Chagas disease is a neglected parasitosis caused by the protozoan parasite Trypanosoma cruzi. This infection is present in most Latin American countries, although, due to migratory movements, it is a growing cause for concern in non-endemic countries. The only two drugs currently available for its treatment—benznidazole and nifurtimox—were marketed 50 years ago. While they are very effective for acute and recent infection, and for the prevention of maternofoetal transmission, their efficacy declines in people who have chronic infection, especially those older than 18 years of age. In the presence of visceral involvement, parasiticidal treatment is of little or no value. The safety profile of both drugs is far from ideal, with frequent adverse events and high rates of drug discontinuation, mainly in adults. So far, new drugs and new strategies have not been shown to improve the results of the current nitroimidazoles, although the results are promising. In this review, we focus on the aspects that allow clinicians to make the best use of currently available drugs. In addition, we discuss new therapeutic options and ongoing research in the field.
La enfermedad de Chagas es una parasitosis desatendida causada por el parásito protozoico Trypanosoma cruzi. Esta infección está presente en la mayoría de los países de América Latina aunque, debido a los movimientos migratorios, es un motivo de creciente preocupación en los países no endémicos. Los 2 únicos fármacos disponibles en la actualidad para su tratamiento (benznidazol y nifurtimox) se comercializaron hace 50 años. Aunque son muy eficaces para las infecciones agudas y recientes, así como para la prevención de la transmisión maternofetal, su eficacia disminuye en las personas que padecen infecciones crónicas, especialmente en mayores de 18 años. En presencia de afectación visceral, el tratamiento parasiticida tiene poco o ningún valor. El perfil de seguridad de ambos fármacos dista mucho de ser ideal, con efectos adversos frecuentes y altas tasas de abandono del tratamiento, especialmente en adultos. Hasta ahora no se ha demostrado que los nuevos medicamentos y las nuevas estrategias mejoren los resultados de los nitroimidazoles actuales, aunque los resultados son prometedores. En esta revisión nos centramos en los aspectos que permiten a los médicos hacer el mejor uso de los medicamentos disponibles en la actualidad. Además, analizamos las nuevas opciones terapéuticas y las investigaciones en curso en este campo.
Trypanosoma cruzi was recognized by Carlos Chagas in 1909 as the etiological agent of Chagas disease.1 This parasitosis, is endemic in 21 countries in Latin America and is present from the south of the United States to the north of Argentina and Chile, where it causes 12,000 deaths per year. Estimations from the year 2010 show that nearly 6 million people are infected.2 Most of them (62.4%) live in the Southern Cone, which has an at-risk population of 70.2 million people, and 38,593 new cases per year (8668 congenital cases). Historically, Chagas disease was confined to rural areas in Latin America. However, as a result of globalisation and an increase in international migrations during the preceding decades, it has become a cause of concern in non-endemic countries. Estimates indicate that up to 347,000 persons are infected in the United States3 and that up to 123,078 are infected in European countries.4
In endemic areas, people contract the disease through contact with the urine or faeces of a T. cruzi-infected blood-sucking triatomine insect (also known as the “kissing bug”), through mucous membranes or non-intact skin.5 Oral transmission is another potential route that is increasing in prevalence in areas like the Amazon basin.6 Other routes of infection, which are of significant importance in non-endemic areas, are congenital transmission (during pregnancy or childbirth),7 transmission through blood and blood products8 or organ transplantation,9 and transmission through laboratory accidents.10
Chagas disease presents two clinical phases: acute and chronic. Acute infections are typically asymptomatic and can occur at any age. Clinical manifestations include fever, lymphadenopathy, hepatosplenomegaly, and inflammation at the inoculum site. Severe forms are rare and account for 1–5% of patients.5 This phase lasts 4–8 weeks before resolving spontaneously and leaving most patients chronically infected if untreated. Nearly 30–40% of chronically infected patients can develop visceral involvement in a 10–30-year timespan after infection.11 Most common visceral involvement comprises cardiomyopathy (affecting the conduction system and myocardium) or megaviscera (megaoesophagus and/or megacolon). In 10% of cases, mild polyneuropathy can be present.5 Patients with chronic infection, whether symptomatic or not, constitute the vast majority of people with T. cruzi infection.
Acute infections, congenital disease, and reactivations in immunosuppressed patients can be diagnosed through direct visualization or molecular biology methods (mainly, PCR techniques). PCR can also be used for monitoring therapeutic failure.5,12 During the chronic phase, parasitemia levels are low and intermittent, so diagnosis is based on 2 positive IgG antibodies tests against different T. cruzi antigens.13
Treatment of T. cruzi infection still relies on drugs licensed over 50 years ago: nifurtimox (launched by Bayer in 1965) and benznidazole (launched by Roche in 1971).14 Their safety and efficacy profiles are far from ideal and are influenced principally by the infection's phase and the age of the patients. In this review, we focus on the factors that allow clinicians to make the best use of currently available drugs. In addition, we discuss new therapeutic options and ongoing research in the field.
Current drugs used for the treatment of t. Cruzi infectionOnly two drugs with proven efficacy against T. cruzi are available today: benznidazole and nifurtimox.
