) |
| Other | 8 (12.9%) |
| Missing values | 2 |
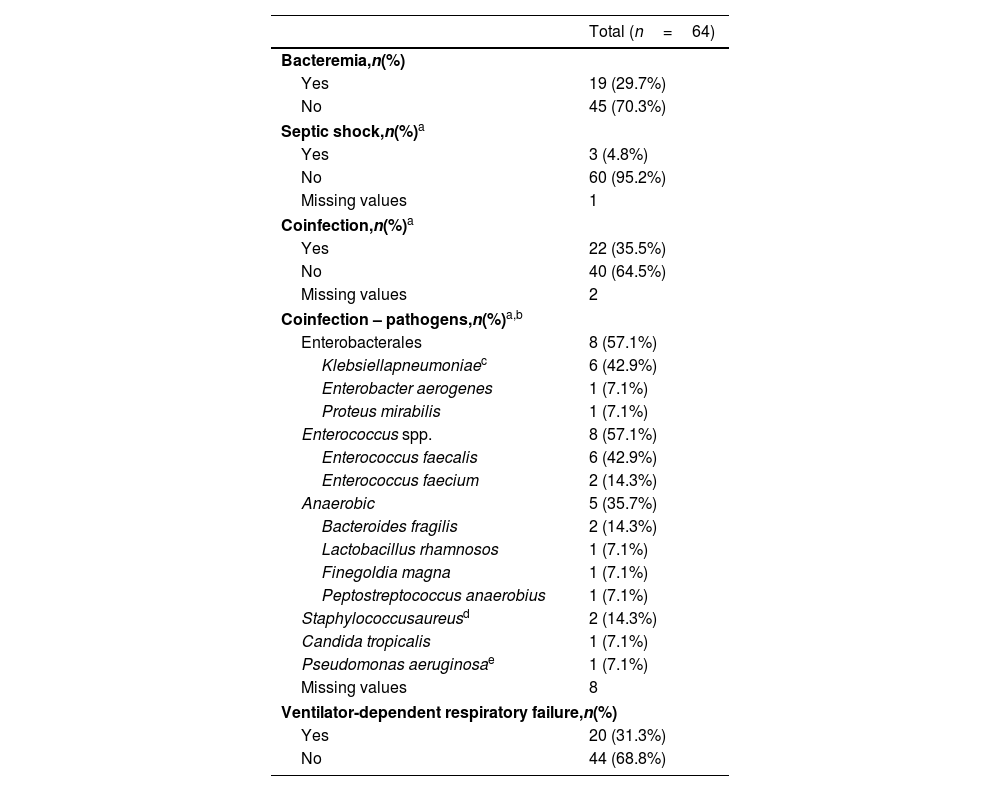
Patients presented with bacteremia and septic shock in 29.7% and 4.8% of C/T courses, respectively – Table 3. Coinfection with other pathogens was observed in 22 patients (35.5%). The most common co-pathogens were Enterococcus spp. and Enterobacterales, each being found in eight of the 14 co-infections (57.1%) for which pathogens were recorded.
Patients’ clinical presentation during ceftolozane/tazobactam treatment courses.
