The reported incidence of parapneumonic pleural effusion, including empyema, has shown fluctuations in the last decades. It has been related to the implementation of different types of conjugate pneumococcal vaccines.
MethodsWe have retrospectively reviewed data from all 10 public hospitals in Alicante Province (Spain) covering a population of 279,000 children under 15 years of age, between 2010 and 2018. Effusions less than 10 mm (PE−) and those of 10 mm or more (PE+) were separated.
ResultsA total of 366 episodes of parapneumonic pleural effusion have been analyzed, 178 PE− (48.6%) and 188 PE+ (51.4%), with a median age of 4 years (interquartile range: 2–7 years) and marked seasonality with the maximum in winter and the minimum in summer. A culture proven bacterial agent was identified in 34 patients (9.3%), mainly Streptococcus pneumoniae (24 patients) followed by Streptococcus pyogenes (7 patients). The most frequent S. pneumoniae serotype was 19A (6 patients) and 3 vaccine failures were observed. The mean annual incidence rate was 14.3 cases per 100,000 children under 15 years of age (7.0 for PE− and 7.3 for PE+). No significant changes were observed in incidence over time, but noticeable differences in incidence were observed in different health departments.
ConclusionsWe have not found temporal variations in incidence of parapneumonic effusion despite the implementation of the 13-valent pneumococcal conjugate vaccine. The unexplained disparity in incidence between close departments is noteworthy.
La incidencia del derrame pleural paraneumónico, incluyendo el empiema, ha sufrido variaciones en las últimas décadas, que se han relacionado con la implantación de distintos tipos de vacuna antineumocócica conjugada.
MétodosSe han revisado retrospectivamente los datos de los 10 hospitales públicos de la provincia de Alicante (España), que abarcan una población de 279.000 niños menores de 15 años, entre 2010 y 2018. Se desglosaron los derrames menores de 10 mm (DP−) y los de 10 mm o más (DP+).
ResultadosSe han analizado 366 episodios de derrame pleural paraneumónico, 178 DP− (48,6%) y 188 DP+ (51,4%), con una mediana de edad de 4 años (rango intercuartílico: 2–7 años) y una evidente estacionalidad con máximo en invierno y mínimo en verano. Se identificó al agente etiológico por cultivo en 34 pacientes (9,3%), destacando Streptococcus pneumoniae (24 pacientes) seguido por Streptococcus pyogenes (7 pacientes). El serotipo de S. pneumoniae más frecuente fue el 19A (6 pacientes) y se han identificado 3 fallos vacunales. La tasa anual media de incidencia fue de 14,3 casos por 100.000 menores de 15 años (7,0 para DP− y 7,3 para DP+), sin cambios significativos a lo largo del tiempo, aunque sí se apreciaron diferencias marcadas de la incidencia entre los distintos departamentos sanitarios.
ConclusionesNo hemos encontrado variaciones temporales en la incidencia del derrame paraneumónico pese a la implementación de la vacuna antineumocócica conjugada de 13 serotipos. Es destacable la variabilidad de la incidencia entre departamentos vecinos sin motivo aparente.
Parapneumonic pleural effusion, including empyema (PPE/E), is the most common complication of pneumonia in children and Streptococcus pneumoniae is its primary causal agent.1 Its incidence is not well known and it may vary geographically and over time. In recent decades, an apparent increase in the incidence of empyema had been observed in children and adults in Spain and in other parts of the world.2–4. Although there had been speculation about its relationship with the introduction of the 7-valent pneumococcal conjugate vaccine (PCV7) as of the year 2000 due to the predominance of infections by serotypes not included in this vaccine the increase had already been observed before it was introduced, hence the causes of this increase are not clear. Other more recent studies have observed a decrease in the incidence of PPE/E in the last decade, related to the introduction of the 13-valent pneumococcal conjugate vaccine (PCV13)5–8, although there are also discordant works.9
Our group carried out a retrospective review of all patients hospitalised for PPE/E in the well-defined setting of a province between 2010 and 2018. Some clinical and therapeutic aspects have already been published.10 The objective of this work is to understand patients' epidemiological characteristics and to calculate the incidence of PPE/E and its evolution in a complete population-based cohort over time. It should be borne in mind that the 10-valent pneumococcal conjugate vaccine was authorised in 2009, that the PCV13 was authorised in 2010 and was included in the routine vaccination schedule in the Valencian Community with public funding for everyone born after 1 January 2015.