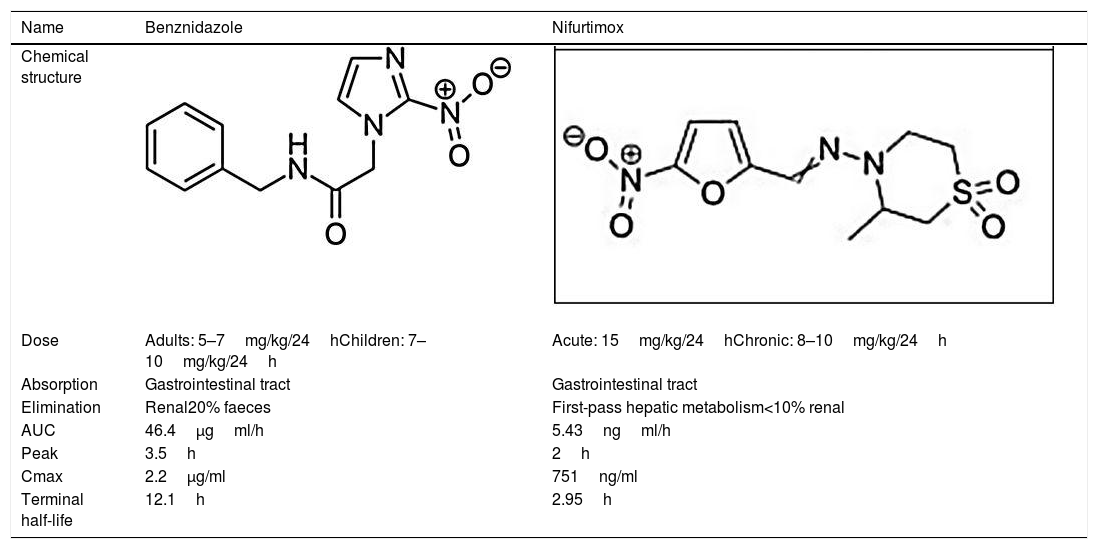
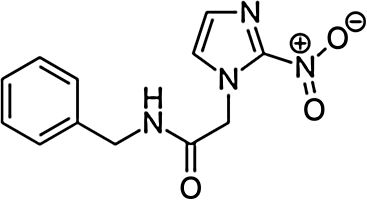
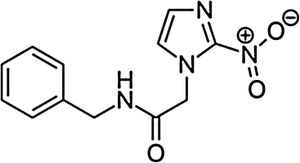
BenznidazoleBenznidazole (2-nitro-N-[phenylmethyl]-1H-imidazole-1-acetamide) is a nitroimidazole derivative that was first described as active against T. cruzi in the late 1960s.15 It was introduced for human treatment in 1971 and showed an efficacy similar to that of nitrofurazone, though with fewer toxic effects.16 Afterward, different clinical and experimental studies were published using benznidazole as a treatment for acute and chronic Chagas disease; it became the first-line treatment in both situations.17,18
As a prodrug, benznidazole is activated by trypanosomal I nitroreductase, releasing other molecules such as dialdehyde glyoxal, which bonds to guanosine bases in DNA and RNA, resulting in its blockade and making the parasite susceptible to oxidative damage in all stages of the T. cruzi life cycle.19,20 In fact, benznidazole acts in a complex manner, resulting in protein, RNA, or DNA synthesis as well as promoting parasitic clearance in infected hosts.21
Benznidazole is mainly absorbed from the gastrointestinal tract. Elimination is predominantly renal, though approximately 20% is excreted in the faeces. It has a good oral bioavailability of over 90% following a one-compartment model. After a single oral dose of 100mg, pharmacokinetics parameters resulted in an area under the curve (AUC) of 46.4μgh/ml, peak plasma concentrations of 3.5h, maximal concentrations of 2.2μg/ml, and a terminal half-life of 12.1h.22 Although its pharmacokinetic profile has not been traditionally considered, its understanding can contribute to a proper design of new protocols and reduce bioaccumulation and the risk of toxicity.
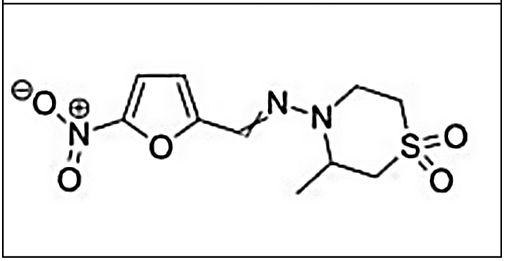
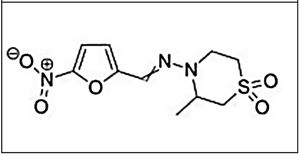
NifurtimoxNifurtimox (3-methyl-N-′[(5-nitro-2 furanyl)-methylene]-4-morpholinamine 1,1 dioxide) is a nitrofuran derivative that was first clinically used in 1969.23,24 Later studies showed different results based on disease phase, treatment duration, patient age, and geographical area.
Nifurtimox generates nitroanion radicals by nitroreductases that, in the presence of oxygen, produce free radicals that damage T. cruzi.25 Along with benznidazole, this radicals also blocks DNA synthesis and accelerates its degradation. It is effective in eradicating the amastigote, epimastigote, and reproductive forms.
Nifurtimox is absorbed from the gastrointestinal tract and undergoes extensive first-pass hepatic metabolism involving cytochrome P-450 and p-450 reductase. It has little renal excretion (less than 1%). A single oral dose of 15mg/kg resulted in an AUC of 5.43ngml/h and a peak plasma concentration at 2h with a maximal concentration of 751ng/ml and a terminal half-life of 2.95h.26 Due to its worse toxicity profile, nifurtimox was discontinued and its commercialization suspended in Brazil, Argentina, Chile, and Uruguay from the early 1980s. However, due to a lack of alternatives, it has retained its indication as a second-line treatment when BNZ fails or toxicity occurs.14 The main pharmacological characteristics of benznidazole and nifurtimox are summarized in Table 1.
Main characteristics of benznidazole and nifurtimox.
| Name | Benznidazole | Nifurtimox |
|---|---|---|
| Chemical structure | ||
| Dose | Adults: 5–7mg/kg/24hChildren: 7–10mg/kg/24h | Acute: 15mg/kg/24hChronic: 8–10mg/kg/24h |
| Absorption | Gastrointestinal tract | Gastrointestinal tract |
| Elimination | Renal20% faeces | First-pass hepatic metabolism<10% renal |
| AUC | 46.4μgml/h | 5.43ngml/h |
| Peak | 3.5h | 2h |
| Cmax | 2.2μg/ml | 751ng/ml |
| Terminal half-life | 12.1h | 2.95h |
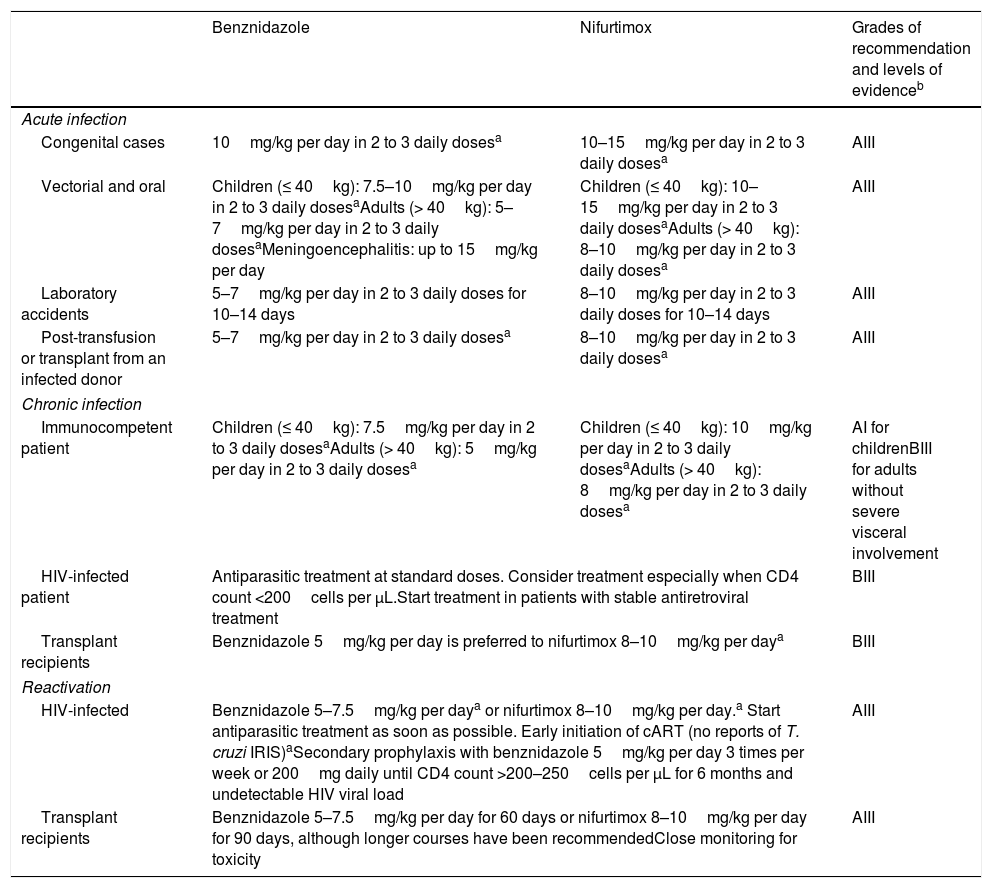
Treatment during the acute phase of Chagas disease is always recommended, regardless of the mechanism of infection, as well as in reactivations in immunosuppressed patients.27–29 In acute infection, treatment produces symptomatic improvement and the clearance of parasitemia as well as serological negativity in most cases. In immunosuppressed patients, early parasiticidal treatment during reactivations can prevent the development of severe disease.30
Although there are no randomized clinical trials that compare benznidazole and nifurtimox, benznidazole is generally preferred due to its better tolerability and tissue penetration, as well as its possibly higher efficacy.5,28 Benznidazole engages in significant activity during the acute and early phases of T. cruzi infection: Serological cure is achieved in up to 100% of patients with congenital disease31,32 treated during the first year of life and in 76% of patients with acute disease.33,34 Newborns of mothers with Chagas disease should be tested for T. cruzi infection as soon as possible28,35 because trypanocidal treatment in this setting is highly effective and well-tolerated. A delay in treatment initiation can result in poorer efficacy.31