| Total (n=64) | |
|---|---|
| Bacteremia,n(%) | |
| Yes | 19 (29.7%) |
| No | 45 (70.3%) |
| Septic shock,n(%)a | |
| Yes | 3 (4.8%) |
| No | 60 (95.2%) |
| Missing values | 1 |
| Coinfection,n(%)a | |
| Yes | 22 (35.5%) |
| No | 40 (64.5%) |
| Missing values | 2 |
| Coinfection – pathogens,n(%)a,b | |
| Enterobacterales | 8 (57.1%) |
| Klebsiellapneumoniaec | 6 (42.9%) |
| Enterobacter aerogenes | 1 (7.1%) |
| Proteus mirabilis | 1 (7.1%) |
| Enterococcus spp. | 8 (57.1%) |
| Enterococcus faecalis | 6 (42.9%) |
| Enterococcus faecium | 2 (14.3%) |
| Anaerobic | 5 (35.7%) |
| Bacteroides fragilis | 2 (14.3%) |
| Lactobacillus rhamnosos | 1 (7.1%) |
| Finegoldia magna | 1 (7.1%) |
| Peptostreptococcus anaerobius | 1 (7.1%) |
| Staphylococcusaureusd | 2 (14.3%) |
| Candida tropicalis | 1 (7.1%) |
| Pseudomonas aeruginosae | 1 (7.1%) |
| Missing values | 8 |
| Ventilator-dependent respiratory failure,n(%) | |
| Yes | 20 (31.3%) |
| No | 44 (68.8%) |
Antibiotic susceptibility testing was carried out for all 64 isolated P. aeruginosa strains. Most tested isolates were resistant to piperacillin-tazobactam (98.4%, 63 out of 64), carbapenems (meropenem and/or imipenem) (95.3%, 61 out of 64), quinolones (90.0%, 54 out of 60), and ceftazidime (86.5%, 45 out of 52). All tested isolates were susceptible to colistin (44 out of 44) and more than half to aminoglycosides (59.0%, 36 out of 61). The great majority of P. aeruginosa strains were XDR (85.9%, 55 out of 64) and 12.7% (8 out of 64) were MDR. One strain was susceptible to all tested antibiotics.
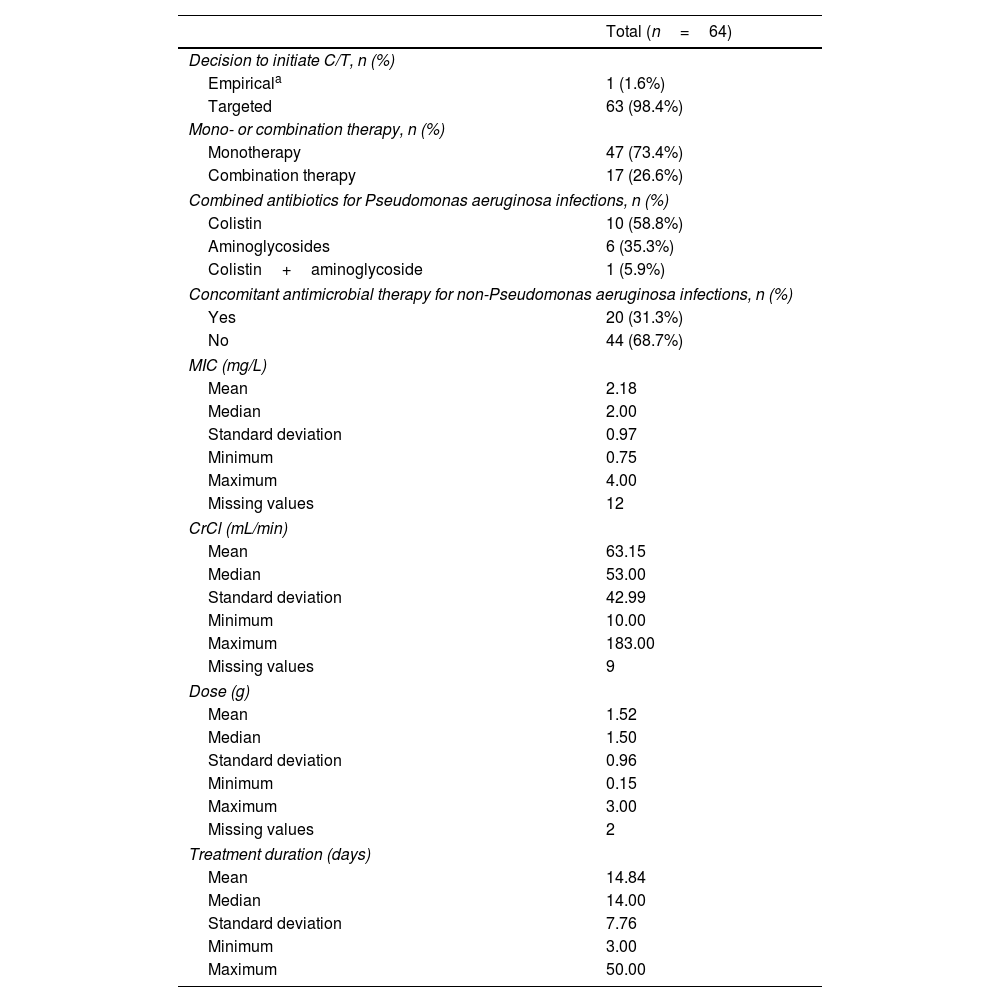
Ceftolozane/tazobactam treatment characterizationC/T was used empirically in one patient only (1.6%), being started after isolation of P. aeruginosa and while waiting for antibiotic susceptibility testing results (Table 4). C/T was most commonly used as monotherapy (73.4%). In the cases in which C/T was used in combination with other antibiotics (26.6%) for the treatment of P. aeruginosa infections, the most frequently used were colistin (58.8%) and aminoglycosides (35.3%). Combination therapy was used in approximately half of the patients with bacteremia (47.4%, 9 out of 19) and in 87.5% (7 out of 8) of neutropenic patients.