MethodsParticipating hospitals and geographic scopeThe 10 public health system hospitals in the province of Alicante that are the reference centres for their respective health areas and cover the entire population of the province participated in the study. According to data from the National Institute of Statistics, the average annual population of the province during the study period was 279,031 children under 15 years of age, with a discreet progressive decrease from a maximum of 284,640 in 2011 to a minimum of 272,818 in 2018. All paediatric patients are entitled to treatment in the 10 hospitals of the public network. Paediatric hospitalisation in private hospitals is rare and patients are transferred to public hospitals when they suffer from diseases that require complex or prolonged care.
Patient selectionThe admission services of the participating hospitals prepared a list of episodes that met the following criteria:
- -
Hospitalisation occurring between 2010 and 2018 inclusive.
- -
Patients under 15 years of age.
- -
Diagnosis of pleural effusion or empyema, either as a primary or secondary diagnosis, of an infectious nature or not. Patients with the following diagnostic codes ICD-9: 510.0, 510.9, 511.1, 511.81, 511.89, 511.9; or ICD-10: J86.0, J86.9, J90, J91, J91.8 were selected.
These hospital admissions were reviewed individually to exclude cases of pleural effusion of a non-infectious cause and those caused by tuberculosis.
VariablesSex, age (in completed years), year and month of admission and place of residence were recorded. Episodes of pneumonia that started during hospital admission for another reason were considered to be of nosocomial origin. PPE/E size was classified in two groups, based on the maximum thickness observed in any of the imaging tests (chest radiography, ultrasound or computed tomography) performed during hospitalisation: PPE/E less than 10 mm (PPE−) and PPE/E of 10 mm or greater (PPE+). The blood culture and pleural fluid culture results were recorded. The aetiological agent was considered reliable when the growth of typically pathogenic bacteria was reported, excluding possibly contaminating or doubtfully pathogenic bacteria. The antibiogram and serotyping of the strains of Streptococcus pneumoniae isolated in the cultures, as well as the vaccination status of the patients in whom this bacterium was isolated, were reviewed. The results of microbiological tests other than culture were not considered because they are performed rarely and with differing frequency in the participating hospitals.
