Crimean-Congo haemorrhagic fever has been reported in more than 30 countries in Africa, Asia, the Middle East and Eastern Europe, with an increasing incidence in recent years, especially in Europe. Because no specific treatments have demonstrated efficacy, supportive treatment is essential, as well as the provision of a centre with the appropriate means to guarantee the safety of its healthcare professionals. Laboratory monitoring of thrombocytopenia, severe coagulopathy or liver failure is of critical importance. Patients with Crimean-Congo haemorrhagic fever should be admitted to High Level Isolation Units where appropriate biocontainment procedures can prevent nosocomial transmission through infected fluids or accidents with contaminated material. In case of high-risk exposures, early administration of ribavirin should be considered.
La fiebre hemorrágica de Crimea-Congo afecta a más de 30 países de África, Asia, Europa oriental y Oriente Medio, con una creciente incidencia durante los últimos años, especialmente en Europa. Sin un tratamiento específico eficaz, las medidas terapéuticas de soporte son fundamentales, así como disponer de un centro con los medios adecuados para garantizar la seguridad de los trabajadores. La monitorización analítica es esencial para el manejo de la trombocitopenia, la coagulopatía grave o el fallo hepático. La atención a los pacientes con fiebre hemorrágica de Crimea-Congo debe llevarse a cabo en Unidades de Aislamiento de Alto Nivel, capaces de aplicar procedimientos de biocontención que eviten la transmisión nosocomial a través de fluidos infectados o accidentes con material contaminado. En caso de exposiciones de alto riesgo podría plantearse la administración precoz de ribavirina.
Crimean-Congo haemorrhagic fever (CCHF) virus has a broad global distribution.1,2 In 2010, ticks belonging to the genus Hyalomma infected with CCHF virus, whose lineage matched virus strains from Mauritania and Senegal, were identified in Extremadura.3 However, until the first two cases of CCHF were diagnosed in August 2016 in Spain,4 no human cases of autochthonous acquisition had been reported in western Europe.5
CCHF virus belongs to the family Bunyaviridae and the genus Nairovirus. Its main vectors are ticks belonging to the genus Hyalomma.1 Different animals, both domesticated and wild, act as reservoirs for the virus (cats, sheep, goats, horses, donkeys, pigs, hares, hedgehogs, etc.).6 Livestock is one of the most important hosts. Human beings may be infected by either a tick bite or direct handling of infected meat or fluids.6 Nosocomial transmission has been reported by healthcare staff with accidental needle sticks or unprotected exposure to blood, bodily fluids or droplets from patients diagnosed with CCHF.6,7 Infection has also been reported in healthcare workers involved in the care of ill patients, without the exact route of transmission having been identified (in some articles, these cases of infection with no clear source account for up to 10–15% of nosocomial cases).7–9
The spectrum of seriousness of CCHF is highly variable. Seroprevalence studies in endemic areas have suggested that up to 80% of cases are asymptomatic.6,10 The classic clinical course of CCHF has been divided into four periods: incubation, pre-haemorrhagic phase, haemorrhagic phase and convalescence.6,11 Reported mortality ranges from 3% to 30%, depending on the outbreak studied. Mortality is primarily caused by fulminant hepatitis, thrombocytopenia and massive bleeding.6,10,12 In Turkey, since the first cases were identified in 2002, more than 9700 cases have been reported with a mortality rate of approximately 5% which has remained stable over the years.13
CCHF virus is classified as a biosafety level 4 pathogen14 capable of interpersonal transmission. It is advisable to manage patients with a suspected or confirmed diagnosis of CCHF using strict precautions for contact under which the patient is isolated in an individual room, preferably with a separate entrance and exit. Viruses are to be isolated in biosafety level 4 high-containment laboratories, and patients with CCHF are to be managed in high-level isolation units.14,15
Supportive therapy is essential, since there is no effective specific aetiological treatment. Although its use is controversial as no conclusive studies have demonstrated its efficacy, in the most serious cases, it is advisable to use high-dose ribavirin. Other treatments, such as hyperimmune serum from convalescent patients, have not been shown to be useful. Furthermore, there are no vaccines with proven efficacy or safety in humans.2
Protective measures in a hospital setting against Crimean-Congo haemorrhagic feverThe CCHF virus is a biosafety level 4 pathogen that is transmissible and capable of causing nosocomial outbreaks with a high mortality rate.7 Contact with contaminated materials and fluids, contact with blood from gastrointestinal bleeding, accidental needle sticks and surgical operations in patients with an unknown diagnosis have been reported as the main routes of infection in a healthcare setting. Close interpersonal contact with the patient and manipulation of the respiratory tract are routes of transmission that are still subject to debate16; however, nosocomial outbreaks of CCHF have indeed been reported during orotracheal intubation.8 The virus's limited repercussions for domestic settings shows that invasive procedures and unprotected contact in the haemorrhagic phase cause most secondary cases.
