The aim of this study was to analyse the incidence, treatment and evolution of infections in children treated with ECMO.
MethodsA retrospective study based on a prospective database was performed. Children under the age of 18 years treated with ECMO from September 2006 to November 2015 were included. The patients’ clinical characteristics were collected, together with ECMO technique, cultures and treatment of infection.
ResultsOne hundred patients with a median age of 11 months were analysed. Heart disease was diagnosed in 94 patients. An infection was suspected and antibiotic treatment was initiated in 51 patients, although only 22 of them were microbiologically confirmed. The most common infection was sepsis (49%), followed by pneumonia (35.3%) and urinary tract infection (9.8%). There were no differences in haematological parameters and acute phase reactants between children with infection and those without. Children who died had a higher incidence of infection during ECMO (60.4%) than the survivors (40.3%), but the difference did not reach statistical significance (p=0.07). The duration of admission in the PICU was 57 days in patients with infection vs. 37 days in patients without infection but the difference was not statistically significant (p=0.067).
ConclusionsInfection in children with ECMO is common. There are no specific infection parameters and less than half of the clinical infections are confirmed microbiologically. There was no statistically significant correlation between infection and mortality or duration of PICU stay.
El objetivo de este estudio es analizar la incidencia de infección en los niños tratados con ECMO, el tratamiento y su evolución.
MétodosSe realizó un estudio retrospectivo basado en una base de datos prospectiva en el que se incluyeron los niños menores de 18 años asistidos con ECMO entre septiembre de 2006 y noviembre de 2015. Se recogieron las características de los pacientes, la ECMO, los cultivos y el tratamiento de la infección.
ResultadosSe estudiaron 100 pacientes de 11 meses de edad mediana. El diagnóstico fue de cardiopatía en 94 pacientes. Se sospechó una infección y se inició antibioterapia en 51 pacientes, aunque solo se confirmó microbiológicamente en 22. Las infecciones más frecuentes fueron la sepsis (49%), neumonía (35,3%) e infección urinaria (9,8%). No existieron diferencias en los parámetros hematológicos y reactantes de fase aguda entre los niños con infección y el resto. Los niños que fallecieron presentaron mayor incidencia, no estadísticamente significativa, de infección durante la ECMO (60,4%) que los supervivientes (40,3%) (p=0,07). La duración de ingreso en la UCIP fue mayor, sin significación estadística, en los pacientes con infección que en el resto: 57 frente a 37 días (p=0,067).
ConclusionesLa frecuencia de infección en niños con ECMO es elevada, pero menos de la mitad son confirmadas microbiológicamente, sin existir parámetros específicos de infección. No se ha encontrado relación estadísticamente significativa de la infección con la mortalidad y la duración de ingreso en la UCIP.
Extracorporeal membrane oxygenation (ECMO) is a life-support technique indicated for patients with heart and/or lung failure refractory to conventional treatment.1,2
Patients treated with ECMO have a high risk of complications as the technique is invasive and their clinical condition is serious.3,4 A number of factors converge in patients treated with ECMO to increase their risk of infection. Cannulation of large vessels for ECMO disrupts the body protection barrier and provides a gateway for pathogens. In addition, these patients have other invasive devices such as central venous and arterial catheters, endotracheal tubes and urinary catheters, which create an increased risk of nosocomial infection.1,5 Moreover, a significant percentage of patients are immunocompromised due to their disease itself, surgery or the treatments that they require.
Diagnosis of infections in patients who undergo ECMO is complicated due to a lack of specific early signs and symptoms. The technique itself and the underlying diseases cause increased biomarkers of tissue inflammatory response, which decreases their usefulness in diagnosis of infection.6,7 Furthermore, many patients receive broad-spectrum antibiotic therapy, which complicates the diagnosis of infection and predisposes them to infections with multidrug-resistant micro-organisms. All these factors impede the diagnosis and treatment of infection in children with ECMO, and may increase their morbidity and mortality.
The primary objective of this study was to examine the incidence, risk factors and type of infection in children treated with ECMO and their relationship to mortality and length of stay on the paediatric intensive care unit (PICU). The secondary objective was to assess the usefulness of a complete blood count and acute-phase reactants in early diagnosis of infection in children with ECMO.
Patients and methodsAn observational study was conducted through a retrospective analysis of a prospective database of children admitted to the PICU at Hospital General Universitario Gregorio Marañón and treated with ECMO. The study was approved by the local independent ethics committee, and the standards for access to the data in the patients’ medical records were followed.
