The aim of this study was to evaluate the epidemiological profile of dermatophytoses from 2008 to 2017 in the area of “Barcelonès Nord”, located in north-eastern Spain.
MethodsFrom 2008 to 2017, 13,419 specimens obtained from patients with suspected superficial mycosis were subjected to direct microscopy and culture. Clinical and sociodemographic data were recorded. Proportions were compared using Chi-square and Fisher's exact tests. A logistic regression model was used for multivariate analysis.
ResultsTrichophyton rubrum was the most frequently isolated fungus (76.1%), followed by Trichophyton mentagrophytes/Trichophyton interdigitale (11.9%) and Microsporum canis (2.9%). Among the population over 15 years of age, tinea unguium pedum (40.4%) and tinea corporis (29.1%) were the predominant dermatophyte infections. Tinea capitis was mostly prevalent (53.6%) among patients up to 15 years of age, followed by tinea corporis (21.4%). We observed an increase in non-endemic anthropophilic dermatophytes (Trichophyton soudanense, Microsporum audouinii and Trichophyton violaceum) in the last few years. These species were associated with the population up to 15 years of age (p<0.001), having tinea capitis (p=0.0017) and being of African/Hindustani origin (p<0.001).
ConclusionsWe confirmed the spread of T. rubrum as the predominant dermatophyte in our area and reported an increase in non-endemic anthropophilic dermatophytes in the last few years, especially in the African and Hindustani population up to 15 years of age.
La finalidad del presente estudio fue evaluar el perfil epidemiológico de las dermatofitosis entre los años 2008 y 2017 en el área de «Barcelonès Nord» ubicada en el noreste de España.
MétodosEntre los años 2008 y 2017, 13.419 muestras obtenidas de pacientes con sospecha de micosis superficial fueron remitidas para microscopía directa y cultivo. Se registraron datos clínicos y sociodemográficos. Las proporciones se compararon mediante las pruebas de Chi-cuadrado y Fisher. Para el análisis de variables múltiples, se utilizó un modelo de regresión logística.
ResultadosTrichophyton rubrum fue el hongo más frecuentemente aislado (76,1%), seguido de Trichophyton mentagrophytes/Trichophyton interdigitale (11,9%) y Microsporum canis (2,9%). Entre la población mayor de 15 años, la tinea unguium pedum (40,4%) y la tinea corporis (29,1%) fueron las dermatofitosis predominantes. La tinea capitis prevaleció principalmente (53,6%) entre los pacientes menores de 15 años, seguida de la tinea corporis (21,4%). Se percibió un aumento de los dermatofitos antropófilos no endémicos (Trichophyton soudanense, Microsporum audouinii y Trichophyton violaceum) en los últimos años. Estas especies se asociaron con la población menor de 15 años (p<0,001), la presencia de tinea capitis (p=0,0017) y el origen africano/indostánico (p<0,001).
ConclusionesConfirmamos el predominio de Trichophyton rubrum como el dermatofito más prevalente en nuestra área, y describimos un aumento en los dermatofitos antropófilos no endémicos en los últimos años, especialmente en población africana e indostana menor de 15 años.
Dermatophytes are a group of filamentous fungi able to invade keratinized tissues such as hair, skin and nails, resulting in superficial infections.1 According to the adaptation of each species to different animals or other ecological reservoirs, they have also been classified into geophilic, zoophilic and anthropophilic species.1 The identification of current main genera Epidermophyton, Microsporum and Trichophyton are mainly based on the microscopic appearance of the reproductive organs in culture, called conidia (L, s: conidium), as well as in some other secondary structures of the vegetative mycelium (coiled, pectinate or antler-like hyphae, chlamydoconidia or nodular organs).2 These fungi represent a frequent reason of consultation, and it is estimated that around 20–25% of the global population are, at some stage, affected by these mycoses.3
The epidemiology of superficial dermatophytoses has changed radically throughout the 20th century in relation to factors such as life conditions, socio-economic status or migration.3 In the 1930s, anthropophilic species such as Microsporum audouinii and Trichophyton schoenleinii were the principal agents of tinea capitis in Europe and America, while Trichophyton mentagrophytes was the dermatophyte most frequently isolated in tinea pedis and tinea corporis.4 After World War II, tinea pedis increased together with global immigration.4 The epidemiology of these infections changed with the spread of Microsporum canis, T. mentagrophytes and Trichophyton verrucosum in Europe and Trichophyton tonsurans in North America as agents of tinea capitis. In the last decades, however, Trichophyton rubrum has become the predominant species in most of the studies performed, particularly in skin and nails.3,4
In Spain, the lack of recent data on superficial mycoses makes it difficult to develop a reliable map of dermatophytoses. However, a change from zoophilic to anthropophilic species has been observed over the last decades.5,6 Species such as T. tonsurans, Trichophyton violaceum and M. audouinii are being reintroduced in this and other European countries, often related to people from endemic countries,5,7,8 and their capacity to produce outbreaks and to persist in healthy carriers represents a public health problem.9–11
In this context, the aim of the present study was to evaluate the epidemiology of the dermatophytoses occurring from 2008 to 2017 in the health area of Barcelonès Nord, located in Northeastern Spain, and to compare it with the results of previous studies conducted in Spain and other European countries.