Ideally, patients with CCHF should receive care in high-level isolation units equipped for critical care, as CCHF may progress with rapid deterioration. The room should be equipped with negative pressure systems should procedures that generate droplets need to be performed.14,15
Healthcare staff should be well informed about the disease and its possible mechanisms of infection. They should also be suitably educated and have regular training in putting on and taking off personal protective equipment (PPE).6,17 Emergency departments in endemic regions should receive instruction in upholding strict contact precautions and ensuring isolation of any suspected case from the start.16 Some retrospective studies have found that around 50% of cases of nosocomial infection originate in patients not yet diagnosed with CCHF.7
Although universal basic protective barriers could suffice to prevent most cases of nosocomial infection, it should be noted that hospital care is provided to more seriously ill patients with greater viraemia. Existing evidence on nosocomial outbreaks and World Health Organisation (WHO) recommendations for other highly lethal haemorrhagic fevers justify the use of PPE comprising at least the following items: a waterproof gown, gloves, a mask and goggles (or a face screen).8,16 For clinical management of confirmed cases, it is advisable to use the same PPE used to manage Ebola virus disease: a waterproof body suit, double layers of footwear and gloves, a hood, a mask and airtight goggles. PPE must always be suited to the type of healthcare to be provided, as the risk of infection may vary.2,18
Therapeutic managementSymptomatic treatmentDrugs should not be administered via intramuscular injection so as to prevent haematomas and local bleeding at puncture sites.6
It is advisable to administer paracetamol as an antipyretic and to avoid non-steroidal anti-inflammatory drugs to the extent possible due to their potential repercussions for clotting.6
Proton pump inhibitors may be used to prevent gastrointestinal bleeding, which may occur due to either disease complications or stress.6,17,19 In women, inhibition of menstrual bleeding through administration of progesterone may be indicated.17
AntibioticsAlthough antimicrobials should not be used in a confirmed case of CCHF, unless superinfection is suspected,17 they must be considered in an investigational case by assessing the different entities included in the differential diagnosis and the patient's geographic origin.2 The differential diagnosis of CCHF is broad. If the patient's geographic origin is taken into account, it includes Alkhurma haemorrhagic fever and Rift Valley fever in the Middle East; Omsk haemorrhagic fever in Russia; Kyasanur forest disease in India; hantavirus in Europe and Asia; Lassa virus, Ebola virus, Marburg virus, Rift Valley fever and yellow fever in Africa; and dengue fever mainly in Asia and central Africa. In tropical and subtropical countries, malaria is the most important alternative diagnosis to be ruled out. If the transmission vector is taken into account, the following must also be included: Rickettsia spp., Ehrlichia spp., Borrelia, Anaplasma and Babesia. In addition, many other infectious diseases may feature a similar initial clinical picture: tularaemia, Q fever (Coxiella burnetii), viral hepatitis, influenza virus infection, meningococcal meningitis, leptospirosis, typhoid fever, sepsis due to staphylococci or Gram-negative bacilli, toxic shock syndrome, salmonellosis, shigellosis, psittacosis, trypanosomiasis, septic infection due to Yersinia pestis, rubella and measles.2
GlucocorticoidsThe efficacy of glucocorticoids for the treatment of CCHF has not been confirmed.19 Studies dedicated to evaluating their usefulness are limited and consist of small case series in both adults and paediatric patients. They have found that administration of high-dose methylprednisolone (20–30mg/day) seems to promote early haematological recovery, reverse haemorrhagic lesions and decrease the need for transfusion of blood products. Their results have been inconclusive due to patients simultaneously receiving ribavirin.20,21 Should a patient develop a haemophiliac syndrome secondary to CCHF, treatment with corticosteroids would be more clearly indicated, since this does have demonstrated efficacy in cases secondary to infections by other microorganisms.22,23