Ceftolozane/tazobactam treatment-related characteristics.
| Total (n=64) | |
|---|---|
| Decision to initiate C/T, n (%) | |
| Empiricala | 1 (1.6%) |
| Targeted | 63 (98.4%) |
| Mono- or combination therapy, n (%) | |
| Monotherapy | 47 (73.4%) |
| Combination therapy | 17 (26.6%) |
| Combined antibiotics for Pseudomonas aeruginosa infections, n (%) | |
| Colistin | 10 (58.8%) |
| Aminoglycosides | 6 (35.3%) |
| Colistin+aminoglycoside | 1 (5.9%) |
| Concomitant antimicrobial therapy for non-Pseudomonas aeruginosa infections, n (%) | |
| Yes | 20 (31.3%) |
| No | 44 (68.7%) |
| MIC (mg/L) | |
| Mean | 2.18 |
| Median | 2.00 |
| Standard deviation | 0.97 |
| Minimum | 0.75 |
| Maximum | 4.00 |
| Missing values | 12 |
| CrCl (mL/min) | |
| Mean | 63.15 |
| Median | 53.00 |
| Standard deviation | 42.99 |
| Minimum | 10.00 |
| Maximum | 183.00 |
| Missing values | 9 |
| Dose (g) | |
| Mean | 1.52 |
| Median | 1.50 |
| Standard deviation | 0.96 |
| Minimum | 0.15 |
| Maximum | 3.00 |
| Missing values | 2 |
| Treatment duration (days) | |
| Mean | 14.84 |
| Median | 14.00 |
| Standard deviation | 7.76 |
| Minimum | 3.00 |
| Maximum | 50.00 |
Among the 14 patients with pneumonia, ten (71.4%) received a dose that is currently recommended for the treatment of these infections. Of these ten, the dose was correctly adjusted to renal function in six patients (60.0%). In the four patients for whom the dose was not correctly adjusted to renal function, three received a dose higher than that recommended, and one patient received a dose lower than recommended. Three of the four patients for whom the dose was not correctly adjusted were on hemodialysis.