Material/MethodsFrom 2008 to 2017, 13,419 specimens obtained from patients with a superficial mycosis suspicion were submitted to the “Microbiology” Department, Laboratori Clínic Metropolitana Nord, Hospital Universitari Germans Trias i Pujol, “Department of Genetics and Microbiology”, Universitat Autònoma de Barcelona, Spain, the reference hospital of “Barcelonès Nord”, an area of 1,400,000 inhabitants. The samples came both from patients at the hospital and from primary health facilities. Only one sample per patient and location was included to avoid bias in the epidemiological analysis, consequently, the positive samples included in the study belonged to different patients. Cultures with isolated non-dermatophyte fungi were excluded.
All specimens (hair, skin and nail) were examined by direct wet mount microscopy using 20% KOH. Samples were cultured on Sabouraud dextrose agar plates with chloramphenicol (BioMérieux, Marcy-L’Étoile, France), Sabouraud-dextrose agar plates with chloramphenicol and gentamicin (BioMérieux, Marcy-L’Étoile, France) and Sabouraud dextrose agar tubes with and without cycloheximide (BioMérieux, Marcy-L’Étoile, France). All cultures were incubated at 25°C for at least 4 weeks and examined weekly. When no sporulation was observed, a subculture was incubated on Borelli's Lactrimel media (wheat flour 2% (w/v), cow's milk 20% (v/v), honey 1% (v/v), agar 2% (w/v), chloramphenicol 0,010% (w/v), water). The identification of dermatophytes was based on microscopic and macroscopic features of the fungi.
Patients were categorized depending on their age (until 15 and >15 years old) and their origin. Patients from South America and China were clustered with Spaniards under the heading of Caucasian. Most of these patients were long-term residents without documented travels and, accordingly, it was assumed that the dermatophytic infection took place in Spain. Patients from Africa, India and Pakistan were grouped together because, in many cases, the country of origin was difficult to determine. However, various sociodemographic data supported an African/Hindustani origin.
Clinical and sociodemographic data in positive cases, such as age, sex, origin, type of sample, species isolated and location of the tinea were recorded if possible.
Proportions were compared with Chi-square and Fisher's exact tests. The odds ratio (OR) was calculated when possible. A logistic regression model was used for multivariate analysis; the level of statistical significance was p<0.05. Statistical data were generated using the software package Stata 14.
ResultsOut of the 13,419 samples submitted, 607 (4.5%) were included in the study [313 from males (51.6%) and 294 from females (48.4%)]. Among these, 84 (13.8%) specimens were obtained from patients up to 15 years of age. Regarding the participants’ origins, 458 were Spaniards, 10 were from South America, 10 from China, 17 from Morocco, 14 from Pakistan, 6 from India and 11 from sub-Saharan countries. The country of origin of the remaining 81 patients could not be determined, although various sociodemographic data supported an African/Hindustani origin.