The diagnosis of acute and congenital infection can be carried out by the direct microscopic visualization of trypomastigotes in the blood,27 though concentration methods such as microhematocrit, and the Strout method.36 PCR techniques have proven to be very useful in the diagnosis of mother-to-child transmission during the first trimester of life, when such techniques are more sensitive than concentration techniques.37,38 Also, in neonatal infection, diagnosis can be confirmed based on positive serology results beyond the eighth month of life.28
In children, treatment is administered at higher doses than it is in adults (Table 2).29 Benznidazole is available in dispersal tablets of 12.5mg, while nifurtimox in dispersal tablets of 30mg will be available for paediatric use.39
Treatment recommendations for acute and chronic Chagas disease.
| Benznidazole | Nifurtimox | Grades of recommendation and levels of evidenceb | |
|---|---|---|---|
| Acute infection | |||
| Congenital cases | 10mg/kg per day in 2 to 3 daily dosesa | 10–15mg/kg per day in 2 to 3 daily dosesa | AIII |
| Vectorial and oral | Children (≤ 40kg): 7.5–10mg/kg per day in 2 to 3 daily dosesaAdults (> 40kg): 5–7mg/kg per day in 2 to 3 daily dosesaMeningoencephalitis: up to 15mg/kg per day | Children (≤ 40kg): 10–15mg/kg per day in 2 to 3 daily dosesaAdults (> 40kg): 8–10mg/kg per day in 2 to 3 daily dosesa | AIII |
| Laboratory accidents | 5–7mg/kg per day in 2 to 3 daily doses for 10–14 days | 8–10mg/kg per day in 2 to 3 daily doses for 10–14 days | AIII |
| Post-transfusion or transplant from an infected donor | 5–7mg/kg per day in 2 to 3 daily dosesa | 8–10mg/kg per day in 2 to 3 daily dosesa | AIII |
| Chronic infection | |||
| Immunocompetent patient | Children (≤ 40kg): 7.5mg/kg per day in 2 to 3 daily dosesaAdults (> 40kg): 5mg/kg per day in 2 to 3 daily dosesa | Children (≤ 40kg): 10mg/kg per day in 2 to 3 daily dosesaAdults (> 40kg): 8mg/kg per day in 2 to 3 daily dosesa | AI for childrenBIII for adults without severe visceral involvement |
| HIV-infected patient | Antiparasitic treatment at standard doses. Consider treatment especially when CD4 count <200cells per μL.Start treatment in patients with stable antiretroviral treatment | BIII | |
| Transplant recipients | Benznidazole 5mg/kg per day is preferred to nifurtimox 8–10mg/kg per daya | BIII | |
| Reactivation | |||
| HIV-infected | Benznidazole 5–7.5mg/kg per daya or nifurtimox 8–10mg/kg per day.a Start antiparasitic treatment as soon as possible. Early initiation of cART (no reports of T. cruzi IRIS)aSecondary prophylaxis with benznidazole 5mg/kg per day 3 times per week or 200mg daily until CD4 count >200–250cells per μL for 6 months and undetectable HIV viral load | AIII | |
| Transplant recipients | Benznidazole 5–7.5mg/kg per day for 60 days or nifurtimox 8–10mg/kg per day for 90 days, although longer courses have been recommendedClose monitoring for toxicity | AIII | |
Benznidazole is not commercialized in Spain, so it can be obtained through two methods depending on the autonomous region and hospital policy. It can be acquired through the foreign drug supply section of the Department of Health of each autonomous region upon the presentation of the Spanish health insurance card, an official medical prescription, and a medical report specifying the patient and physician data, the diagnosis that motivates the prescription, and the drug, dosage, and duration of the treatment.40 The drug can also be acquired at the hospital pharmacy if the hospital had previously requested it from the Department of Health of each autonomous region. In the United States, benznidazole was recently approved by the FDA for patients between 2 and 12 years of age.41
Due to an agreement with Bayer, nifurtimox is available free of charge upon a request to the WHO.42 In Spain, this drug can be obtained free of charge upon request from the WHO through a patient-named programme. It is not currently approved by the FDA, so in the United States, it could be used under the CDC's investigational protocols.43
Trypanocidal treatment of chronic Chagas diseaseThe vast majority of patients who do not receive treatment during the acute phase will progress to chronic infection. Most of them will never develop visceral involvement, resulting in a chronic indeterminate phase characterized by a good prognosis, the absence of clinical signs and symptoms, and normal radiological and electrocardiography studies.44
The efficacy of treatment seems to decrease as time passes since the incidence of primary infection and is very poor when visceral involvement is established.45,46 Parasiticidal treatment is generally offered to patients with chronic Chagas disease in the indeterminate phase, especially patients under 18 years of age and patients with mild to moderate disease (Table 2). These patients would likely benefit from treatment and should be informed about the risk-benefit balance of treatment with parasiticidal drugs.29
Nifurtimox's disclosed cure rates in the chronic indeterminate phase range from 86% in children below 14 years to 7–8% in adults.14,47 As for benznidazole, cure rates range from 60 to 94% in children aged up to 13 years48,49 to 2–40% in adults with late chronic disease, although these values improve with longer follow-up.33,50–52 Some reports have shown that only 30 days of treatment with benznidazole can be useful for chronically infected adults.31,50