The inclusion criterion was having received ECMO circulatory assistance on the PICU between September 2006 and November 2015.
The following variables were collected: clinical data (date of birth, sex, weight, height, body surface area and diagnosis), data on ECMO (start and end dates, type of ECMO, type of cannulation, prior surgery and characteristics thereof, time of placement of ECMO, and number of circuit changes), infection-related data (suspicion of clinical infection; leukocytes; CRP and PCT at baseline, 24h, 48h, 72h, 5 days and 7 days; cultures during and after the end of ECMO; antibiotic therapy; and course of the infection) and data on the patient's clinical course (survival and length of stay on the PICU).
There were no previously established criteria for infection. Suspicion of clinical infection was determined by the physician in charge of the patient according to general clinical symptoms (fever and worsening of general condition) or local clinical symptoms (e.g. worsening of respiratory condition accompanied by new infiltrates on chest X-ray) as well as increased leukocytosis and acute-phase reactants.
A blood culture, bronchial aspirate and urine culture were collected every 72h as well as before the start of antibiotic treatment in suspected infection.
Patients on ECMO with cervical cannulation received surgical prophylaxis if they were taking cefazolin at doses of 30mg/kg/8h for 48h in the postoperative period following heart surgery. Patients on ECMO with transthoracic cannulation and open-heart surgery received treatment with piperacillin-tazobactam and teicoplanin. Statistical analysis was performed with the IBM SPSS (version 21.0) software programme. In the descriptive study, the quantitative variables that had a normal distribution were presented with means and standard deviations (SDs), and those that did not meet the assumption of normality (according to the Kolmogorov–Smirnov test and the Shapiro–Wilk test) were presented with medians and interquartile ranges (IQRs) (p25-p75). Qualitative variables were presented as percentages. The χ2 test and Fisher's exact test were used to compare qualitative variables. Student's t test was used to compare means in quantitative variables. The Wilcoxon signed-rank test was used for variables without a normal distribution. A p value <0.05 was considered statistically significant.
ResultsA total of 100 children treated with ECMO were studied. Their median age was 11 months (IQR: 4–66 months), and their median weight was 7.4kg (IQR: 4.8–16.8kg). Of these children, 67% were male. In addition, 94% had heart disease on admission, 5% had respiratory failure and 1% had septic shock. In 64% of cases, ECMO was started in the postoperative period following heart surgery. ECMO was performed through cervical cannulation in 55 patients, transthoracic cannulation in 30 patients and both types of cannulation in 15 patients. Their median duration of ECMO was 5.5 days (IQR: 3–9 days).
InfectionsDuring ECMO, an infection was suspected in 51 patients (51%). The most common infection was sepsis (49%), followed by respiratory infection (35.3%), urinary infection (9.8%), intra-abdominal infection (3.9%) and surgical wound infection (2%). The infection was microbiologically confirmed in just 22 cases (43.1%).
The rates of confirmed infection per 1000 days of ECMO assistance were as follows: sepsis (40), respiratory infection (34), urinary infection (10), surgical wound infection (4.5) and intra-abdominal infection (3).