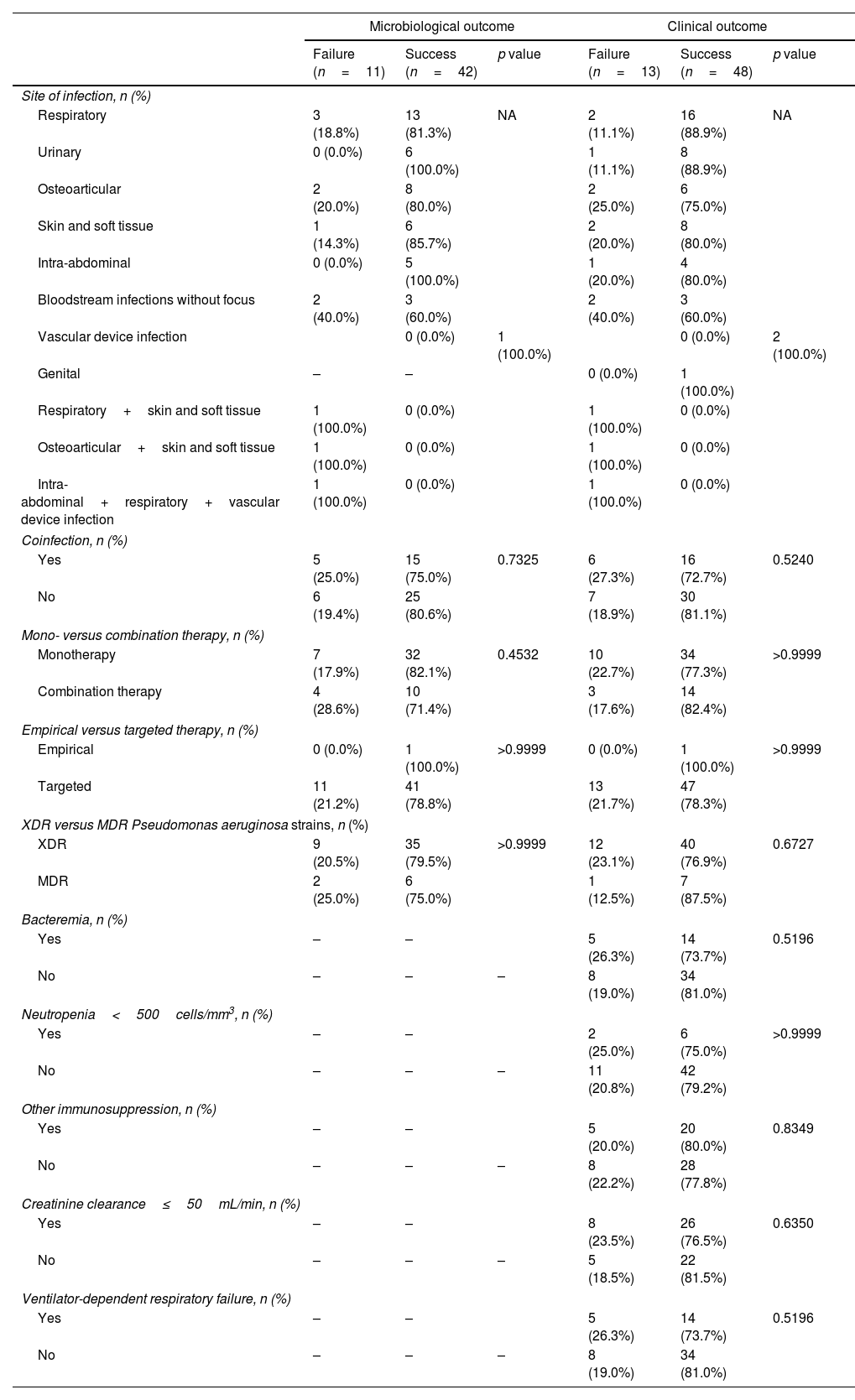
Outcomes following ceftolozane/tazobactam treatmentSuccessful microbiological and clinical outcomes were observed for most patients with available data on these variables – 79.2% (42 out of 53) and 78.7% (48 out of 61), respectively. Regarding infection site, the lowest percentages of microbiological and clinical success were observed for bloodstream infections without focus (n=3, 60.0%). No statistically significant differences in microbiological and clinical success percentages were found between compared subgroups – Table 5.
Microbiological and clinical outcomes by subgroups.