Treatment is generally not indicated in patients with moderate to severe cardiomyopathy, as it has not been shown to reduce clinical cardiac events and death as compared to placebo.45 For patients older than 50 years of age who do not have severe cardiomyopathy, treatment should be individualized.5,28,29 The treatment of women of childbearing age with chronic Chagas disease has the additional benefit of preventing mother-to-child transmission of T. cruzi.53–56 Therefore, the screening of women who are currently living, or who have lived, in endemic areas, as well as women born to mothers from endemic regions, is key to the prevention of vertical transmission.35,57
Pregnancy must be ruled out before the initiation of trypanocidal treatment. The use of benznidazole and nifurtimox is not recommended during pregnancy, mainly because of the scarcity of safety data.28,29 Nifurtimox has been related to delayed intra-uterine development and lower body weight in rats and mice foetuses born to mothers treated with high doses of up to 125mg/kg.58 Benznidazole crosses the placental barrier of pregnant rats and reaches the foetus, binding reactive metabolites to foetal proteins.59 Both drugs have been associated with chromosomal aberrations in infected children after treatment.60,61 The accidental intake of trypanocidal drugs during pregnancy is not a criterion for its termination.28 Parasiticidal treatment is generally recommended In cases of acute infection or reactivation during pregnancy.62
Close monitoring of both mother and foetal conditions is necessary in cases of the diagnosis of any phase of Chagas disease during pregnancy. Vertical transmission can occur at any time but is more common during the third trimester of pregnancy.28,63 The congenital transmission rate for mothers with positive PCR is around 8–18.8% compared to anecdotal cases of transmission in PCR-negative mothers.12,54,56,64 The infection itself does not justify a Caesarean section.5,28
Treatment during breastfeeding is generally discouraged because, most of the time, there is no urgent need for therapy. It can be considered when this period represents the sole opportunity for the woman's treatment, as happens in some rural Latin American areas.65 Recent studies have demonstrated a limited and low transference of both trypanocidal drugs through breast milk. Median benznidazole and nifurtimox milk concentrations were 3.8mg/L (range 0.3–5.9) and 2.15mg/L (interquartile range 1.32–4.55), respectively.65,66 Both concentrations are below the 10% cut-off used to guide the risk evaluation of drugs during lactation. Treatment for chronically infected women should be considered after delivery and the breastfeeding period.28,35
Trypanocidal treatment in immunosuppressed patientsChagas disease in this population may represent an acute infection in a patient with pre-existing immunosuppression or a reactivation in a patient chronically infected with T. cruzi.30
Apart from vectorial transmission, an acute T. cruzi infection can be acquired through organ or bone marrow transplantation or through blood product infusion. Treatment with benznidazole (see the acute infection treatment in Table 2) is mandatory to control the acute symptoms and improve the prognosis.30,67
Chronically infected patients with Chagas disease can present a reactivation in the case of immunosuppression, characterized by an increase in parasitaemia even in the absence of symptoms. The incubation period can be prolonged and symptoms—such as prolonged fever, panniculitis, myocarditis, or meningoencephalitis—can be atypical and serious.30,67 The risk of reactivation can vary depending on the immunosuppression and the organ transplanted, from 1.8% in liver transplantation to 23.3%, 27.3%, and 31%, in bone marrow, kidney, and heart transplantation, respectively.29 Administration of antitrypanosomal prophylaxis to infected recipients is generally not recommended, though it could be considered a pretransplant treatment in potential heart transplant recipients or in living donors infected with T. cruzi.5 Close monitoring (preferably with the Strout method and quantitative PCR) is recommended in order to obtain an early diagnosis of reactivation episodes.28,30,68 There are no data supporting the reduction of the risk of reactivation with the administration of benznidazole prophylaxis prior to or immediately after transplantation.68,69 Nevertheless, transplant recipients may benefit from trypanocidal treatment before transplantation in the presence of patent parasitemia.68
As for patients coinfected with T. cruzi and HIV, reactivation is closely related to late HIV diagnosis, severe immunosuppression (CD4 cell counts <200mm3), and a lack of antiretroviral treatment. In these circumstances, the rate of reactivation has been estimated as 39.6%.5,29,30 Treatment of reactivations should begin with the prompt initiation of antiretroviral therapy as soon as possible after diagnosis. Secondary prophylaxis (see Table 2) is generally needed until the patient reaches CD4 cell counts higher than 200–350mm3 and an undetectable HIV-ARN viral load for longer than 6 months.70 Chronically infected patients may benefit from trypanocidal treatment as non-HIV-infected patients.