Supportive therapyCare for patients with CCHF requires careful monitoring of vital signs to detect organ failure early and start the necessary supportive care immediately.6
CCHF requires close laboratory monitoring: complete blood count, alanine non-transferable (ALT), aspartame non-transferable (AST), total Rubin, creating, international normalised ratio (INR), activated partial thromboplastin time (aPTT), creatine kinase and lactate dehydrogenase. In case of a platelet count below 40,000mm−3, it would be advisable to perform at least two rounds of laboratory testing per day. If disseminated intravascular coagulation (DIC) is suspected, d-dimer and arterial blood gas values should be obtained.17,19
Suitable electrolyte replacement must be ensured and fluid therapy must be adjusted; hypotonic solutions are to be avoided.19 In the event of kidney failure, which would require renal replacement therapy, haemodialysis is preferable to peritoneal dialysis.17
The patient may develop level-of-consciousness abnormalities, seizures, coma and respiratory failure,6 necessitating endotracheal intubation. This should be done extremely carefully to prevent bleeding, and the healthcare team should be strictly protected.17
Transfusion treatment in haemorrhagic complicationsIn serious cases, haemorrhagic signs occur 3–6 days from the onset of fever.6,10
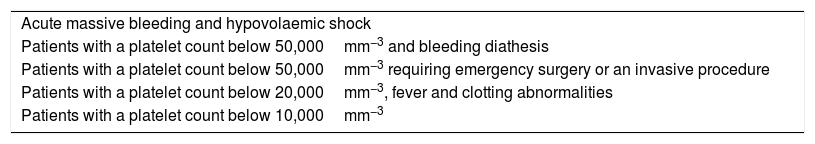
Treatment with platelet concentrate (Table 1).
Situations in which platelet transfusion is recommended.
| Acute massive bleeding and hypovolaemic shock |
| Patients with a platelet count below 50,000mm−3 and bleeding diathesis |
| Patients with a platelet count below 50,000mm−3 requiring emergency surgery or an invasive procedure |
| Patients with a platelet count below 20,000mm−3, fever and clotting abnormalities |
| Patients with a platelet count below 10,000mm−3 |
Platelet concentrate obtained by apheresis from a single donor is recommended because it involves separation and selection of blood components from the same donor; this decreases its immunogenic potential compared to transfusion from multiple donors. The volume to transfuse is 0.1 units per kg of body weight (the difference lies in the fact that to obtain equivalent platelet units, in the case of multiple donors, 4–6 different transfusions are required versus one in plateletpheresis). If transfusion is indicated due to active bleeding, it shall be performed on demand based on clinical response. Laboratory testing is to be performed 18–24h following administration. If there is no increase around 30–50,000platelets/μl, then the possibility of an alloimmunisation event due to anti-HLA antibodies, DIC or a complication of sepsis must be considered.17,19
Fresh plasmaThis may be useful in cases of massive haemorrhage, liver failure, thrombocytopenic thrombotic purpura, dilutional coagulopathy, DIC, vitamin K deficiency, INR 1.5 times above normal limits and decreased aPTT. It offers the advantage of a higher concentration of clotting factors compared to other blood products. The dose to be administered is 10–15ml/kg or a dose dependent on laboratory response monitoring. It should be used with caution in heart failure.19 At present, commercial preparations derived from human plasma proteins are available, which are very useful in clotting disorders. They undergo cell filtration and are treated with solvents/detergents to neutralise anti-leukocyte antibodies, reduce bioactive lipids and eliminate viral agents. Their role in the context of CCHF has not been studied.
ImmunoglobulinsThese might be useful in refractory thrombocytopenia thanks to their immunomodulating activity; however, data on their use in CCHF are anecdotal.17
Aetiological treatmentThere is no antiviral drug with proven efficacy against CCHF virus. One of the main problems for research on active drugs against the virus is the lack of animal model. CCHF virus is not pathogenic in animals. With the exception of human beings, no disease has been reported in any mammal with an intact immune system.
To date, the only model that is available is an infant or immunodeficient mouse model.