| Microbiological outcome | Clinical outcome | |||||
|---|---|---|---|---|---|---|
| Failure (n=11) | Success (n=42) | p value | Failure (n=13) | Success (n=48) | p value | |
| Site of infection, n (%) | ||||||
| Respiratory | 3 (18.8%) | 13 (81.3%) | NA | 2 (11.1%) | 16 (88.9%) | NA |
| Urinary | 0 (0.0%) | 6 (100.0%) | 1 (11.1%) | 8 (88.9%) | ||
| Osteoarticular | 2 (20.0%) | 8 (80.0%) | 2 (25.0%) | 6 (75.0%) | ||
| Skin and soft tissue | 1 (14.3%) | 6 (85.7%) | 2 (20.0%) | 8 (80.0%) | ||
| Intra-abdominal | 0 (0.0%) | 5 (100.0%) | 1 (20.0%) | 4 (80.0%) | ||
| Bloodstream infections without focus | 2 (40.0%) | 3 (60.0%) | 2 (40.0%) | 3 (60.0%) | ||
| Vascular device infection | 0 (0.0%) | 1 (100.0%) | 0 (0.0%) | 2 (100.0%) | ||
| Genital | – | – | 0 (0.0%) | 1 (100.0%) | ||
| Respiratory+skin and soft tissue | 1 (100.0%) | 0 (0.0%) | 1 (100.0%) | 0 (0.0%) | ||
| Osteoarticular+skin and soft tissue | 1 (100.0%) | 0 (0.0%) | 1 (100.0%) | 0 (0.0%) | ||
| Intra-abdominal+respiratory+vascular device infection | 1 (100.0%) | 0 (0.0%) | 1 (100.0%) | 0 (0.0%) | ||
| Coinfection, n (%) | ||||||
| Yes | 5 (25.0%) | 15 (75.0%) | 0.7325 | 6 (27.3%) | 16 (72.7%) | 0.5240 |
| No | 6 (19.4%) | 25 (80.6%) | 7 (18.9%) | 30 (81.1%) | ||
| Mono- versus combination therapy, n (%) | ||||||
| Monotherapy | 7 (17.9%) | 32 (82.1%) | 0.4532 | 10 (22.7%) | 34 (77.3%) | >0.9999 |
| Combination therapy | 4 (28.6%) | 10 (71.4%) | 3 (17.6%) | 14 (82.4%) | ||
| Empirical versus targeted therapy, n (%) | ||||||
| Empirical | 0 (0.0%) | 1 (100.0%) | >0.9999 | 0 (0.0%) | 1 (100.0%) | >0.9999 |
| Targeted | 11 (21.2%) | 41 (78.8%) | 13 (21.7%) | 47 (78.3%) | ||
| XDR versus MDR Pseudomonas aeruginosa strains, n (%) | ||||||
| XDR | 9 (20.5%) | 35 (79.5%) | >0.9999 | 12 (23.1%) | 40 (76.9%) | 0.6727 |
| MDR | 2 (25.0%) | 6 (75.0%) | 1 (12.5%) | 7 (87.5%) | ||
| Bacteremia, n (%) | ||||||
| Yes | – | – | 5 (26.3%) | 14 (73.7%) | 0.5196 | |
| No | – | – | – | 8 (19.0%) | 34 (81.0%) | |
| Neutropenia<500cells/mm3, n (%) | ||||||
| Yes | – | – | 2 (25.0%) | 6 (75.0%) | >0.9999 | |
| No | – | – | – | 11 (20.8%) | 42 (79.2%) | |
| Other immunosuppression, n (%) | ||||||
| Yes | – | – | 5 (20.0%) | 20 (80.0%) | 0.8349 | |
| No | – | – | – | 8 (22.2%) | 28 (77.8%) | |
| Creatinine clearance≤50mL/min, n (%) | ||||||
| Yes | – | – | 8 (23.5%) | 26 (76.5%) | 0.6350 | |
| No | – | – | – | 5 (18.5%) | 22 (81.5%) | |
| Ventilator-dependent respiratory failure, n (%) | ||||||
| Yes | – | – | 5 (26.3%) | 14 (73.7%) | 0.5196 | |