In the case of cancer and T. cruzi infection, reactivations have been described in patients with compromised cellular immunity (mainly haematological malignancies). As a means of preventing reactivations, these patients may benefit from trypanocidal treatment prior to chemotherapy or immunotherapy.71
Reactivation in patients with systemic autoimmune diseases is rare. Available information is limited to some case reports of patients with systemic lupus erythematosus treated with immunosuppressive drugs (azathioprine, mycophenolate mofetil, or ciclosporin).71–73 In these cases, whenever possible, trypanocidal treatment should be administrated before immunosuppression, and close monitoring is indicated to detect reactivations.74
Evaluation of the therapeutic response to trypanocidal treatmentThe only current criterion for a cure is to revert the outcome of conventional serological tests to negative.27 Time to cure depends on the phase of the disease and can vary: 1 year in congenital infection, 3–5 years in the acute phase, 5–10 years in early chronic infection, and 10–25 years in chronically infected adults.28 A number of surrogate biomarkers (some of them related to the parasite itself) for the cure or progression of the disease are under study. Additionally, immunological and biochemical markers are related to the host response to the parasite. Nevertheless, so far, none of them have been validated for clinical use.75 The main limitations in the validation of new markers are the possibility of reinfection in endemic areas, the long follow-up period needed to establish the current cure criterion, the lack of consensus over an early therapeutic response, and the role of the parasite genotype.5,76
After parasiticidal treatment, it is recommended that patients engage in annual follow-ups that evaluate the serological response5,28,77 or determine whether there has been a clinical progression, to start prompt therapy for the visceral complications.5 PCR is useful for detecting therapeutic failure when T. cruzi become detectable after treatment, although undetectable values do not indicate a cure.78
A closer follow-up is recommended for children, as they achieve cure earlier than do adults. In acute, early congenital infection and reactivations, direct parasitological methods and PCR techniques can be used for monitoring response to treatment.28,78 In coinfected transplant recipients, a weekly or biweekly follow-up is indicated during the first 6 months to achieve an early detection of reactivation.68
Treatment failure is usually seen as a positive PCR result. If the original treatment indication remains unchanged, retreatment with the same or a different drug for 60 or 90 days is suggested. Combination of therapies and longer drug courses may be considered in this situation.
Toxicity of trypanocidal treatmentToxicity of benznidazoleAlthough drug toxicity is the main limitation for the treatment of Chagas disease, the benznidazole safety profile is still not well understood. Most of the available safety information about benznidazole is based on post-marketing studies, given that no pre-marketing safety studies were performed.79
Benznidazole's plausible mechanism of action is through highly reactive products formed during its anaerobic nitroreduction. These products form molecular complexes from covalent bonds with DNA, RNA, proteins, and low-molecular-weight thiols. Nitro radical anion derivates are also generated when benznidazole is processed by cytochrome P450 reductase and mitochondrial dihydrolipoamide dehydrogenase.77 The interaction between these metabolites and the host DNA, proteins, lipids, or other relevant cellular components is believed to be one of the causes of benznidazole toxicity.80 In addition, moderate and severe cutaneous reactions seem to be caused by delayed (non-IgE-mediated) hypersensitivity reactions associated with the presence of the HLA-B*3505 allele.81
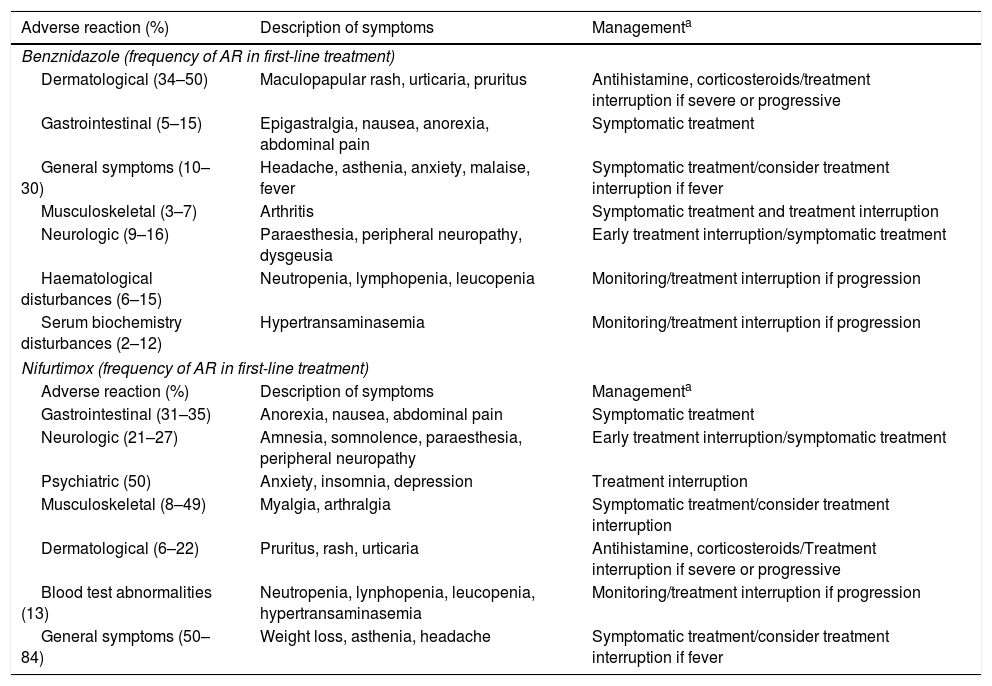
Overall, benznidazole causes adverse reactions in 44.1% (95% CI 37.2–51.2) of patients—more frequently in adults than in children (51.6 vs. 24.5%). As a consequence, 11.4% (95% CI 8.5–14.5) of them must interrupt their treatment—again, more frequently in adults than in children (14.2 vs. 3.8%).82 In general, toxicity is moderate and reversible, with severe reactions being reported in only 3% of cases.82 The majority of patients had more than one AR, with a median of 3 AR.79,83
AR tend to appear within the first 12–15 days of treatment. The most common are dermatological in nature, that use to be mild to moderate in most cases.79,83 An infrequent (<1%) exception is DRESS syndrome (drug reaction with eosinophiia and systemic symptoms), characterized by rash and severe general symptoms such as fever, lymphadenopathy, and blood test abnormalities. DRESS syndrome leads to treatment discontinuation and inpatient management for treatment and follow-up.
Gastrointestinal AR are usually the first expression of toxicity. Similar to general symptoms they usually are mild, do not cause treatment interruption and do not require additional treatment.79,83 Arthritis is a rare and late AR (day 27, IQR:35) more frequently affecting women, who should interrupt benznidazole use.79
Blood cell disturbances (most commonly neutropenia and lymphopenia) tend to appear around 14 days after treatment initiation (IQR:14) generally are mild, though, in the case of persistence, the treatment should be discontinued promptly.79 Severe thrombocytopenia is a very rare event that can be severe and lead to treatment interruption.84 Serum biochemistry disturbances (mainly the alteration of the liver function test) appear with a median time onset of around 28.5 days (IQR: 35) and are generally mild and transitory; nevertheless, close monitoring is recommended.79
Neurological toxicity (paraesthesia and dysgeusia) typically appears late in the treatment course (approximately 40 days after treatment) and often entails treatment discontinuation.83 Other rare AR such as psychiatric symptoms (sleeping disorders and anxiety or panic attacks), amenorrhoea, erectile dysfunction, and bronchospasm with basal lung infiltrations have been described.79 The general incidence, description of symptoms, and recommended management for benznidazole AR are summarized in Table 3.