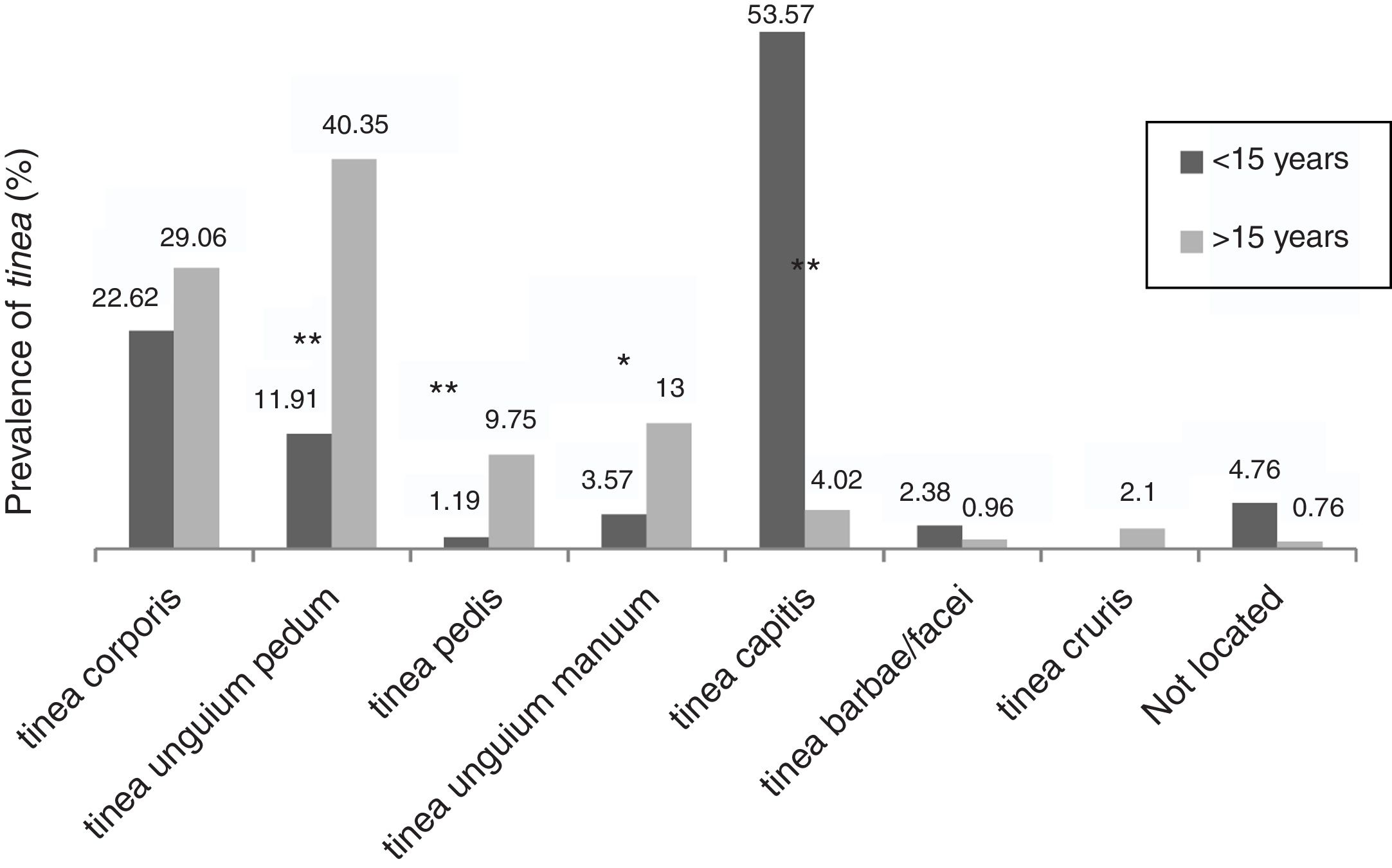
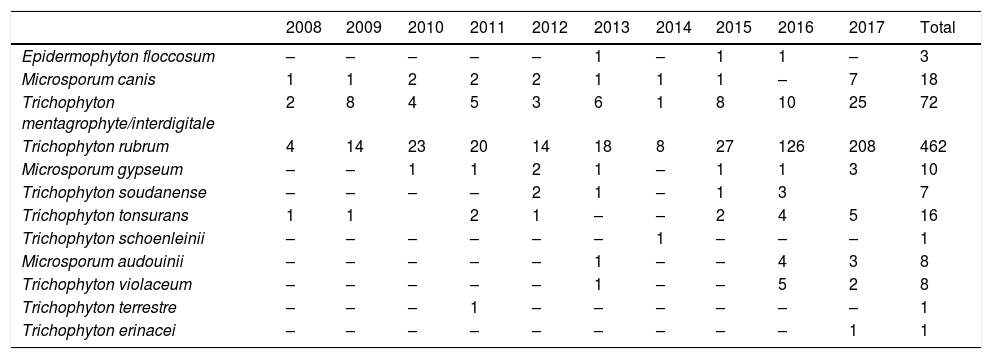
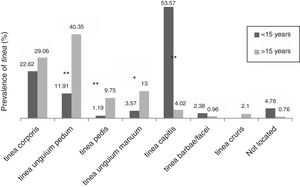
The dermathophyte species isolated per year are shown in Table 1. T. rubrum was the predominant fungus (76.1%) in all dermatophytoses unless tinea barbae/tinea facei, showing a higher prevalence among the population older than 15 (Fig. 1). T. mentagrophytes/Trichophyton interdigitale was isolated in 11.9% of cases, mainly in tinea corporis and tinea unguium pedum (Table 2). The increase of non-endemic dermatophytes over time was also noteworthy (11 T. tonsurans, 7 M. audouinii, 4 Trichophyton soudanense and 7 T. violaceum isolated in the last three years) (Table 1). The location of the tinea could not be determined in eight patients (five T. rubrum and three T. mentagrophytes/T. interdigitale).