Statistical analysisThe data obtained were processed using the SPSS® program, version 22. The frequency and percentage were calculated for the qualitative variables and the median and interquartile range for the quantitative variables. To calculate the annual incidence rate (AIR), the numerator used was the number of patients who met the inclusion criteria each year and who lived in one of the health areas of the province of Alicante, with patients who lived outside the province excluded. In the denominator, the annual population under 15 years of age in the province was calculated as the mean of the population registered between 1 January of that year and 1 January of the following year with the data obtained from the National Statistics Institute (www.ine.es). To calculate the AIR by areas, the denominator used the mean of the monthly data of the population under 15 years of age for each area and each year obtained from the Population Information System of the Valencian Community health system. The Pearson χ2 test was used for the analysis of qualitative variables and the Mann-Whitney U test for comparisons between quantitative variables. The Pearson (r) linear correlation coefficient was calculated to determine the relationship between the AIR of the PPE+ and PPE− in the different areas. Hypothesis tests were two-tailed, with a significance of 0.05.
EthicsThe study was approved by the Medicines Research Ethics Committee of the Department of Health of Alicante - Hospital General.
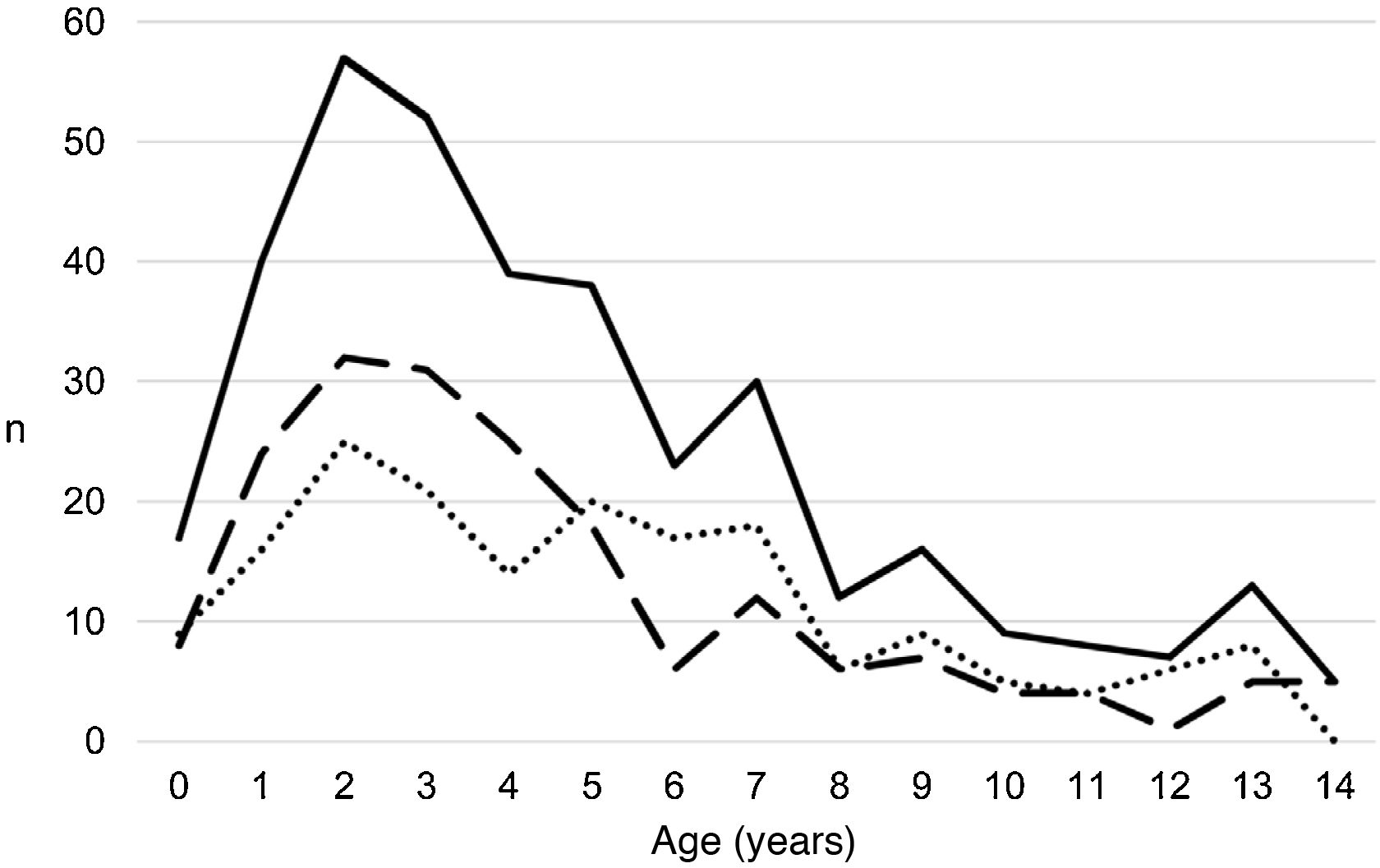
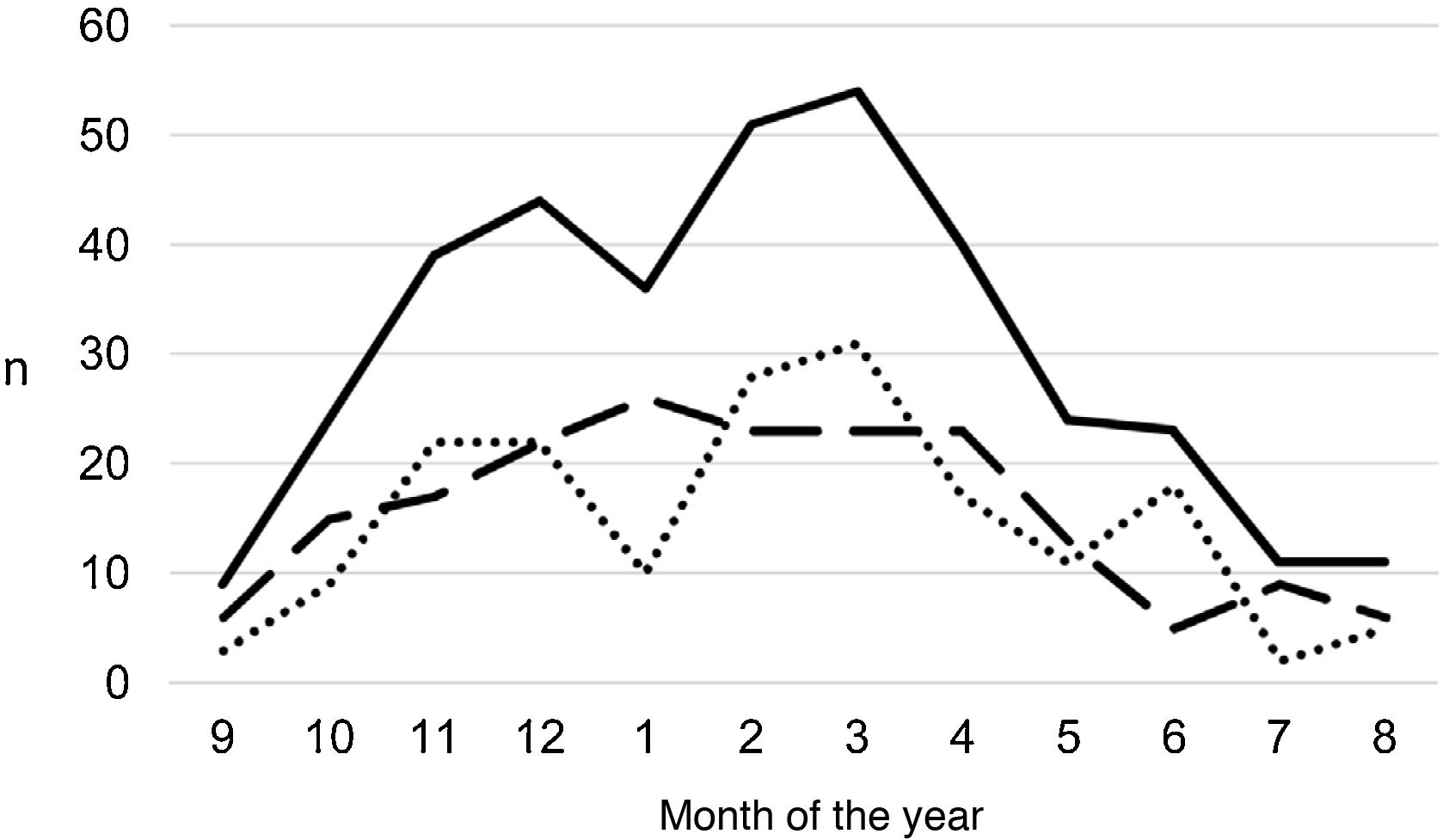
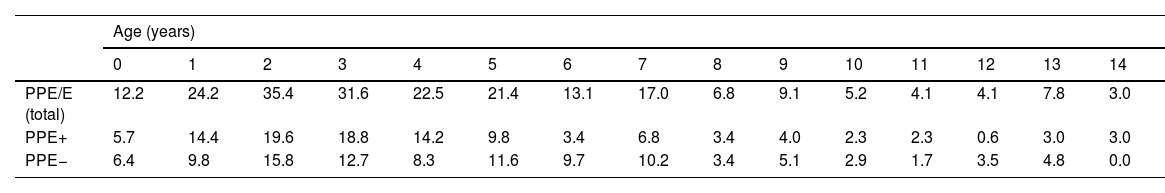
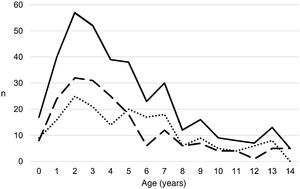
ResultsA total of 366 PPE/E episodes that met the inclusion criteria were collected, 361 of which were considered community-based and five (1.4%) hospital-acquired. Only 1 patient presented 2 episodes in the study period, separated by almost 3 years, and this patient's data were analysed as independent episodes. Of the 366 episodes, 178 (48.6%) corresponded to a PPE− and 188 (51.4%) to a PPE+. There was a slight predominance of males (54.6%, p = 0.076) and the median age was 4 years (interquartile range: 2–7 years). As can be seen in Fig. 1, 62% of the patients were between 1 and 5 years