Mycoplasma genitalium is now a well-recognized sexually transmitted infection (STI), known to cause urethritis and other adverse reproductive tract conditions in both men and women.1 Antimicrobial resistance has become a major concern in treating M. genitalium infections. The macrolide azithromycin, given as an extended 1.5g-dose (500mg day 1, 250mg days 2–5), has been the recommended first-line treatment against uncomplicated infections.2 However, macrolide resistance has rapidly emerged, exceeding 50% in many countries,3 likely enhanced by the widespread use of this antibiotic. The fourth-generation fluoroquinolone moxifloxacin is currently the second-line antibiotic used to treat macrolide resistant infections.2 Even so, in vivo resistances are increasingly being reported in many countries, including Spain.4,5 Fluoroquinolone resistance is associated with single nucleotide polymorphisms (SNPs) in the quinolone determining region (QRDR) of the parC gene affecting amino-acids S83 and D87.6,7
Since data regarding fluoroquinolone resistance in M. genitalium remains limited in local population, the aim of this retrospective study was to estimate the rate of fluoroquinolone resistance-associated mutations among a 2016–2017 series of individuals in Barcelona, Spain, using the recently validated commercial MG+parC (beta) assay (SpeeDx, Australia).8
Specimens were collected at the Microbiology Department of the Vall d’Hebron University Hospital, as a part of a previous study,9 between December 2016 and February 2017. Demographic and clinical data are published elsewhere.9 A total of 82 baseline samples from 80 patients, coming from different clinical settings (hospitals, primary care centers and STI clinics), were included.
Briefly, the samples were processed as follows. First, samples were tested for M. genitalium by real-time PCR (qPCR) using the Allplex™ STI Essential Assay (Seegene, South Korea). Then, positive samples were retrospectively screened for macrolide resistance genotypic markers using the commercial qPCR ResistancePlus® MG assay (SpeeDx, Australia) and Sanger sequencing. DNA extracts were finally stored at −20°C for subsequent analyses. For the current study, samples were tested for fluoroquinolone resistance-associated mutations using the MG+parC (beta) assay.8 The MG+parC (beta) test is a novel two-well qPCR assay that detects M. genitalium through the mgpB (MG191) gene and five parC mutations linked with moxifloxacin treatment failure: G248T (S83I) in well 1, and A247C (S83R), G259A (D87N), G259T (D87Y) and G259C (D87H) in well 2. Ethical approval for the study was obtained from the Vall d’Hebron University Hospital Ethics Committee (351/2018).
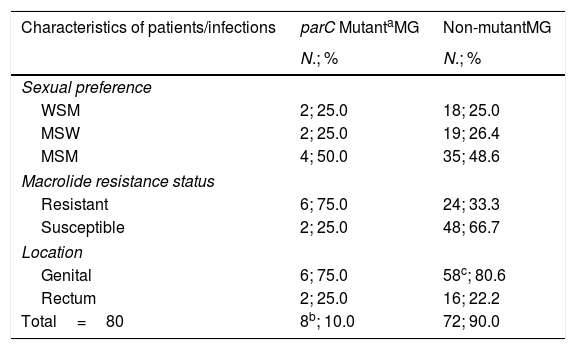
Among the 80 infections studied, fluoroquinolone resistance-associated mutations were detected in eight cases (10.0% [95% confidence interval (CI), 4.4–18.8%]). Results are described in Table 1. Of them, three infections (37.5% [95% CI, 8.5–75.5%]) harbored mutation G248T (S83I), individually reported by the MG+parC (beta) assay. Detection of the remaining parC mutants were reported through a single channel in well 2. Furthermore, macrolide resistance was significantly more prevalent among parC mutants (75.0% [95% CI, 34.9–96.8%]) compared to wild-type (WT) infections (33.3% [95% CI, 22.7–45.4%]); (OR 6.0 [95% CI, 1.1–32.0]), p=0.036.
Prevalence of parC fluoroquinolone resistance-associated mutations in M. genitalium.
| Characteristics of patients/infections | parC MutantaMG | Non-mutantMG |
|---|---|---|
| N.; % | N.; % | |
| Sexual preference | ||
| WSM | 2; 25.0 | 18; 25.0 |
| MSW | 2; 25.0 | 19; 26.4 |
| MSM | 4; 50.0 | 35; 48.6 |
| Macrolide resistance status | ||
| Resistant | 6; 75.0 | 24; 33.3 |
| Susceptible | 2; 25.0 | 48; 66.7 |
| Location | ||
| Genital | 6; 75.0 | 58c; 80.6 |
| Rectum | 2; 25.0 | 16; 22.2 |
| Total=80 | 8b; 10.0 | 72; 90.0 |
Abbreviations: MG, Mycoplasma genitalium; MSW, men who have sex with women; MSM, men who have sex with men; WSM, women who have sex with men.
Mutant category includes the mutants targeted in the MG+parC (beta) assay – A247C (S83R), G248T (S83I), G259T (D87Y), G259C (D87H) and G259A (D87N).
The research provides further data regarding fluoroquinolone resistance in M. genitalium in Spain, where estimates remain limited. In the present study fluoroquinolone resistance-associated mutations were detected in 10% of infections, similar to previous investigations reporting a prevalence of 5%-9%.4,5,8 Additionally, the presence of fluoroquinolone resistance-associated mutations was strongly associated with macrolide resistance in our series (p=0.036). In fact, the prevalence of resistance to both classes of antibiotics was (7.5% [95% CI, 2.8–15.6%]) in the study population. Consequently, multi-drug resistant infections may gradually appear in our settings against which therapeutic options are very scarce.
Recently, a resistance-guided sequential treatment utilizing the novel ResistancePlus® MG assay has been successfully evaluated with promising results in terms of infection eradication and antibiotic resistance selection control.10 In this scenario, the novel commercial MG+parC (beta) assay, demonstrating to be rapid, simple and accurate, could complement this resistance-guided therapy approach with the additional detection of fluoroquinolone resistance-associated mutations in M. genitalium, optimizing and refining antimicrobial stewardship. Nevertheless, despite the test detects known fluoroquinolone resistance-associated SNPs, further studies are required to fully establish the contribution of other newly parC mutations in fluoroquinolone resistance.6,7 Moreover, more investigations on new antibiotics and novel combinations with existing treatments are imperative to fight against M. genitalium that may soon become the next sexually transmitted superbug.
FundingSpeeDx Pty Ltd. supplied all the reagents for molecular testing of M. genitalium and fluoroquinolone resistance.
Conflicts of interestJ.S.P and J.E. employed by the “Vall d’Hebron University Hospital”, have received remuneration for contract work from SpeeDx Pty Ltd. M.E. employed by the “Corporació Sanitària Parc Taulí”, has received remuneration for contract work from SpeeDx Pty Ltd. M.F.H. and M.E. have participated in symposiums organized by SpeeDx Pty Ltd.