Number of dermatophytes isolated per year.
| 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | Total | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Epidermophyton floccosum | – | – | – | – | – | 1 | – | 1 | 1 | – | 3 |
| Microsporum canis | 1 | 1 | 2 | 2 | 2 | 1 | 1 | 1 | – | 7 | 18 |
| Trichophyton mentagrophyte/interdigitale | 2 | 8 | 4 | 5 | 3 | 6 | 1 | 8 | 10 | 25 | 72 |
| Trichophyton rubrum | 4 | 14 | 23 | 20 | 14 | 18 | 8 | 27 | 126 | 208 | 462 |
| Microsporum gypseum | – | – | 1 | 1 | 2 | 1 | – | 1 | 1 | 3 | 10 |
| Trichophyton soudanense | – | – | – | – | 2 | 1 | – | 1 | 3 | 7 | |
| Trichophyton tonsurans | 1 | 1 | 2 | 1 | – | – | 2 | 4 | 5 | 16 | |
| Trichophyton schoenleinii | – | – | – | – | – | – | 1 | – | – | – | 1 |
| Microsporum audouinii | – | – | – | – | – | 1 | – | – | 4 | 3 | 8 |
| Trichophyton violaceum | – | – | – | – | – | 1 | – | – | 5 | 2 | 8 |
| Trichophyton terrestre | – | – | – | 1 | – | – | – | – | – | – | 1 |
| Trichophyton erinacei | – | – | – | – | – | – | – | – | – | 1 | 1 |
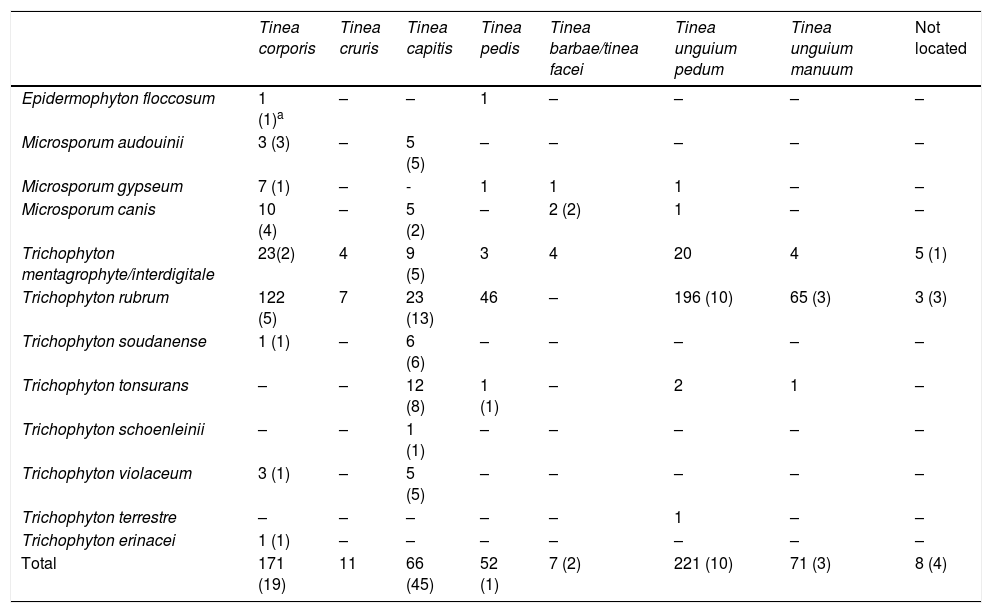
Number of dermatophytes isolated by location.