old and 76% were between 1 and 7 years old, with the patients with PPE+ being younger than the patients with PPE− (median 3 vs. 5 years, p = 0.030). There were no differences in the size of the effusions between males and females (p = 0.229). There was a clear seasonality of the PPE/E with the maximum in winter and the minimum in summer (Fig. 2). Blood culture results were retrieved from 296 patients, 47 of whom were positive, although only 27 (9.1%) were considered reliable. Pleural fluid culture results were obtained from 63 patients, with growth obtained in 15, of whom 11 (17.5%) were considered reliable. A reliable aetiological agent was identified in 34 patients: 7 with PPE− (3.9%) and 27 with PPE+ (14.4%) (p = 0.001). The agents identified were Streptococcus pneumoniae in 24 patients (17 by blood culture, 5 in pleural fluid and 2 in both cultures), Streptococcus pyogenes in 7 (4 by blood culture, 1 in pleural fluid and 2 in both cultures), and Staphylococcus aureus (blood culture), Haemophilus influenzae (pleural fluid) and Pseudomonas aeruginosa (blood culture in 1 patient with myeloid leukaemia) in 1 patient each. In another 22 patients, the growth of germs of dubious pathogenicity, possibly contaminants, was obtained, mainly Staphylococcus hominis and Staphylococcus epidermidis. The serotypes of Streptococcus pneumoniae observed were: 19A (6 isolates), 1 (3 isolates), 3 (3 isolates), 7 F (2 isolates), 33 F (2 isolates), 6A and 6C (1 isolate each), it being unknown in the remaining 6 cases. Of the patients in whom Streptococcus pneumoniae was isolated, 5 had received the PCV7 (with an incomplete schedule in 1 case), all of them hospitalised between 2010 and 2011, and 8 had received the PCV13 (with an incomplete schedule in 2 cases), all of them hospitalised between 2013 and 2017. Nine (9) patients were not vaccinated against Streptococcus pneumoniae, while the datum was unknown in 2 patients. Three (3) of the patients correctly vaccinated with PCV13 had an infection by Streptococcus pneumoniae serotypes, theoretically covered by the vaccine: 3, 7 F and 19A, respectively. Of the 24 isolates of Streptococcus pneumoniae, 15 were sensitive to penicillin, 5 had some degree of resistance (4 serotype 19A and 1 unknown serotype) and in 4 this datum was unknown, while 18 were sensitive to cefotaxime, 1 had intermediate resistance (serotype 19A) and the datum was unknown in 5.