Description of the most common adverse reactions to benznidazole and nifurtimox.
| Adverse reaction (%) | Description of symptoms | Managementa |
|---|---|---|
| Benznidazole (frequency of AR in first-line treatment) | ||
| Dermatological (34–50) | Maculopapular rash, urticaria, pruritus | Antihistamine, corticosteroids/treatment interruption if severe or progressive |
| Gastrointestinal (5–15) | Epigastralgia, nausea, anorexia, abdominal pain | Symptomatic treatment |
| General symptoms (10–30) | Headache, asthenia, anxiety, malaise, fever | Symptomatic treatment/consider treatment interruption if fever |
| Musculoskeletal (3–7) | Arthritis | Symptomatic treatment and treatment interruption |
| Neurologic (9–16) | Paraesthesia, peripheral neuropathy, dysgeusia | Early treatment interruption/symptomatic treatment |
| Haematological disturbances (6–15) | Neutropenia, lymphopenia, leucopenia | Monitoring/treatment interruption if progression |
| Serum biochemistry disturbances (2–12) | Hypertransaminasemia | Monitoring/treatment interruption if progression |
| Nifurtimox (frequency of AR in first-line treatment) | ||
| Adverse reaction (%) | Description of symptoms | Managementa |
| Gastrointestinal (31–35) | Anorexia, nausea, abdominal pain | Symptomatic treatment |
| Neurologic (21–27) | Amnesia, somnolence, paraesthesia, peripheral neuropathy | Early treatment interruption/symptomatic treatment |
| Psychiatric (50) | Anxiety, insomnia, depression | Treatment interruption |
| Musculoskeletal (8–49) | Myalgia, arthralgia | Symptomatic treatment/consider treatment interruption |
| Dermatological (6–22) | Pruritus, rash, urticaria | Antihistamine, corticosteroids/Treatment interruption if severe or progressive |
| Blood test abnormalities (13) | Neutropenia, lynphopenia, leucopenia, hypertransaminasemia | Monitoring/treatment interruption if progression |
| General symptoms (50–84) | Weight loss, asthenia, headache | Symptomatic treatment/consider treatment interruption if fever |
AR: Adverse reaction; T: toxicity.
Some authors discontinue transiently or decrease the daily drug dose in the case of moderate AR and after the patient's recovery; reintroduce the drug progressively.83,87
Factors that have been related to benznidazole toxicity are gender and age. Females seem to have a higher incidence of AR but without higher treatment discontinuation rates than males, while older patients (mainly adults as compared to children) also showed a poorer tolerability profile.82,85 Other characteristics related to an increased risk of toxicity are being of the white or mulatto races and having a better level of education.85 No relationship has been found between benznidazole toxicity and its serum concentrations, the use of benznidazole from different manufacturers, or the use of daily doses higher than 300mg compared to less than 300mg83,86,87 Other strategies that have failed in decreased benznidazole toxicity are the use of progressive doses of benznidazole combined with methylprednisolone, the use of progressive doses of benznidazol lasting 5 days, and the decrease of the duration of treatment to 30 days.50,87,88
Toxicity of nifurtimoxNifurtimox, a nitrofurane, is reduced by liver microsomes to a nitro anion-free-radical-mediated through NADPH-cytochrom Pe-450 reductase. The free radical formation may be the basic cause of nifurtimox toxicity in mammals.89 It has been related to defects in spermatogenesis in mice, a lower weight increase and less activity in pregnant rats, and the dose-dependent reduction of body weight in rat foetuses.58 It has also been related to severe central nervous system toxicity in experimental studies involving rats and dogs.90 Studies of male rats exposed to oral nifurtimox demonstrated a significantly higher percentage of malignant and benign tumours than in control groups, though so far this effect has not been demonstrated in human beings.80,91
As a first-line treatment, nifurtimox presents a high incidence of AR (80.3–100%) and treatment discontinuation (18.4–43.8%).91–94 Most frequent and earlier AR are gastrointestinal, with a median time onset of 21 days (1–119), that usually are mild and controlled. General symptoms typically appear early after treatment initiation and use to be of mild to moderate intensity. Neurologic and psychiatric symptoms tend to develop later in the course of treatment and frequently imply treatment discontinuation.91,93 The global frequency, description, and general management of nifurtimox AR are summarized in Table 3.
The safety profile of nifurtimox, when this drug is prescribed after benznidazole intolerance, is similar to that reported in the literature for patients given nifurtimox as a first-line treatment. Nevertheless, while the rates of AR and treatment discontinuation are equivalent for patients treated in Europe, those rates seem to be higher than the rates reported in endemic areas and in the United States.95 The overall frequencies of AR and TD in this setting were 64.2% and 49.1%, respectively, with the most common AR being cutaneous hypersensitivity (24.1%), digestive disorders (22.2%), fever (12.9%), neurologic disturbances (11.1%), and depression, anxiety, or insomnia (9.2%).95
The toxicity of nifurtimox seems to not be related to the mean starting dosage (600.8mg vs 580.7mg).91 As with benznidazole, the combination of nifurtimox and betamethasone does not improve tolerance.96
New approaches to the treatment of T. cruzi infectionImidazole derivatives experienceTriazole derivatives act as selective inhibitors of T. cruzi ergosterol synthesis with potent intrinsic activity against the parasite in vitro and in vivo models and had been considered promising drugs for the treatment of Chagas disease. Several studies using different ergosterol synthesis inhibitors (ESI) for the treatment of Chagas disease have been published, including ketoconazole, itraconazole, or posaconazole in murine models of acute and chronic Chagas disease and in small human series, with good results.97–99
Consequently, different clinical trials were developed to evaluate the efficacy and safety of various triazole derivatives. The CHAGASAZOL trial was designed to compare posaconazole at different doses to benznidazole in qPCR-positive patients. Even in patients receiving the maximum dose approved for human use, posaconazole showed higher treatment failure rates of as much as 80.7% compared to a rate of 38.4% obtained with benznidazole.100 Attempts to optimize available treatments were made in the STOP-CHAGAS trial, in which the authors compared benznidazole and posaconazole in monotherapy and in combination. In this trial, benznidazole in monotherapy showed a parasitological cure of 86.7%—higher than posaconazole in monotherapy (13.3%) and even higher than both treatments in combination (80%).101 Later, a ravuconazole prodrug was tested at different doses and compared to standard treatment with benznidazole. After a 12-month follow-up, only 29% of patients treated with high-dose ravuconazol had sustained parasite response, compared to 82% of patients treated with benznidazole.102
A trypanostatic effect on quiescent amastigote forms with low replicative taxes in the late chronic stage of Chagas disease has been proposed to explain these results.103 As far as we know, despite the contradictory evidence of ESI in the animal model, all these results suggest that ESI at the current treatment regimens fails to cure patients with Chagas disease.