| Tinea corporis | Tinea cruris | Tinea capitis | Tinea pedis | Tinea barbae/tinea facei | Tinea unguium pedum | Tinea unguium manuum | Not located | |
|---|---|---|---|---|---|---|---|---|
| Epidermophyton floccosum | 1 (1)a | – | – | 1 | – | – | – | – |
| Microsporum audouinii | 3 (3) | – | 5 (5) | – | – | – | – | – |
| Microsporum gypseum | 7 (1) | – | - | 1 | 1 | 1 | – | – |
| Microsporum canis | 10 (4) | – | 5 (2) | – | 2 (2) | 1 | – | – |
| Trichophyton mentagrophyte/interdigitale | 23(2) | 4 | 9 (5) | 3 | 4 | 20 | 4 | 5 (1) |
| Trichophyton rubrum | 122 (5) | 7 | 23 (13) | 46 | – | 196 (10) | 65 (3) | 3 (3) |
| Trichophyton soudanense | 1 (1) | – | 6 (6) | – | – | – | – | – |
| Trichophyton tonsurans | – | – | 12 (8) | 1 (1) | – | 2 | 1 | – |
| Trichophyton schoenleinii | – | – | 1 (1) | – | – | – | – | – |
| Trichophyton violaceum | 3 (1) | – | 5 (5) | – | – | – | – | – |
| Trichophyton terrestre | – | – | – | – | – | 1 | – | – |
| Trichophyton erinacei | 1 (1) | – | – | – | – | – | – | – |
| Total | 171 (19) | 11 | 66 (45) | 52 (1) | 7 (2) | 221 (10) | 71 (3) | 8 (4) |
Among the population older than 15, tinea unguium pedum (40.3%) was the main dermatophyte infection, followed by tinea corporis (29.1%) (Fig. 1). Tinea capitis was the most prevalent (53.6%) among patients until 15 years of age (Fig. 1).
Accordingly, tinea pedis (p<0.001), tinea unguium manuum (p=0.010; OR=3.25) and tinea unguium pedum (p<0.001; OR=4.9) were significantly linked to those over 15 (Fig. 1). Tinea capitis was statistically associated with patients up to 15 years of age (p<0.001; OR=20.74) and caused by a wide variety of fungi, highlighting anthropophilic dermatophytes such as M. audouinii (n=5), T. soudanense (n=6), T. tonsurans (n=12), T. schoenleinii (n=1) and T. violaceum (n=5) (Table 2).
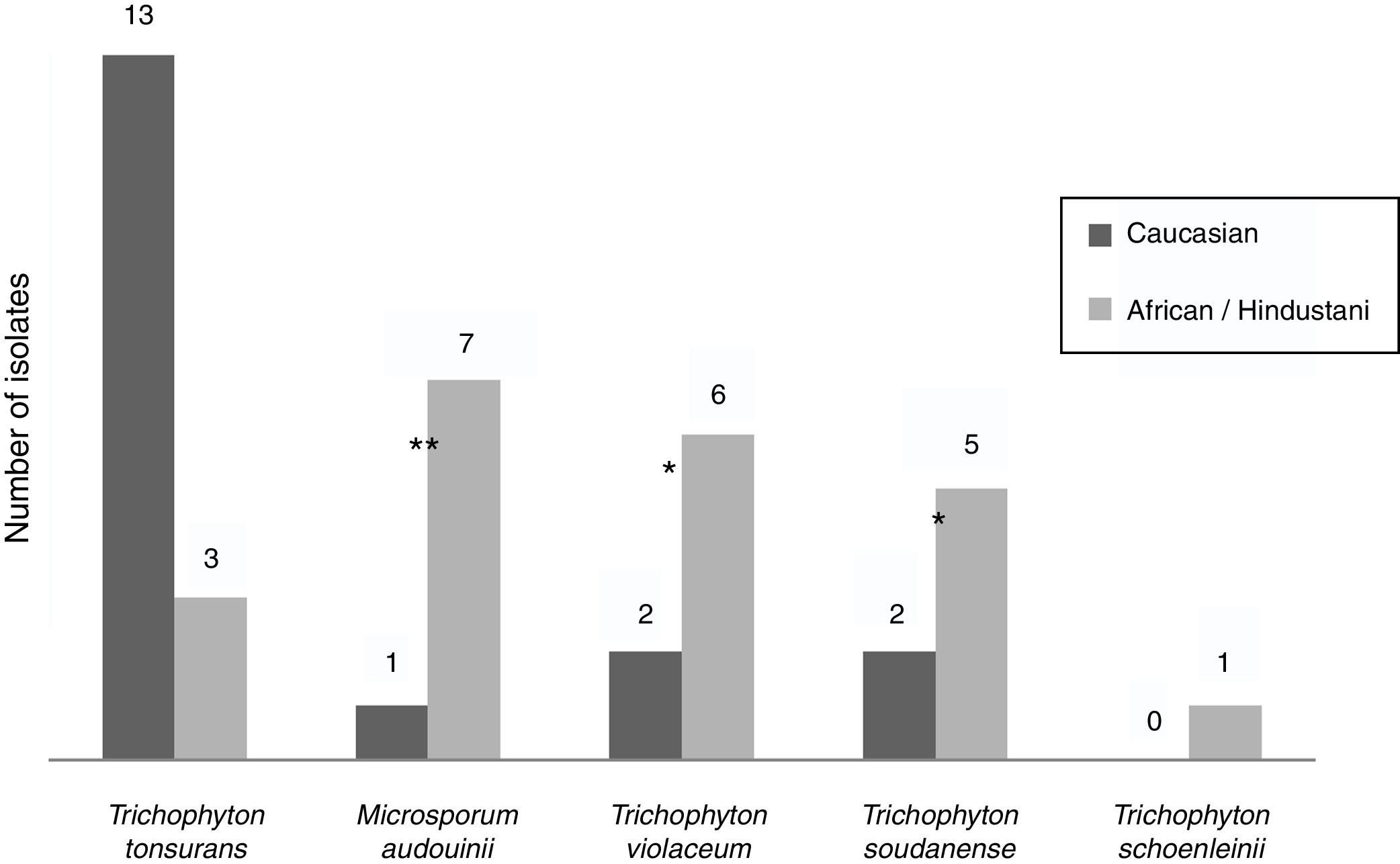
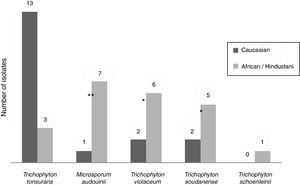