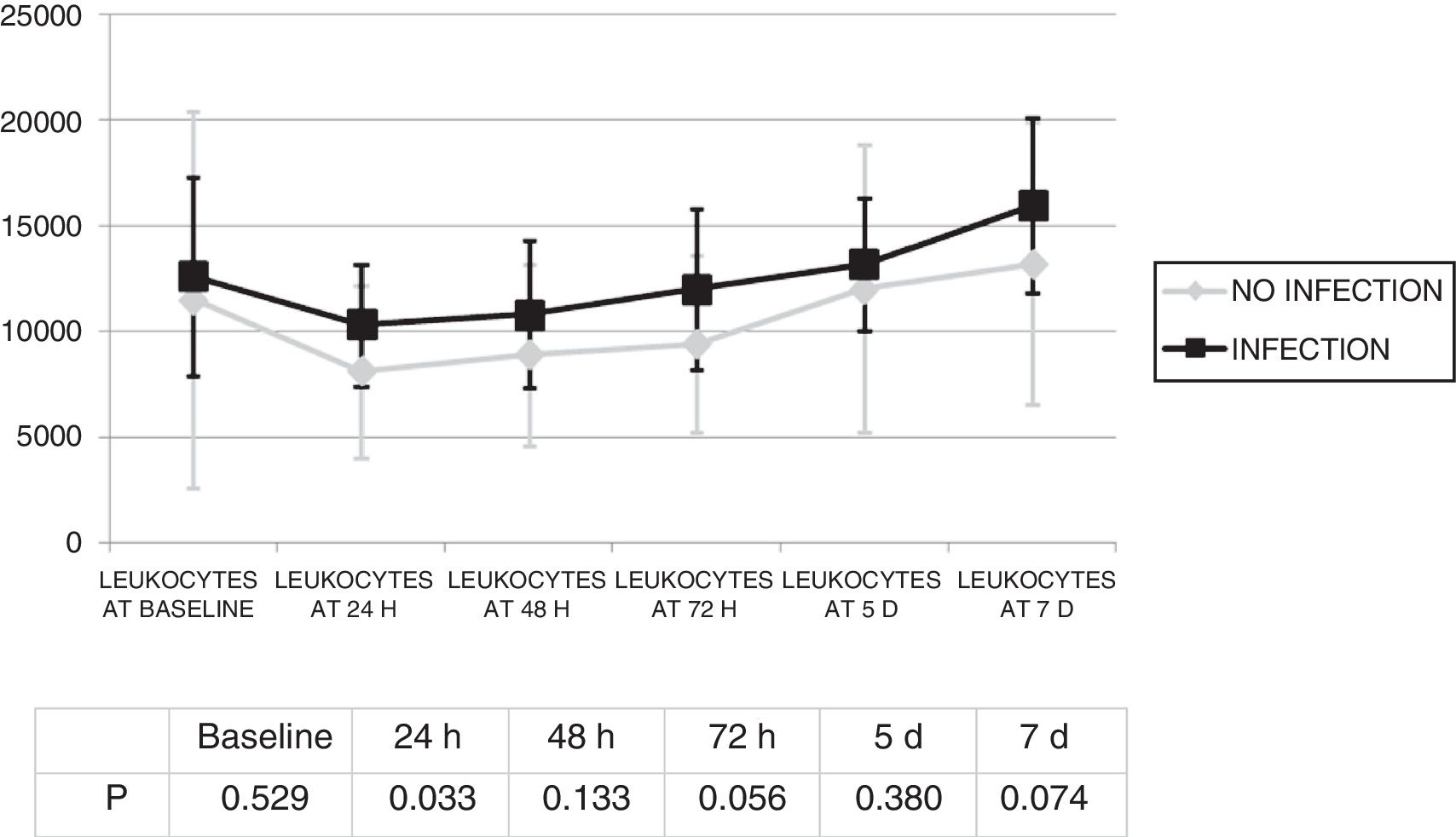
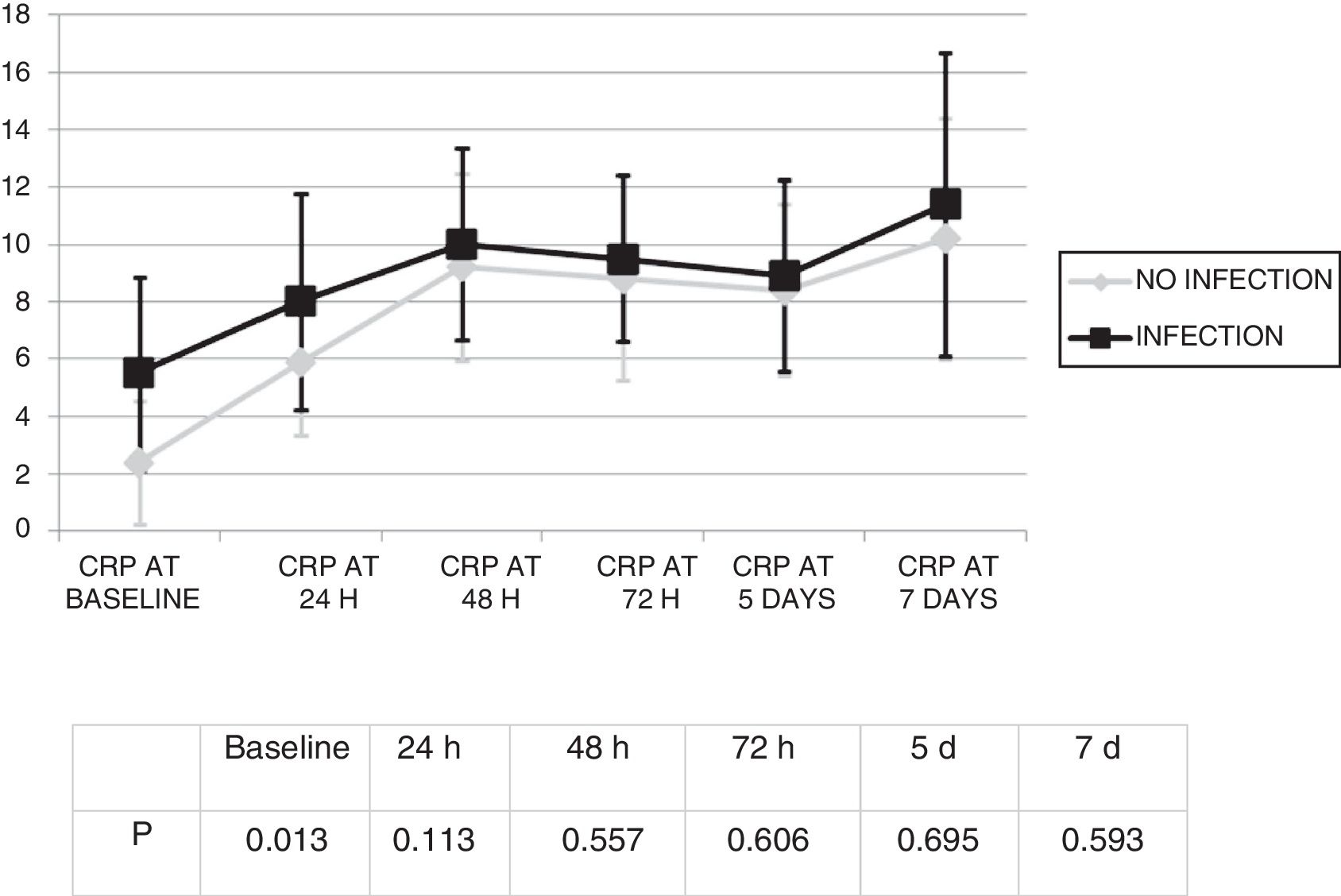
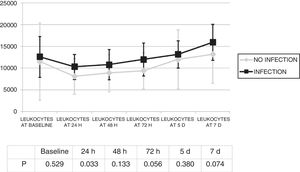
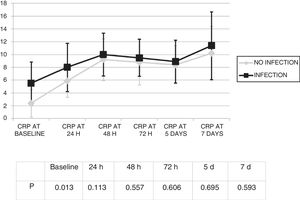
Changes in leukocytes and CRP were analysed for the first 7 days after ECMO was started. There were no significant differences in terms of leucocyte levels (Fig. 1) or CRP values (Fig. 2) between infected patients and non-infected patients, except in leukocytes at 24h and in baseline CRP.
The infection was diagnosed 4.8 (SD: 4) days from the start of ECMO. There were no statistically significant differences in terms of leukocytes on the day of the diagnosis of the infection (12,805.7) (SD: 7367.6) compared to leukocytes at the start of ECMO (1934.2) (SD: 9883.4) (p=0.945).