| No | – | – | – | 8 (19.0%) | 34 (81.0%) | |
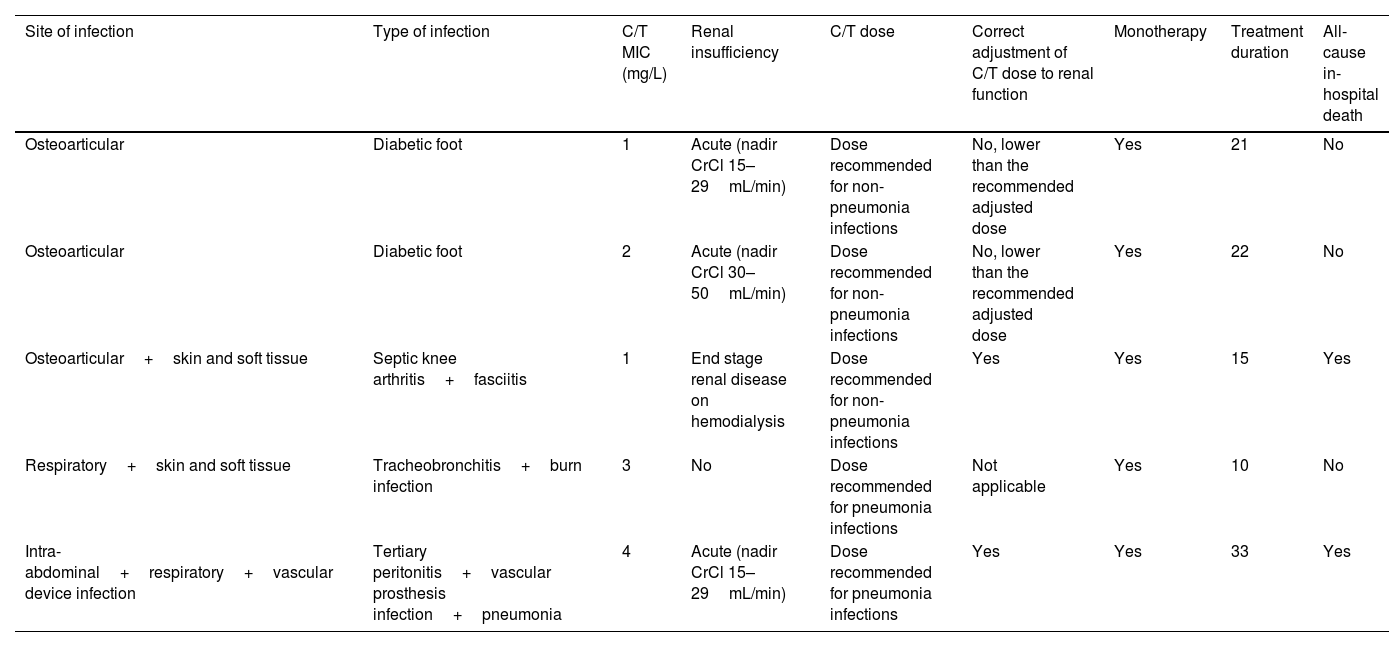
Development of C/T resistance was documented in five patients (7.8%) – Table 6. All these patients received monotherapy for difficult to treat infections, namely osteoarticular and device-associated infections. The C/T dose was correctly adjusted to renal function in three of the five patients. In the two remaining patients, although the initial dose was correctly defined according to the patients’ renal function at the time of treatment start, no adjustment (i.e., dose increase) was made as renal function recovered during the treatment period. Thus, these patients were treated with doses lower than those recommended after renal function improved. Despite not having pneumonia, one patient received the dose recommended for the treatment of these infections in patients without renal insufficiency. The three patients with multiple-site infections developed resistance. Median treatment duration was 21 days (range: 10–33 days) – higher than that observed for patients not developing resistance (14 days [range: 1–50 days]). Two of the patients who developed resistance died during the hospitalization.
Characterization of patients who developed Ceftolozane/tazobactam resistance.
| Site of infection | Type of infection | C/T MIC (mg/L) | Renal insufficiency | C/T dose | Correct adjustment of C/T dose to renal function | Monotherapy | Treatment duration | All-cause in-hospital death |
|---|---|---|---|---|---|---|---|---|
| Osteoarticular | Diabetic foot | 1 | Acute (nadir CrCl 15–29mL/min) | Dose recommended for non-pneumonia infections | No, lower than the recommended adjusted dose | Yes | 21 | No |
| Osteoarticular | Diabetic foot | 2 | Acute (nadir CrCl 30–50mL/min) | Dose recommended for non-pneumonia infections | No, lower than the recommended adjusted dose | Yes | 22 | No |
| Osteoarticular+skin and soft tissue | Septic knee arthritis+fasciitis | 1 | End stage renal disease on hemodialysis | Dose recommended for non-pneumonia infections | Yes | Yes | 15 | Yes |
| Respiratory+skin and soft tissue | Tracheobronchitis+burn infection | 3 | No | Dose recommended for pneumonia infections | Not applicable | Yes | 10 | No |
| Intra-abdominal+respiratory+vascular device infection | Tertiary peritonitis+vascular prosthesis infection+pneumonia | 4 | Acute (nadir CrCl 15–29mL/min) | Dose recommended for pneumonia infections | Yes | Yes | 33 | Yes |
CT, ceftolozane/tazobactam; MIC, minimum inhibitory concentration; CrCl, creatinine clearance.