Epidemiological data were recorded from non-endemic dermatophytes. Among the eight T. violaceum specimens isolated, six belonged to patients until 15 (none of them exceeded 7 years) (Table 2). Five patients were of African origin, one was from Pakistan and two were Spaniards (Fig. 2). Further epidemiological data could be obtained from five patients, of which four had moved to Spain a few months ago.
T. soudanense was the causal agent of seven dermatophytoses, of wich five corresponded to African children (three from Gambia and one from Senegal) and two from Spanish children (Fig. 2). Among the latter, one shared school with another African child and the other had a mother of African origin. No data about travels or time of residence could be recorded from African patients.
T. schoenleinii was the dermatophyte agent of tinea capitis favosa (n=1) (Table 2) in a Moroccan woman of 41 years, diagnosed in early childhood in his country of origin (Fig. 2).
M. audouinii was the causative agent of eight dermatophytoses (Table 2). Out of eight patients, seven were African children (two from Guinea and one from Mali) and one from Spain (Fig. 2). Further epidemiological information could only be recorded from four patients, of which two became infected in their country of origin.
T. tonsurans was the etiological agent of 16 dermatophyte infections (Table 2). Nine patients were Spaniards, four from South America and three from Africa (Fig. 2). Further epidemiological data could be recorded in eight cases. One local patient contracted the disease in South America, while another reported an outbreak within the family.
T. soudanense (p<0.0001), M. audouinii (p<0.0001), T. tonsurans (p=0.0002; OR=7.18) and T. violaceum (p=0.0002; OR=18.68) were significantly associated with patients up to 15 years of age.
Moreover, T. soudanense (p=0.0047; OR=10.20), M. audouinii (p=0.0004; OR=24.74) and T. violaceum (p=0.0002; OR=18.68) were associated with patients from Africa/Hindustan. An analysis of T. schoenleinii could not be performed due to the scarcity of isolates from this species.