Antivirals- •
Ribavirin: ribavirin is the antiviral drug with the most extensive experience of use in CCHF, with controversial data. It is a synthetic antiviral and a guanosine analogue. It shows a broad antiviral spectrum including DNA and RNA viruses.19 It has been most widely used in combination with interferon for the treatment of hepatitis C virus infection.24Although it has demonstrated antiviral activity against CCHF virus in studies in vitro and in an infant mouse model,19,25,26 data on its efficacy in humans are based on observational studies,27 a single open-label clinical trial and two meta-analyses. Ribavirin would be indicated early, in the pre-haemorrhagic phase, since this is when viraemia is greatest. Once the haemorrhagic phase begins, viraemia usually decreases and pathogenic factors for coagulopathy, disseminated intravascular coagulation and cytokine storm predominate.28–30 With this pathophysiology, in order to evaluate the efficacy data for the use of ribavirin, it is critical to know the point in the course of CCHF at which drug administration was started.In 2010, Koksal et al. conducted the only randomised clinical trial, at a hospital centre in Turkey, where they compared adding versus not adding ribavirin to supportive therapy (72 versus 64 patients, respectively). They found no differences between groups in terms of either survival or length of stay.30Two meta-analyses performed in 2010 and 2011 also did not find the use of ribavirin to have any benefit.28,29 It should be noted that the studies included in each meta-analysis were non-randomised, non-blinded prospective studies, with the exception of the above-mentioned clinical trial30; furthermore, they were limited in number.28,29 Overall, the mortality rate in patients receiving ribavirin is 2–9%; by contrast, the mortality rate in patients not receiving ribavirin is 5–11%.31 The WHO recommends the use of ribavirin for CCHF, although the recommendation with the most support is for Lassa fever.32 Ultimately, the evidence on the efficacy of ribavirin for the treatment of CCHF must be improved by conducting clinical trials,33 although conducting placebo-controlled clinical trials may pose ethical problems.34 At present, 2 non-placebo-controlled clinical trials are being conducted with intravenous ribavirin, one in CCHF and the other in Lassa fever. They are being led by the US Army Medical Research and Material Command (NCT00992693 and NCT02483260).The current consensus among experts is to use ribavirin in serious cases, preferably intravenously, to prevent the hepatic first pass effect and achieve plasma levels more quickly. Recommended doses of ribavirin vary by source consulted: regimens of 10 days, with an initial loading dose of 30mg/kg, followed by 15mg/kg every 6h for 4 days and then 7.5mg/kg every 8h for 6 days are recommended.6 For oral administration, the WHO recommends administering an initial loading dose of 2g, following it with a dose of 1g every 6h for 4 days and finishing the regimen between the fifth and sixth day of treatment with 500mg every 6h.35Side effects, which are known from experience with treatment for HCV infection — haemolytic anaemia, aplastic anaemia, kidney failure, hypocalcaemia and hypomagnesaemia, and acute cholestatic hepatitis — might appear more often as a dose is administered that is four times greater.6 Ribavirin is contraindicated in pregnant women.6
- •
Favipiravir: this is a nucleoside analogue active against RNA viruses. At present, it is approved in Japan for the treatment of influenza A virus infection.36 It is active in vitro against Bunyavirus, Arenavirus and Filovirus and has demonstrated activity against CCHF virus in mouse models.36 In the case of CCHF virus, a study conducted in mice compared the efficacy of ribavirin, umifenovir and favipiravir. All animals treated with favipiravir in the first hour and for at least two days survived. Ribavirin prolonged the time to death but did not increase the survival rate. Umifenovir did not yield any benefit.26 Ultimately, while favipiravir could be a treatment for CCHF, there are no data on its use in humans, nor is there any record of clinical trials in progress.34
- •
Chloroquine and chlorpromazine have demonstrated certain antiviral effects. Chloroquine has shown activity against RNA viruses (and intracellular bacteria). Chlorpromazine has demonstrated activity against adenovirus, Ebola virus and coronavirus through inhibition of viral endocytosis.37Studies in vitro have shown that both drugs have direct activity against CCHF virus. In addition, combined use with ribavirin was synergistic.37
- •
Interferon type I: this has demonstrated activity in vitro and in mouse models.19
- •
MxA: this interferon-inducible GTPase seems to inhibit intracellular virus replication and virion production by interacting with nucleocapsid components.19
- •
ISG20: this belongs to the group of genes stimulated by interferon; it is an exonuclease which degrades viral RNA, this having been demonstrated in cell cultures with vesicular stomatitis virus and with HIV. In 2007, Weber and Mirazimi demonstrated its effect on CCHF virus.19
- •
Serum from convalescent patients: there are published cases of infection with CCHF virus treated both intramuscularly and intravenously with hyperimmune serum from convalescent patients. Their results were encouraging, especially in groups of patients with very high viral loads, with survival rates around 90% for ill patients.38,39 The lack of clinical trials and a control group in observational studies is an obstacle to recommendation of their routine use.27,34
- •
Monoclonal antibodies: in a study conducted in mouse models, just one subgroup achieved protection following administration of antibodies. Some non-neutralising antibodies were seen to protect animals from death, suggesting that antibody-mediated response depends not only on these but also on host factors.34,40
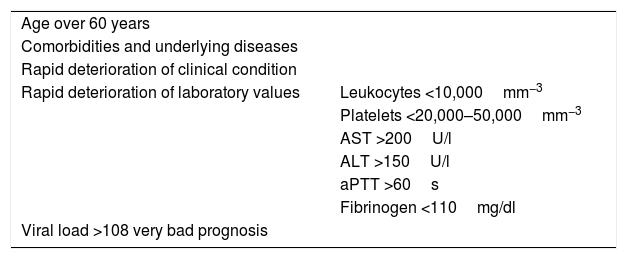
General factors associated with a poor prognosis include the following: age over 60 years, underlying comorbidities, rapid deterioration of clinical condition and accelerated worsening of laboratory values (Table 2).10
Data for poor prognosis in CCHF virus infection.