By contrast, a significant increase in CRP values was seen, from 3.3 (SD: 4.7) mg/dl to 10.6 (SD: 8.6) mg/dl (p<0.01).
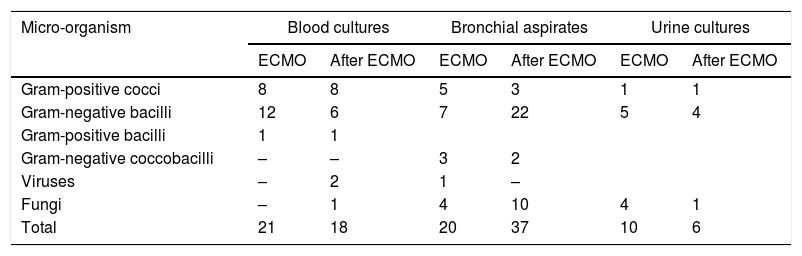
Table 1 shows the results of the microbiological cultures. Some 15.1% (21 out of 139) blood cultures performed during ECMO were positive, and some 11.5% (21 out of 182) performed in the 15 days after ECMO were positive. Of the bronchial aspirates performed during ECMO assistance, 25.9% (20 out of 77) were positive; of those performed after completing ECMO, 22.6% (37 out of 163) were positive. Some 10.4% (10 out of 96) of the 96 urine cultures performed during ECMO were positive, and some 6.4% (6 out of 94) of the urine cultures performed subsequently were positive. Just one extended-spectrum β-lactamase–producing Klebsiella pneumoniae was isolated.