All-cause in-hospital mortality rate was 34.4%.
Safety and tolerabilityOne adverse drug reaction (ADR) – leucopenia – was reported in the 64 patients for whom C/T was administered. This ADR occurred in an HIV patient with a diffuse large B-cell lymphoma, who did not have chemotherapy-induced marrow aplasia at the time of the event. This was a profound and prolonged neutropenia (nadir leucocyte of 260cells/μL, with zero neutrophils) that developed at the fourth day of treatment with C/T. The treatment was suspended at the tenth day and the patient was treated with human granulocyte colony stimulating factor. The leucopenia event was resolved 15 days after treatment suspension.
DiscussionThis project aimed at describing the experience of five Portuguese public hospitals with using C/T for the treatment of patients with P. aeruginosa infections. To the best of our knowledge, this is the first analysis of cases regarding the real-world use of C/T in Portugal.
As mentioned in the introduction, the treatment of P. aeruginosa infections is particularly challenging due to the growing resistance of this pathogen to various antibiotics, namely carbapenems.25,26 Indeed, in the present case series, most isolated strains of P. aeruginosa were resistant to carbapenems (95.3%), as well as to piperacillin-tazobactam (98.4%), quinolones (90.0%), and ceftazidime (86.5%). The high rate of resistance among P. aeruginosa strains likely represented the main reason for using C/T for the targeted treatment of high-risk patients with severe infections, who accounted for the majority of patients in our case series.
This case series suggests that C/T is an effective option for the treatment of P. aeruginosa infections, as evidenced by the high percentages of microbiological and clinical success observed for included patients (79.2% and 78.7%, respectively). The significance of these results is further highlighted when one considers that the great majority of isolated P. aeruginosa strains were XDR (85.9%). Clinical success rate results are in line with past research.1,18–23
Previous studies focusing on P. aeruginosa infections have reported no differences in clinical response when comparing patients who received C/T as monotherapy with those receiving combination therapy, indicating that the former may be sufficient for the treatment of these infections.2,18,19,21,22,27 In this case series, percentages of clinical success were similar for cases treated with C/T monotherapy (77.3%) versus combination therapy (82.4%). The slight difference observed for microbiological success (10.7% higher among courses during which patients received monotherapy) may be due to selection bias, as proposed by Gallagher JC et al.20 That is, physicians likely choose to administer additional antibiotics in patients performing worse. This explanation is supported by the fact that a considerably higher proportion of patients treated with combination therapy presented bacteremia when compared to those that received monotherapy (52.9% vs. 21.3%, respectively).
In line with past research,18 our data also support the safety and tolerability profile of C/T treatment, in a wide range of P. aeruginosa infections. Leucopenia was the only ADR reported during the 64 courses of treatment with this antimicrobial, occurring once in one patient.
Development of resistance to novel β-lactam/β-lactamase inhibitor combinations – the introduction of which have lighten the pressing demand of new agents for the treatment of MDR/XDR P. aeruginosa infections – is a matter of concern.28 Emergence of resistance to C/T has indeed been documented in previous studies, ranging from 3% to 14% of patients treated for P. aeruginosa infections.18,19,21 Our data fall within the range reported in the literature, with five patients (7.8%) developing resistance to C/T. Whether combination therapy is able to protect against the development of resistance is still up to debate.29 Our results seem to support this hypothesis, as all five patients who developed resistance received monotherapy. Still, one should note that use of monotherapy was considerably more frequent in this case series (73.4% vs. 26.6% of combination therapy).