Distribution of the number of cases of parapneumonic pleural effusion and empyema by age. Total cases are represented with a continuous line, cases with a thickness of 10 mm or greater are represented with a dashed line, and cases with a thickness of less than 10 mm are represented with a dotted line.
Distribution of the number of cases of parapneumonic pleural effusion and empyema by months of the year, beginning with the ninth month of the year (September). Total cases are represented with a continuous line, cases with a thickness of 10 mm or greater are represented with a dashed line and cases with a thickness of less than 10 mm are represented with a dotted line.
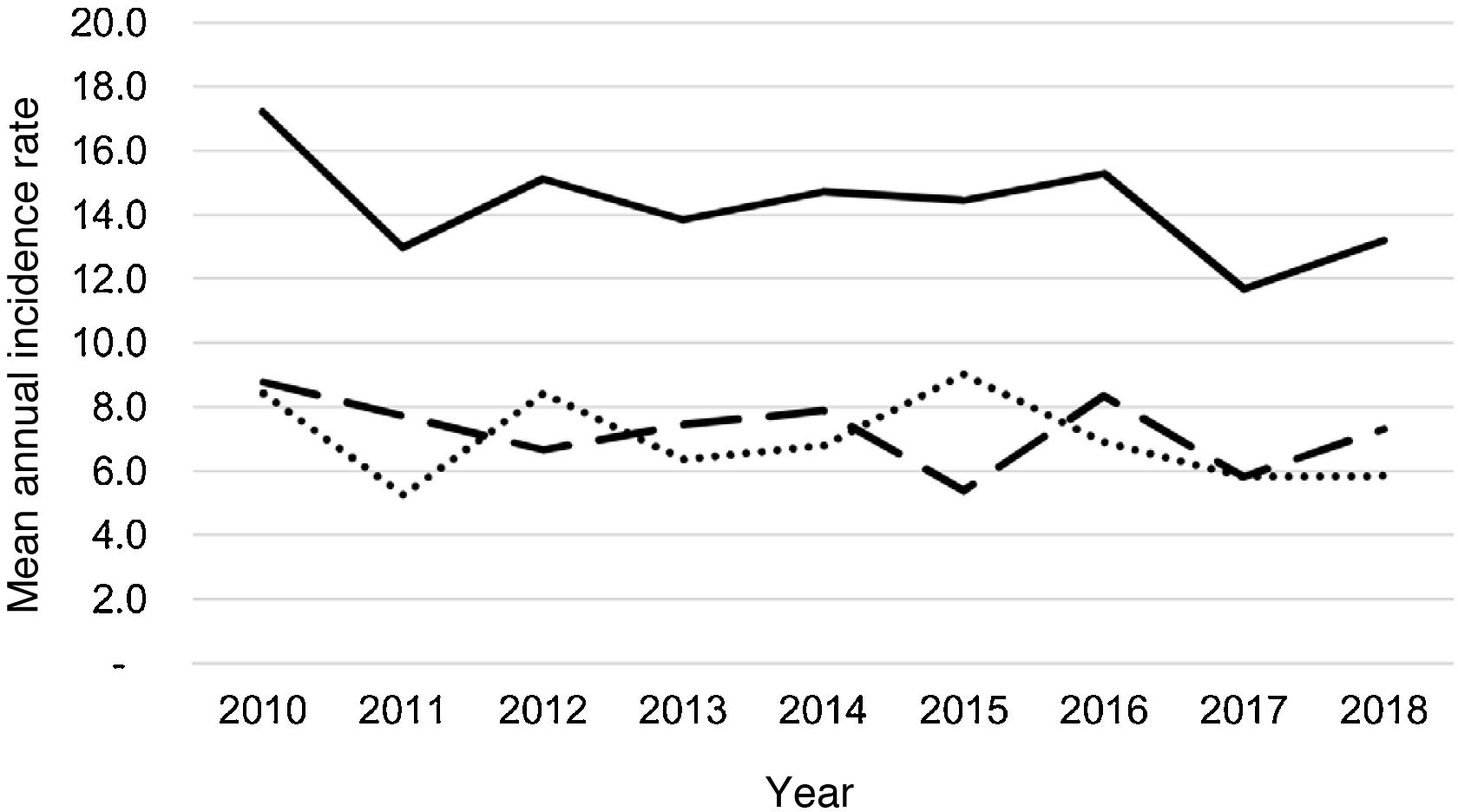
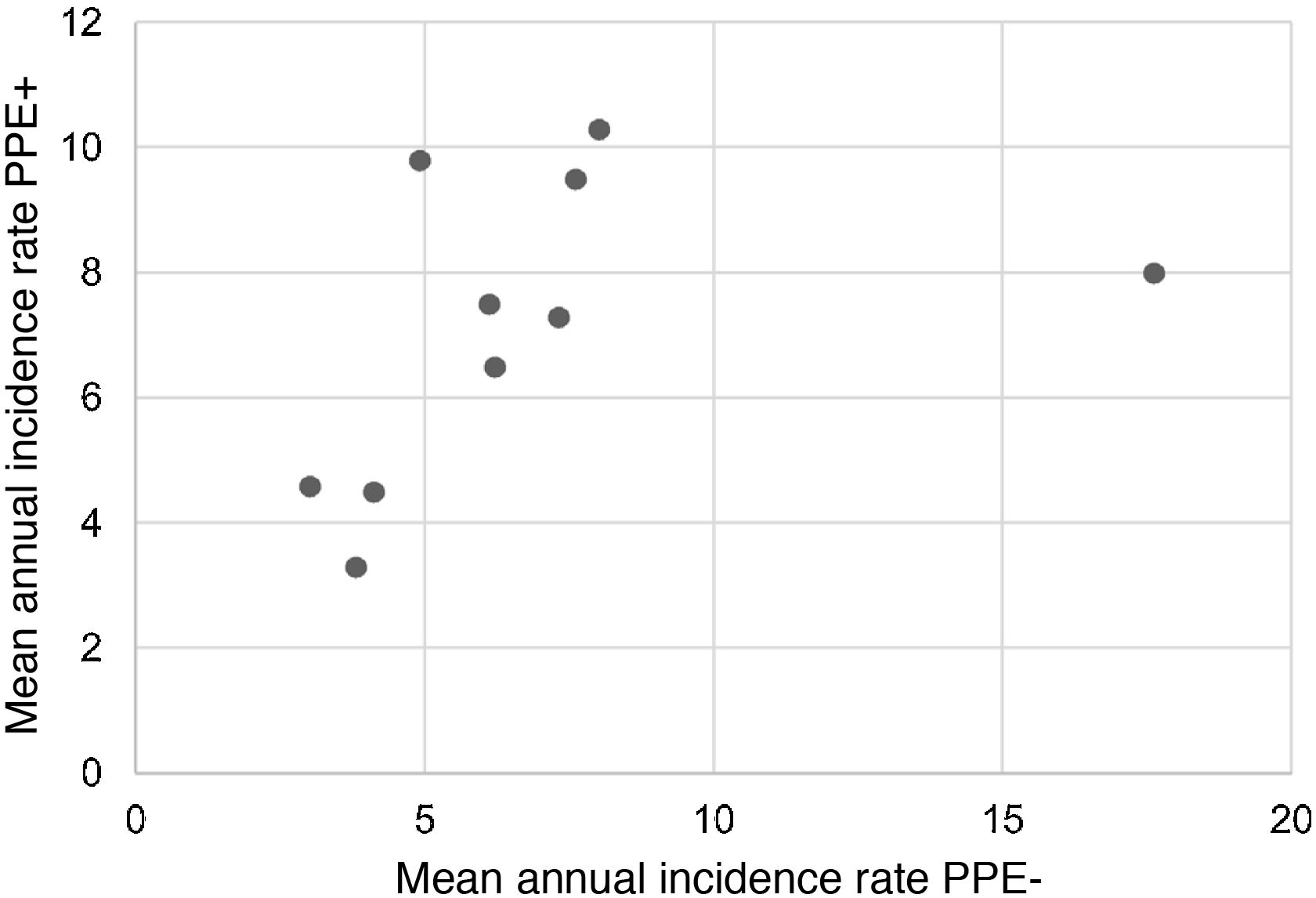
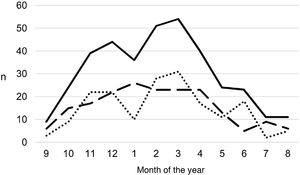
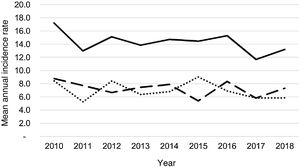
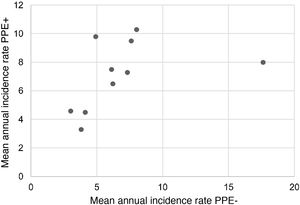
During the study period, an average of 41 patients with PPE/E were admitted per year (maximum 50 in 2010 and minimum 33 in 2017). To calculate the AIR, data from 359 episodes were analysed, after 7 patients who lived outside the province of Alicante had been excluded. The average AIR of PPE/E in residents of the province of Alicante between 2010 and 2018 was 14.3 cases per 100,000 children and adolescents under 15 years of age, corresponding to 7.0 per 100,000 for PPE− and 7.3 per 100,000 for PPE + . As can be seen in Fig. 3, the AIR remained relatively stable or slightly decreased in the course of the study, both globally and when small and large effusions are itemised. The incidence rate by age is shown in Table 1, with a slightly different distribution for PPE+ and PPE−, superimposable to that observed in Fig. 1. A wide variation of the mean AIR was observed in the province's health areas, ranging from 7.1 to 25.6 per 100,000 