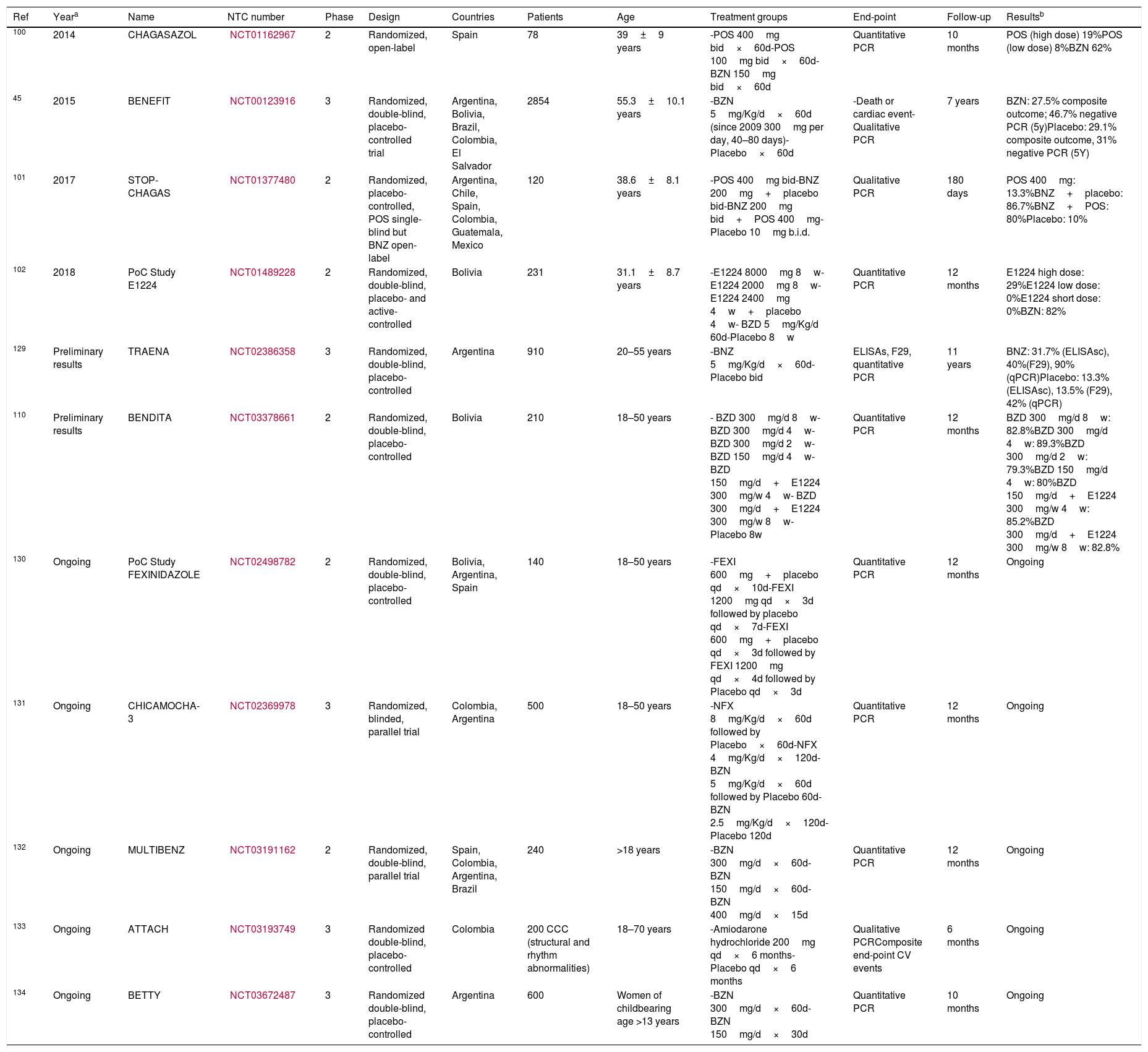
Among other candidates, fexinidazole—a new nitroimidazole—is the only one that has reached clinical trials in humans. Its anti-trypanocidal activity has been demonstrated in phase II and phase III clinical development for human African trypanosomiasis.104 Two proofs-of-concept clinical trials have been carried out (ClinicalTrials.gov Identifier: NCT02498782 and NCT03587766) in Bolivia and Spain, respectively, without any published result yet. A brief description of the recent clinical trials performed with trypanocidal treatment for chronic Chagas disease is summarized in Table 4.
Recent clinical trials including etiological treatment for chronic Chagas disease.
| Ref | Yeara | Name | NTC number | Phase | Design | Countries | Patients | Age | Treatment groups | End-point | Follow-up | Resultsb |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 100 | 2014 | CHAGASAZOL | NCT01162967 | 2 | Randomized, open-label | Spain | 78 | 39±9 years | -POS 400mg bid×60d-POS 100mg bid×60d-BZN 150mg bid×60d | Quantitative PCR | 10 months | POS (high dose) 19%POS (low dose) 8%BZN 62% |
| 45 | 2015 | BENEFIT | NCT00123916 | 3 | Randomized, double-blind, placebo-controlled trial | Argentina, Bolivia, Brazil, Colombia, El Salvador | 2854 | 55.3±10.1 years | -BZN 5mg/Kg/d×60d (since 2009 300mg per day, 40–80 days)-Placebo×60d | -Death or cardiac event- Qualitative PCR | 7 years | BZN: 27.5% composite outcome; 46.7% negative PCR (5y)Placebo: 29.1% composite outcome, 31% negative PCR (5Y) |
| 101 | 2017 | STOP-CHAGAS | NCT01377480 | 2 | Randomized, placebo-controlled, POS single-blind but BNZ open-label | Argentina, Chile, Spain, Colombia, Guatemala, Mexico | 120 | 38.6±8.1 years | -POS 400mg bid-BNZ 200mg+placebo bid-BNZ 200mg bid+POS 400mg-Placebo 10mg b.i.d. | Qualitative PCR | 180 days | POS 400mg: 13.3%BNZ+placebo: 86.7%BNZ+POS: 80%Placebo: 10% |
| 102 | 2018 | PoC Study E1224 | NCT01489228 | 2 | Randomized, double-blind, placebo- and active-controlled | Bolivia | 231 | 31.1±8.7 years | -E1224 8000mg 8w-E1224 2000mg 8w-E1224 2400mg 4w+placebo 4w- BZD 5mg/Kg/d 60d-Placebo 8w | Quantitative PCR | 12 months | E1224 high dose: 29%E1224 low dose: 0%E1224 short dose: 0%BZN: 82% |
| 129 | Preliminary results | TRAENA | NCT02386358 | 3 | Randomized, double-blind, placebo-controlled | Argentina | 910 | 20–55 years | -BNZ 5mg/Kg/d×60d-Placebo bid | ELISAs, F29, quantitative PCR | 11 years | BNZ: 31.7% (ELISAsc), 40%(F29), 90% (qPCR)Placebo: 13.3% (ELISAsc), 13.5% (F29), 42% (qPCR) |
| 110 | Preliminary results | BENDITA | NCT03378661 | 2 | Randomized, double-blind, placebo-controlled | Bolivia | 210 | 18–50 years | - BZD 300mg/d 8w- BZD 300mg/d 4w- BZD 300mg/d 2w- BZD 150mg/d 4w- BZD 150mg/d+E1224 300mg/w 4w- BZD 300mg/d+E1224 300mg/w 8w- Placebo 8w | Quantitative PCR | 12 months | BZD 300mg/d 8w: 82.8%BZD 300mg/d 4w: 89.3%BZD 300mg/d 2w: 79.3%BZD 150mg/d 4w: 80%BZD 150mg/d+E1224 300mg/w 4w: 85.2%BZD 300mg/d+E1224 300mg/w 8w: 82.8% |
| 130 | Ongoing | PoC Study FEXINIDAZOLE | NCT02498782 | 2 | Randomized, double-blind, placebo-controlled | Bolivia, Argentina, Spain | 140 | 18–50 years | -FEXI 600mg+placebo qd×10d-FEXI 1200mg qd×3d followed by placebo qd×7d-FEXI 600mg+placebo qd×3d followed by FEXI 1200mg qd×4d followed by Placebo qd×3d | Quantitative PCR | 12 months | Ongoing |
| 131 | Ongoing | CHICAMOCHA-3 | NCT02369978 | 3 | Randomized, blinded, parallel trial | Colombia, Argentina | 500 | 18–50 years | -NFX 8mg/Kg/d×60d followed by Placebo×60d-NFX 4mg/Kg/d×120d-BZN 5mg/Kg/d×60d followed by Placebo 60d-BZN 2.5mg/Kg/d×120d-Placebo 120d | Quantitative PCR | 12 months | Ongoing |
| 132 | Ongoing | MULTIBENZ | NCT03191162 | 2 | Randomized, double-blind, parallel trial | Spain, Colombia, Argentina, Brazil | 240 | >18 years | -BZN 300mg/d×60d-BZN 150mg/d×60d-BZN 400mg/d×15d | Quantitative PCR | 12 months | Ongoing |
| 133 | Ongoing | ATTACH | NCT03193749 | 3 | Randomized double-blind, placebo-controlled | Colombia | 200 CCC (structural and rhythm abnormalities) | 18–70 years | -Amiodarone hydrochloride 200mg qd×6 months-Placebo qd×6 months | Qualitative PCRComposite end-point CV events | 6 months | Ongoing |