| Age over 60 years | |
| Comorbidities and underlying diseases | |
| Rapid deterioration of clinical condition | |
| Rapid deterioration of laboratory values | Leukocytes <10,000mm−3 |
| Platelets <20,000–50,000mm−3 | |
| AST >200U/l | |
| ALT >150U/l | |
| aPTT >60s | |
| Fibrinogen <110mg/dl | |
| Viral load >108 very bad prognosis | |
ALT: alanine aminotransferase; aPTT: activated partial thromboplastin time; AST: aspartate aminotransferase.
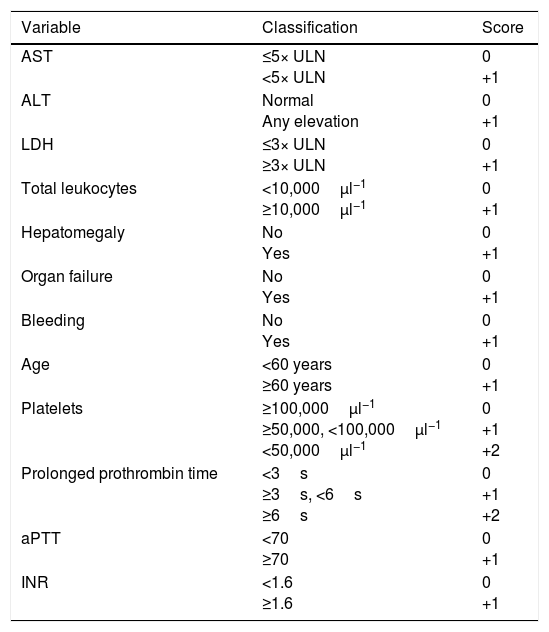
In 1989, Swanepoel et al. reported a mortality rate of 90% in the presence of any of the following laboratory abnormalities in the first five days: leukocytes ≥10×109l−1, platelets ≤20×109l−1, AST ≥200U/l, ALT ≥150U/l, aPTT >60s or fibrinogen ≤110mg/dl.41 Bakir et al. proposed a prognostic score which was subsequently validated in an external cohort (Table 3).
Variables included in Bakir et al.’s prognostic score.
| Variable | Classification | Score |
|---|---|---|
| AST | ≤5× ULN <5× ULN | 0 +1 |
| ALT | Normal Any elevation | 0 +1 |
| LDH | ≤3× ULN ≥3× ULN | 0 +1 |
| Total leukocytes | <10,000μl−1 ≥10,000μl−1 | 0 +1 |
| Hepatomegaly | No Yes | 0 +1 |
| Organ failure | No Yes | 0 +1 |
| Bleeding | No Yes | 0 +1 |
| Age | <60 years ≥60 years | 0 +1 |
| Platelets | ≥100,000μl−1 ≥50,000, <100,000μl−1 <50,000μl−1 | 0 +1 +2 |
| Prolonged prothrombin time | <3s ≥3s, <6s ≥6s | 0 +1 +2 |
| aPTT | <70 ≥70 | 0 +1 |
| INR | <1.6 ≥1.6 | 0 +1 |
ALT: alanine aminotransferase; aPTT: activated partial thromboplastin time; AST: aspartate aminotransferase; INR: international normalised ratio; ULN: upper limit of normal.