As previously stated, options for the treatment of MDR and XDR P. aeruginosa infections are limited.6,7 As C/T is one of the best antipseudomonal agents currently available,30 efforts should be made to avoid the development of resistance to this antimicrobial caused by selective antibiotic pressure. Thus, the authors consider that C/T should mainly be reserved for the targeted treatment of infections caused by carbapenem-resistant MDR and XDR P. aeruginosa infections, while the use of C/T for the treatment of extended-spectrum beta-lactamases-producing Enterobacterales as a carbapenem-sparing strategy should be avoided. Risk factors for MDR P. aeruginosa and carbapenemase-producing Enterobacterales frequently overlap in the context of MDR infections. As carbapenemase-producing Enterobacterales are outside the spectrum of C/T, empirical treatment for high mortality risk infections in this context should be based on new β-lactam/β-lactamase inhibitor combinations (e.g., ceftazidime/avibactam, meropenem/vaborbactam, imipenem/relebactam). In these scenarios of high risk of resistance, the authors consider that the empirical use of C/T should be limited to those cases in which P. aeruginosa has been isolated but the antibiotic susceptibility testing results have not yet become available. In these cases, the association of a non β-lactam antibiotic should be considered to broaden the spectrum of activity. The preference for the use of C/T as a targeted treatment across the participating hospitals is clearly reflected in our results, as only one instance of empirical use of C/T was recorded among the 64 included patients.
Establishing adequate comparisons of mortality rates with past studies is challenging due to the different methodologies adopted. While we considered all-cause in-hospital mortality, previous studies mainly assessed mortality at fixed time points (30- and 90-day rates), with the exception of Munita JM et al.22 In this study, which evaluated C/T treatment for serious infections caused by carbapenem-resistant P. aeruginosa, a lower all-cause in-hospital mortality rate was observed (22.8% vs. 34.4% in our case series). When compared to the studies assessing fixed time point mortality, the in-hospital mortality rate obtained in our case series was higher than the 30-day mortality rates (10.0%–27.6%)1,20,21 and lower than the 90-day mortality rate (48%)21 reported. The patients’ characteristics should be taken into account when interpreting mortality results, as they allow us to infer about the clinical presentation severity of the patients included in this case series. Specifically, included patients were mostly over 60 years old and approximately 32% had cancer. Moreover, in an appreciable proportion of treatment courses, patients were immunosuppressed (39.1%) and presented with bacteremia (29.7%), coinfection with other pathogens (35.5%), and ventilator-dependent respiratory failure (31.3%).
This case series has some limitations. First, it is limited by the retrospective nature of the data and the reliance on medical records to determine clinical and microbiological outcomes. Second, though multiple hospitals participated in this series, our findings might not be representative of other institutions across Portugal. Third, the cases herein reported pertain only to patients treated for at least 72h with C/T during the hospitalization. As a result, more severe cases (e.g., patients who died prior to completing 72h of the antibiotic course) were excluded from this case series. Lastly, as only all-cause in-hospital mortality was collected, it's not possible to determine the proportion of deaths that were due to P. aeruginosa infection.
In conclusion, the present case series contributes to the body of evidence suggesting that C/T is an effective and safe option for treating P. aeruginosa infections, namely those caused by XDR strains, both when used as mono- or combination therapy. Our results are relevant to physicians responsible for the management of in-hospital patients presenting with infections caused by this pathogen.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of InterestDr. Mimoso Santos reports personal fees and non-financial support from Pfizer, MSD and Gilead, outside the submitted work. Dr. André reports personal fees and non-financial support from Pfizer and MSD, outside the submitter work. Dr. Lino reports personal fees and non-financial support from MSD, Gilead, ViiV and Pfizer, outside the submitted work. Dr. Froes reports personal fees and non-financial support from Pfizer, MSD, Sanofi, AstraZeneca and Novartis, outside the submitted work. Dr. Leitão and Dr. Lemos have no relevant financial or non-financial interests to disclose.
Statistical support was provided by Vera Vicente of CTI Clinical Trial & Consulting Services and medical writing and editorial assistance was provided by Diogo Ribeiro of CTI Clinical Trial & Consulting Services.