children under 15 years of age. Fig. 4 shows the marked relationship between the AIR of patients with PPE− and PPE+ in the different health areas, except in one of them, which presented a discordantly high AIR of PPE−. When the data from this discordant area are removed, a strong positive correlation between the AIR of PPE− and PPE+ (r = 0.769, p = 0.015) was observed, which disappeared when the data from that area were also included (r = 0.429, p = 0.216).
Annual incidence rate of parapneumonic pleural effusion and empyema (cases per 100,000 inhabitants under 15 years of age) during the years of the study, calculated in the resident population. Total cases are represented with a continuous line, cases with a thickness of 10 mm or greater are represented with a dashed line and cases with a thickness of less than 10 mm are represented with a dotted line.
Mean annual incidence rate (per 100,000) of parapneumonic pleural effusion distributed by age.
| Age (years) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | |
| PPE/E (total) | 12.2 | 24.2 | 35.4 | 31.6 | 22.5 | 21.4 | 13.1 | 17.0 | 6.8 | 9.1 | 5.2 | 4.1 | 4.1 | 7.8 | 3.0 |
| PPE+ | 5.7 | 14.4 | 19.6 | 18.8 | 14.2 | 9.8 | 3.4 | 6.8 | 3.4 | 4.0 | 2.3 | 2.3 | 0.6 | 3.0 | 3.0 |
| PPE− | 6.4 | 9.8 | 15.8 | 12.7 | 8.3 | 11.6 | 9.7 | 10.2 | 3.4 | 5.1 | 2.9 | 1.7 | 3.5 | 4.8 | 0.0 |
PPE/E: parapneumonic pleural effusion, including empyema; PPE+: parapneumonic pleural effusion of thickness equal to or greater than 10 mm; PPE−: parapneumonic pleural effusion less than 10 mm thick.
Correlation between the mean annual incidence rates of PPE− and PPE+ in the 10 areas included in the study, expressed in cases per 100,000 inhabitants under 15 years of age. Each dot represents a health area. The strong positive correlation between the detection of PPE− and PPE+ is observed, except in one area, where the rate of PPE− (but not PPE+) is discordantly high.