Micro-organisms found in cultures.
| Micro-organism | Blood cultures | Bronchial aspirates | Urine cultures | |||
|---|---|---|---|---|---|---|
| ECMO | After ECMO | ECMO | After ECMO | ECMO | After ECMO | |
| Gram-positive cocci | 8 | 8 | 5 | 3 | 1 | 1 |
| Gram-negative bacilli | 12 | 6 | 7 | 22 | 5 | 4 |
| Gram-positive bacilli | 1 | 1 | ||||
| Gram-negative coccobacilli | – | – | 3 | 2 | ||
| Viruses | – | 2 | 1 | – | ||
| Fungi | – | 1 | 4 | 10 | 4 | 1 |
| Total | 21 | 18 | 20 | 37 | 10 | 6 |
ECMO: extracorporeal membrane oxygenation.
Fungi.
Blood culture: Candida 1.
Bronchial aspirates: Candida 13, Aspergillus 1.
Urine culture: Candida 5.
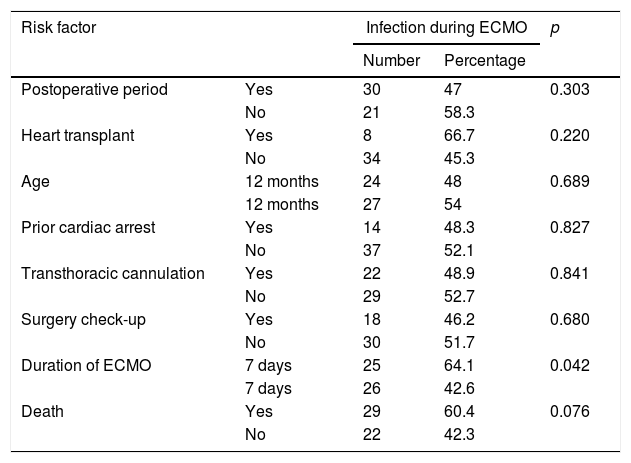
Table 2 shows factors linked to infection during ECMO. Of the variables studied, only a duration of ECMO longer than 7 days was associated with a higher frequency of clinical infection (p=0.042). Patients who died had a higher frequency of clinical infection than survivors; however, the differences were not statistically significant (p=0.076).
Factors associated with infection during ECMO.
| Risk factor | Infection during ECMO | p | ||
|---|---|---|---|---|
| Number | Percentage | |||
| Postoperative period | Yes | 30 | 47 | 0.303 |
| No | 21 | 58.3 | ||
| Heart transplant | Yes | 8 | 66.7 | 0.220 |
| No | 34 | 45.3 | ||
| Age | 12 months | 24 | 48 | 0.689 |
| 12 months | 27 | 54 | ||
| Prior cardiac arrest | Yes | 14 | 48.3 | 0.827 |
| No | 37 | 52.1 | ||
| Transthoracic cannulation | Yes | 22 | 48.9 | 0.841 |
| No | 29 | 52.7 | ||
| Surgery check-up | Yes | 18 | 46.2 | 0.680 |
| No | 30 | 51.7 | ||
| Duration of ECMO | 7 days | 25 | 64.1 | 0.042 |
| 7 days | 26 | 42.6 | ||
| Death | Yes | 29 | 60.4 | 0.076 |
| No | 22 | 42.3 | ||
ECMO: extracorporeal membrane oxygenation.
Surgical prophylactic antibiotic therapy was administered prior to ECMO in 97.4% of patients with cefazolin 60mg/kg/day with a median duration of 2 days (IQR: 2–6).
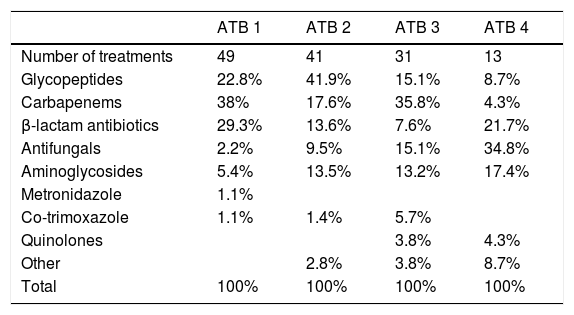
Just 8% of patients did not receive antibiotic therapy during ECMO. Among those who did, 18% received one antibiotic, 21% received 2, 30% received 3 and 23% received 4. The antibiotic most commonly used as initial antibiotic therapy was meropenem (38%), followed by piperacillin/tazobactam (19.6%) and teicoplanin (17.4%). The median duration of antibiotic therapy was 10 days (IQR: 6–15).