An analysis of non-endemic dermatophytes grouping T. soudanense, M. audouinii, T. violaceum and T. schoenleinii as non-endemic dermatophytes was conducted (T. tonsurans was excluded from this analysis based on the univariate analysis). In the univariate analysis, non-endemic dermatophytes were associated with African/Hindustani origin (p<0.0001; OR=16.47) and patients until 15 years of age (p<0.0001; OR=50.20). When stratified by age, non-endemic dermatophytes were associated with African/Hindustani origin only in the population up to 15 years (p<0.0001). In the multivariate analysis, non-endemic dermatophytes (all except T. tonsurans were considered non-endemic) were linked to ages until 15 years (p<0.001), tinea capitis (p=0.0017) and African/Hindustani origin (p<0.001).
DiscussionDermatophytoses are a major public health problem, affecting 20–25% of the global population.3,4 Their epidemiology has changed significantly throughout the 20th century,4 and their spread has been linked to factors such as economic status,3,8,12,13 population growth,4 immigration,5,7,8,11,14 living conditions4 or contact with animals.8,15
In the present study tinea unguium pedum was the predominant dermatophytic infection (40.4%) among the population over 15 years of age, unlike previous studies performed in Spain,16,17 but consistent with a national study performed by Monzon et al.5 in 2001 and with recent surveys in Europe.13,18 The frequency of tinea pedis and tinea unguium pedum increased after World War II,4 and several sociocultural factors, such as occlusive footwear or the use of swimming pools, have been related to this increase.13,18 As expected, tinea unguium manuum (p=0.010; OR=3.25) and tinea unguium pedum (p<0.001; OR=4.9) were associated with patients over 15 years of age (Fig. 1). The increase of tinea unguium with age is well-known. Factors related to this dermatophytose in the elderly include poor peripheral circulation, repeated nail trauma and slower nail growth. By contrast, children and young people have a faster nail growth, a smaller nail surface and are more likely to start treatment at an earlier stage of the disease.13
In parallel, T. rubrum was the main dermatophyte in the present study. This anthropophilic species is distributed worldwide4 and its growth is favored in humid environments.6 It has become the most common etiological agent in the majority of the European surveys performed.5,6,8,19,20 The rise of this dermatophyte and its close relationship with tinea unguium has been widely discussed. Sociodemographic factors, such as the cohabitation of more than one generation, sharing towels and socks or the presence in family members have been related to the spread of the fungus.13
Tinea pedis was poorly represented in our study (9.8% among the population over 15 years and 1.2% up to 15 years), which is remarkable regarding other epidemiological surveys.5,6,16,18,21,22 The lack of clinical signs in a great percentage of cases could explain this finding. A study conducted by Perea et al.23 in Madrid found that 55% of tinea pedis infections in healthy individuals were asymptomatic; the authors also observed a high prevalence of concomitant tinea unguium and tinea pedis, explaining the underrepresentation of tinea pedis in our work. Besides, in many cases, this tinea does not need for microbiological confirmation, and specimens are not usually sent to the laboratory. Similar to tinea unguium, tinea pedis was associated with the population over 15 years (p<0.0001).
Tinea capitis was the most prevalent (53.6%) dermatophytosis among patients up to 15 years, according to other Spanish and international surveys.5,7,12T. rubrum was the most frequent dermatophyte in tinea capitis, which contrasts to previous Spanish studies, in which M. canis predominated.5,16,17,24 Generally, cats and dogs are the reservoir of M. canis,24 and the low prevalence of this fungus in the present study could be explained by the absence of stray animals in this area. We observed other anthropophilic fungi as etiological agents of tinea capitis, such as T. tonsurans, T. soudanense, M. audouinii and T. violaceum. Kieliger and colleagues remarked the spread of these fungi in Zurich, mostly isolated in patients of African origin. They suggested contagion within the family unit as a necessary condition for their transmission.25 In another French study, the authors also noted the high prevalence of such Trichophyton species as etiological agents of tinea capitis, mainly in patients from West Africa and coinciding with the return of children from their holidays in Africa.26
T. rubrum was also the main dermatophyte in tinea corporis, which has been observed in other surveys from Spain, Sweden and Poland.5,10,27 However, in other studies from Italy28 and Greece,22M. canis was still the leading cause of tinea corporis.