As in most series, we observed a slight predominance of the male sex, with a higher incidence of PPE/E in preschool children, more accentuated for PPE+.5,7,9,11–23 The seasonal distribution was consistent with other studies, with a maximum during winter-spring and a minimum in summer.11,13,15,19,21 Although the causal agent could not be identified in most patients, Streptococcus pneumoniae was the most frequently isolated germ, followed by Streptococcus pyogenes, similar to that which is observed in most works.1–3,5,7,9,13,14,16,17,20,24–26Staphylococcus aureus was barely detected in our patients compared to findings in some populations.12,15,18,22,23,27 The Streptococcus pneumoniae serotypes observed in our patients matched those reflected in many studies.2–9,13–16,24,25,28–31 Although most of the isolates were susceptible to penicillin, serotype 19A, predominant in our series, was clearly associated with resistance to penicillin, as described in other studies.4,6,24
The incidence of PPE/E in the province of Alicante remained relatively stable for almost an interpandemic decade, after the 2009 influenza pandemic and before the 2020 coronavirus pandemic, coinciding with the implementation period of the PCV13. We made a distinction between PPE− cases, which are usually less severe and more likely to go undetected, and PPE+, which would largely correspond to complex effusions and empyema.10 The latter represent the most serious spectrum of the disease, hence the hospitalisation, detection and registration related to these cases should be less exposed to biases between hospitals. The AIR was distributed approximately equally between the PPE+ and PPE− and no changes in the trend in either were observed.