Many patients received sequential antibiotic therapy due to suspected infection. A cycle of antibiotic therapy was defined as an occasion on which one or more antibiotics were started due to suspicion of a new infection or non-resolution of the infection with prior antibiotic therapy. The antibiotics used in each cycle of antibiotic therapy are shown in Table 3.
Antibiotics used during treatment. First to fourth cycle of antibiotics.
| ATB 1 | ATB 2 | ATB 3 | ATB 4 | |
|---|---|---|---|---|
| Number of treatments | 49 | 41 | 31 | 13 |
| Glycopeptides | 22.8% | 41.9% | 15.1% | 8.7% |
| Carbapenems | 38% | 17.6% | 35.8% | 4.3% |
| β-lactam antibiotics | 29.3% | 13.6% | 7.6% | 21.7% |
| Antifungals | 2.2% | 9.5% | 15.1% | 34.8% |
| Aminoglycosides | 5.4% | 13.5% | 13.2% | 17.4% |
| Metronidazole | 1.1% | |||
| Co-trimoxazole | 1.1% | 1.4% | 5.7% | |
| Quinolones | 3.8% | 4.3% | ||
| Other | 2.8% | 3.8% | 8.7% | |
| Total | 100% | 100% | 100% | 100% |
ATB: antibiotic.
Twenty-five patients presented infections in the 15 days after ECMO was stopped.
Ventilator-associated pneumonia was the most common infection (40%), followed by sepsis (32%), surgical wound infection (16%) and urinary tract infection (12%).
Clinical courseECMO assistance could be stopped in 76 patients: in 62 because their clinical condition improved, in 7 because they switched to a ventricular assistance system and in another 7 because they underwent a heart transplant.
Fifty-two patients survived to hospital discharge. The mortality of children with suspected infection during ECMO was 60.4%, and the mortality of children without suspected infection was 42.3% (p=0.076). The mortality of patients with suspected infection during ECMO was higher (60.4%) than the mortality of patients with infection subsequent to ECMO (24%) (p=0.017).
There were no statistically significant differences between those who survived and those who died in each type of infection: sepsis (39.5% versus 48.6%), respiratory infection (39.5% versus 31.4%), urinary infection (7% versus 14.3%), surgical wound infection (7% versus 5%) and intra-abdominal infection (4.7% versus 0%) (p=0.520).
The main cause of death in patients with suspected infection was multiorgan failure (57.1%), followed by neurological complications (20%). In patients with no suspected infection, the most common causes of death were neurological complications (30.8%), followed by multiorgan failure (23.1%). There were no statistically significant differences in terms of cause of death between patients with infection and patients without infection (p=0.158).
The median length of stay on the PICU was 45.7 days (SD: 37.3). The length of stay of patients with suspected infection was longer (50.1 days) (SD: 39.6) than that of patients without infection (24.9 days) (SD: 9.4 days); however, this difference did not achieve statistical significance (p=0.06). There were no differences in terms of length of stay on the PICU between children with microbiologically confirmed infection (52.2 days) (SD: 43.7) and children without microbiologically confirmed infection (48.3 days) (SD: 36.4) (p=0.176).
Patients who had infections during ECMO had a longer mean length of stay on the PICU (57 days) (SD: 46) than patients who had infections after ECMO (39.2 days) (SD: 25.9); however, these differences, too, were not statistically significant (p=0.161).
DiscussionIncidence and diagnosis of infectionThe incidence of infection in our study (51% had suspected infection and 22% had microbiologically confirmed infection) was higher than that reported in the ELSO registry (18.7%).3 These differences may be due in part to the fact that the ELSO registry, unlike our study, only includes microbiologically confirmed infections and infections occurring during ECMO.3 We preferred to refer to infection when there was a clinical suspicion (although in patients with ECMO it is very complicated to distinguish infection from other clinical complications) and patients were treated with antibiotics. This probably led us to overestimate the incidence of infection somewhat.