T. mentagrophytes/T. interdigitale was the second most common dermatophyte and was mainly found in tinea corporis and tinea unguium pedum. Zoophilic strains of T. mentagrophytes are difficult to distinguish from anthropophilic T. interdigitale by optic microscopy, requiring molecular assays for this purpose,29 which is a limitation of this study. In India, however, Chowdhary and colleagues have suggested that the epidemic observed in their country was more likely due to an anthropophilic species.29 In the work carried out by Mazon et al. from 1989 to 1994, T. mentagrophytes was the main dermatophyte in tinea corporis,16 while in another survey performed by Monzon et al. in 2001, T. mentagrophytes was the second etiological agent in tinea unguium and tinea pedis and the most isolated fungus in tinea barbae/tinea facei and tinea manuum.5 Boz and collaborators described T. mentagrophytes (var. Mentagrophytes) as the second most common agent of tinea capitis among the pediatric population in Southern Spain. The spread of zoophilic species such as T. mentagrophytes has been widely described in the past century in Spain, mainly associated with rural environments and close contact with animals.3,24
The presence of anthropophilic species such as T. soudanense, T. schoenleinii, M. audouinii, T. tonsurans or T. violaceum as etiological agents of tinea capitis and tinea corporis (mostly in patients up to 15 years of age) is remarkable. Although extensive epidemiological data was difficult to obtain, most of patients were of African origin, except for T. tonsurans (the majority were Spanish). The association of T. tonsurans with Spanish patients is not surprising. In other European countries, such as England and France, T. tonsurans has become the most common cause of tinea capitis.3,26 However, further studies are needed to understand the prevalence and origin of this fungus among the study population.
Univariate and multivariate analyses revealed an association between these species with patients up to 15 years, tinea capitis and with patients from Africa/Hindustan (T. tonsurans was the only one not associated with African/Hindustani origin). Moreover, in some cases (four T. violaceum, one T. schoenleinii and two M. audouinii) there was evidence that these patients contracted the infection in their country of origin. These dermatophytes were highly prevalent in Spain and the rest of Europe at the beginning of the 20th century, mainly associated with tinea capitis.4,6 Nowadays, they are endemic throughout the African continent.12 With advanced globalization, the reappearance of anthropophilic dermatophytes has been observed in Europe, provoking outbreaks in kindergartens and schools.11,14
Previous surveys have already reported the emergence of these anthropophilic species across Europe.5,7–9,21,30 In Spain, a study performed by Monzón et al. documented the significant prevalence of T. violaceum and T. tonsurans among patients of African origin, mainly in the scalp.5 In another study, Teodolinda et al. observed a predominance of T. violaceum as etiological agent of tinea capitis among African patients, although they seemingly contracted the infection in Italy.7
The presence of asymptomatic carriers in some studies is also noteworthy.9,11,14 In a prospective study conducted by Cuetara et al. from 1994 to 1996 with 10,000 healthy children, the authors detected 19 asymptomatic carriers (13 were T. tonsurans).9 In another study in Paris, Gits-Muselli reported a high number of asymptomatic carriers of M. audouinii.26
This study has some limitations. Firstly, it only reflects positive cultures from specimens delivered to the laboratory. Therefore, it may not be fully representative of all clinically diagnosed dermatophytoses. Secondly, the use of partial epidemiological data hampers to determine where the disease was contracted, which would allow us to a better understanding of the epidemics dynamic of the above mentioned non-endemic dermatophytes. Moreover, the African/Hindustani group reflects a heterogeneous population with very diverse origins. This is mainly due to the lack of sociodemographic information for many patients, of whom it was difficult to determine the country of origin. However, the objective of the present study was not to describe the epidemiology of superficial mycoses in these countries, but to study the evolution and possible causes of dermatophytoses in our environment.
In conclusion, we confirmed the spread of T. rubrum as the predominant dermatophyte in our area, replacing zoophilic species such as M. canis or T. mentagrophytes. Moreover, we reported an increase in non-endemic anthropophilic dermatophytes in the last few years, especially in the African and Hindustani population up to 15 years of age. These agents are highly contagious and require continuous monitoring. However, prospective population-based studies are needed to understand the determinants of dermatophytosis in the study area.
Ethical approvalThe project related to this manuscript was approved by the Ethical Committee of the Hospital Germans Trias i Pujol.
FundingThis research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflict of interestNone declared.