The effect of the introduction of pneumococcal conjugate vaccines on the incidence of PPE/E over the last 2 decades is controversial. Following the introduction of PCV7, an increase in the incidence of empyema was observed, related in part to the replacement of pneumococcal infections by serotypes not included in the vaccine, although this increase had also been observed before the introduction of the vaccine and could be due to other causes, including improved diagnostic methods.2–5,11,12,20,26–29,32,33 Following the introduction of PCV13, most studies have observed a decrease in the incidence of pleural effusion and empyema, especially in younger children5–8,22,28,33–35, although other authors present discordant results that have been related to a possible low efficacy against some serotypes covered by PCV13, particularly serotype 3.7,9,13,15,30,31 In our series, three patients correctly vaccinated with PCV13 presented infections due to serotypes included in the vaccine, as observed in other studies.5,15,25,28,30,31
It is not easy to compare our incidence rates with those of other studies carried out in different geographical or time settings due to methodological differences, including different diagnostic methods, inclusion criteria or definitions of PPE/E.2,3 Our mean PPE/E AIR is close to the figure calculated in Navarre between 1995 and 2014 (16.1 cases per 100,000 children under 15 years of age), although in Navarre significant variations were observed over time, with an increase of 6.2–25.6 per 100,000 following the introduction of PCV7 and a progressive tapering off to 12.01 per 100,000 after the introduction of PCV13.5 Our AIR is somewhat lower than the 18.17 cases per 100,000 children under 18 years of age observed in a study conducted between 2009 and 2018 in Germany, where a decrease was observed after the introduction of PCV13, followed by a new increase in the following years.36 Many of the studies screen for patients with complex effusions or empyemas, partially comparable to our PPE+ patients. Between 2010 and 2017, the incidence of this type of effusion in Germany ranged between 1.4 and 1.8 cases per 100,000, with no significant changes in the trend.13 After the introduction of the PCV13, the AIR of empyema fell in the USA, stabilising at around 2 cases per 100,000 children34, while in Australia the AIR increased from 1.4 to 1.8 cases per 100,0009. One notable aspect of our work is the fact that a wide variability in the PPE/E rates in the different areas of the province was found, although no easy explanation has been found for this. The consistency observed between the rates of PPE− and PPE+ demonstrates that these differences are real, due to the lower risk of detection bias of PPE+, while the high AIR of PPE− in one of the areas may be due to an overdiagnosis of this kind of effusion in that hospital. Few studies have pointed out the presence of regional differences in the incidence of pleural empyema in children.18,23 In general, studies from different parts of the world evince the broad variations in AIR due to PPE/E.6,12,19,22,23,28,33,35
Our work has similar limitations to most published studies, due to its retrospective nature and because it depends on the quality of hospital records. The size of the effusion was determined with different imaging techniques in each patient. However, the distinction between a thickness greater or less than 10 mm is relatively straightforward with the chest radiography, which is often the only test conducted for smaller effusions. Although vaccination data were not available for all the patients included, it is clear that the vaccination rate with PCV13 must have increased progressively during the study period, especially after it was introduced into the public health system-funded vaccination schedule. One important limitation of our work is the lack of knowledge of the aetiological agent in most of our patients due to the known low yield of blood cultures and because pleural fluid did not need to be obtained.7,9,10,13,24–26 Although Streptococcus pneumoniae continues to be the most commonly detected germ, the low rate of bacterial isolation limits the conclusions that can be drawn in relation to the predominant serotypes and resistance to antibiotics as well as the impact of vaccination. Despite these limitations, our comprehensive review of all patients hospitalised with any type of pleural effusion enabled us to minimise the possibility of losing patients due to coding errors in a comprehensive population of nearly 280,000 children under 15 years of age over a 9-year period. Other studies have included less well-defined populations and have included only patients with a diagnostic code for parapneumonic pleural effusion or empyema despite the fact that the distinction between these entities is not well defined and that coding may depend on local criteria or on other factors such as imaging techniques or obtaining pleural fluid, which are not always performed.12
To conclude, PPE/E particularly affects young children during the winter months and Streptococcus pneumoniae is the main agent identified. The incidence of PPE/E in the paediatric population of our province has not varied significantly in the last decade despite the introduction of the PCV13. The broad variability of the incidence rate between nearby areas is striking, and not easy to explain, but it is consistent with the differences observed in other studies in different geographical areas. Our data may serve as a reference for studies carried out with the same methodology in other places or at other times in order to understand the influence of different factors such as the introduction of vaccines or epidemic or pandemic outbreaks caused by infectious agents.
FundingNo funding was received for this study.
Conflicts of interestThe authors declare that they have no conflicts of interest.