The most common infections in our patients were sepsis (49%) and ventilator-associated pneumonia (35.3%). This was in line with that reported in the ELSO registry.3,5
The biggest problem in making an early diagnosis of infection is that both CRP and PCT are inflammatory markers which increase due to underlying disease, surgery or even ECMO.6 Therefore, it is also necessary to use other clinical signs, although they are not specific either and may also be abnormal for other reasons, such as tachycardia and hypotension, and an increased need for vasoactive drugs.8
Not all positive cultures should be considered infection because a significant percentage of critically ill patients may be colonised and not infected (as in, for example, most bronchial aspirates positive for Candida). Infection should only be considered when a positive culture is accompanied by consistent clinical and laboratory findings.2 Moreover, broad-spectrum antibiotic therapy started early in patients with a clinical suspicion may mean that some cultures fail to test positive.9
Risk factors for infectionOther studies have linked infection with duration of ECMO,2,4,8,10 urgency with which the device is implanted,8 more serious patient condition, transthoracic cannulation, mechanical ventilation, prolonged parenteral nutrition and older age of children.2,11 In our study, only a duration longer than 7 days was associated with infection during ECMO. However, it must be noted that infection could also influence the need to prolong ECMO. The incidence of infection in children with ECMO following surgery was no higher than in non-surgical patients, nor were there any differences with respect to age.
Antibiotic therapyIn our study, antibiotic therapy was administered prior to ECMO in 97.4% of patients, but as prophylaxis for heart surgery and not due to having started ECMO. The use of prophylactic antibiotic therapy in children with ECMO is a very controversial subject. A systematic review by O’Horo et al. did not find antibiotic prophylaxis to reduce the incidence of infection in children or adults with ECMO.12 According to the ELSO registry, the only patients who have been found to benefit from antibiotic prophylaxis are those who undergo open-heart surgery, since they are at higher risk of mediastinitis, which is linked to a higher mortality rate.4,5,12
In any case, it is important not to use broad-spectrum antibiotic prophylaxis and for this not to be of prolonged duration, as it hinders early diagnosis of infection and predisposes the patient to infections with multidrug-resistant micro-organisms.13
The most commonly used empirical antibiotics in our study were meropenem and piperacillin/tazobactam, in accordance with our protocol for nosocomial infection in critically ill children. However, during the study, our antibiotics policy changed. Now, piperacillin/tazobactam and teicoplanin are used as initial antibiotics,1 and meropenem is reserved for infections with extended-spectrum β-lactamase–producing micro-organisms and micro-organisms resistant to other drugs.
Clinical courseSeveral studies have found a higher mortality rate in patients on ECMO with confirmed infection.3,14 By contrast, our study found no differences in terms of mortality rate between children with infection (microbiologically confirmed or not) and children without infection. However, we found infection during ECMO to be associated with higher mortality than infection after ECMO; this finding had not been analysed in prior studies.
LimitationsOur study had several limitations. First, as it was a retrospective analysis, there were limitations with respect to collection of some data and interpretation of the diagnosis of infection and the indications for antibiotic therapy. Clinical infection was suspected at the discretion of the physician in charge of the patient according to clinical and laboratory findings. Although this does reflect clinical practice, it may have biased the results. Moreover, as the study was a single-centre study conducted on a leading PICU in heart surgery, it may not be entirely possible to extrapolate our data to another centre with another type of patient. Laboratory data was compared at fixed periods of time after the start of ECMO but time to development of infection varied. Finally, the number of patients was relatively small for analysing some variables.
ConclusionsThe frequency of infection in children with ECMO was high. Less than half of clinical infections were microbiologically confirmed, and no specific parameters aided in early diagnosis of infection. We did not find a statistically significant relationship between, on the one hand, infection and, on the other hand, mortality and length of stay on the PICU.
FundingInstituto de Salud Carlos III (PI15/00743). Maternal and Child Health and Development Research Network (SAMID Network). RETICS funded by the PN R&D&i 2008–2011 (Spain), ISCIII Sub-Directorate General for Research Assessment and Promotion and the European Regional Development Fund (ERDF), ref. RD16/0022/0007.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Santiago-Lozano MJ, Barquín-Conde ML, Fuentes-Moreno L, León-Vela RM, Madrid-Vázquez L, Sánchez-Galindo A, et al. Complicaciones infecciosas en niños tratados con oxigenación por membrana extracorpórea. Enferm Infecc Microbiol Clin. 2018;36:563–567.