The score takes the values from the time of admission. All patients with a score less than or equal to 4 survived, those with a score of 5–8 had a mortality rate of 20% and those with a score greater than or equal to 9 had a mortality rate of 100%. Source: Akinzi et al.10
Finally, viral load has also been closely linked to prognosis. Levels in excess of 108copies/ml have been associated with high mortality.6,10
Discharge criteriaThe information published on discharge criteria is diverse and sometimes controversial, in part because most endemic regions for CCHF do not have healthcare systems similar to those in western Europe. The WHO, as a generic recommendation for haemorrhagic fevers, considers it safe to discharge the patient from isolation if the patient experiences clinical improvement and disappearance of symptoms (enabling autonomy in basic vital functions) and has at least one negative blood PCR no less than 48h after the last positive PCR.32 However, in Turkey, discharge is recommended based on clinical data (absence of symptoms for at least three days) and laboratory data (platelets >50,000mm−3; normalisation of clotting and a descending curve of transaminases), with no need for a negative PCR for CCHF virus. Some Turkish centres even limit themselves to confirming the absence of fever for 48–72h or the disappearance of complications particular to the disease.42 These clinical and laboratory discharge criteria seem to be safe for the patient and for preventing community transmission of the disease according to data from a prospective multi-centre analysis conducted in Turkey.42
Management of high-risk exposureRibavirin: because the use of ribavirin as post-exposure prophylaxis is controversial, its recommendation is limited to the highest-risk cases: direct contact with fluids, contamination of the mucosae or conjunctivae with fluids, or accidental needle sticks.2,19 There is no clear consensus in terms of dose (the same as the therapeutic regimen or starting with a loading dose of 2g per day followed by 4g per day for four days and ending with 2g per day for six days, etc.). If administration is indicated, it should be started very early.19,27,43
Vaccination: clinical trials have not identified any vaccine with proven efficacy.18,34 Clinical trials are difficult to conduct as outbreaks are sporadic with irregular numbers of cases.25 In the early 1970s, a trial in Russia of a vaccine derived from mouse brain tissue and inactivated with formaldehyde showed a limited antibody response that increased with revaccination after a year (it demonstrated an increase in antibodies 1–4 weeks after the third dose and a decrease in antibodies 3–6 months later).27 No further data on this vaccine were subsequently published.25
There are two other experimental vaccines. The first is obtained from infected mouse brain cells and inactivated with chloroform. In 1974, a trial conducted in Bulgaria with fewer than 583 participants found a seroconversion rate around 96%. Another study on a population having been vaccinated every two years detected significant antibodies after receiving this biannual booster. The suggested regimen consisted of a first dose, a second dose 30–45 days later and a booster after one year followed by revaccination every five years. The Bulgarian Ministry of Health reported that the number of cases reported due to CCHF had decreased by a factor of four; however, no subsequent studies have fully supported its usefulness.25 The second experimental vaccine is a DNA vaccine based on the M segment of the virus. This vaccine has been shown to develop neutralising antibodies in mice.6 It was not subsequently marketed. None of the above-mentioned vaccines has been approved for use by the European Medicines Agency or the United States Food and Drug Administration.
A clinical trial of a new vaccine against CCHF is currently being conducted in Turkey (NCT03020771).
ConclusionsCCHF virus is the virus responsible for the most widespread haemorrhagic fever in the world. The cornerstone of its treatment is supportive therapy, with a particular focus on clotting abnormalities, the need for transfusion of blood products or fresh frozen plasma and the ion abnormalities which may appear.
Although for the moment the condition lacks specific treatment, ribavirin is the only drug that may provide some benefit, even though the studies published offer contradictory results.
With regard to post-exposure prophylaxis, again, ribavirin is the most commonly used drug and its use is recommended in cases of high-risk exposure. However, there are no specific efficacy data, nor is there a well-established regimen with respect to dose and duration of prophylaxis.
Conflicts of interestThe authors declare that they have no conflicts of interest.
The clinical team of the authorship would like to express their appreciation for the support received from the Spanish Tropical Disease Cooperative Research Network (RICET) (RD12/0018/0023).
Please cite this article as: de la Calle-Prieto F, Martín-Quirós A, Trigo E, Mora-Rillo M, Arsuaga M, Díaz-Menéndez M, et al. Manejo terapéutico de la fiebre hemorrágica de Crimea-Congo. Enferm Infecc Microbiol Clin. 2018;36:517–522.