| 134 | Ongoing | BETTY | NCT03672487 | 3 | Randomized double-blind, placebo-controlled | Argentina | 600 | Women of childbearing age >13 years | -BZN 300mg/d×60d-BZN 150mg/d×30d | Quantitative PCR | 10 months | Ongoing |
Abbreviations: BNZ: Benznidazole, NFX: Nifurtimox, POS: Posaconazole, E1224: Ravuconazole prodrug, FEXI: Fexinidazole, d: day, PCR: Polymerase-chain-reaction, qPCR: quantitative polymerase-chain-reaction, CCC: chronic Chagas cardiomyopathy.
The strategy of repositioning established therapeutic agents has also been used in Chagas disease. Although various attempts have been carried out in murine models, and potential treatments have been identified, few drugs have been tested in humans. Allopurinol, a hypoxanthine analogue used to treat hyperuricemic conditions, was also described as a trypanocidal drug in the early 1980s.105 Discordant results in humans have been published. Apt et al.106 first published a randomized trial comparing itraconazole, allopurinol, or placebo in patients with chronic Chagas disease. Parasitological cure, assessed with xenodiagnosis and lytic antibody activity, was evident in 44% of those treated with allopurinol compared to 53% with itraconazole and 25% with placebo. On the other hand, Rassi et al.107 performed a randomized double-blind clinical trial comparing 900mg of allopurinol daily and placebo with 0% negativization of xenodiagnosis. It was also used in a sequential treatment leading to therapeutic immunological changes and the significant reduction of parasitic loads in murine and human models.108
Amiodarone, an antiarrhythmic agent, has demonstrated potent and selective anti-T. cruzi activity.109 Two clinical trials are being tested against Chagas disease. The ATTACH trial (ClinicalTrials.gov Identifier: NCT01722942) aims to assess the trypanocidal effect and its clinical benefit among individuals with mild to moderate chronic Chagas cardiomyopathy.
Meanwhile, the CHAGASICS trial (ClinicalTrials.gov Identifier: NCT01722942) has been designed to compare the efficacy of treatment using implantable cardioverter defibrillator implantation versus amiodarone in the prevention of all-cause mortality in high-risk patients with Chagas cardiomyopathy.
New experimental regimens for nitroderivative-based therapyBecause the major drawback of nitroderivative-based therapy is its toxicity, which hampers its efficacy rate, different approaches have been designed to improve its tolerance.
Based on population pharmacokinetic studies, a dose reduction has been proposed as a novel approach. This hypothesis has driven the realization of two randomized clinical trials, which are currently being assessed: the BENDITA110 and MULTIBENZ studies (ClinicalTrials.gov Identifiers: NCT03378661 and NCT03191162, respectively). Both studies aim to determine the efficacy and safety of different benznidazole regimens in adults who are in the chronic phase of Chagas disease. The primary efficacy endpoint is the parasitological response measured by PCR at the end of treatment and maintained for 12 months. In the BENDITA trial, a randomized, double-blind, phase-II, placebo-controlled trial conducted in Bolivia between 2016 and 2018, 210 patients were randomized into seven groups (30 patients in each group) of benznidazole on shorter regimens and in combination with E1224: benznidazole 300mg daily, administered for (a) 8 weeks, (b) 4 weeks, or (c) 2 weeks); (d) benznidazole 150mg daily for 4 weeks; (e) benznidazole 150mg daily for 4 weeks in combination with E1224 300mg weekly; (f) benznidazole 300mg daily for 8 weeks in combination with E1224 300mg weekly; and (g) corresponding placebos. The intention-to-treat primary efficacy analysis showed that 89.3% had sustained clearance at 6 months on parasitemia on benznidazole 300mg for 8 weeks and 4 weeks; 82.8% on benznidazole 300mg for 2 weeks; 83.3% on benznidazole 150mg for 4 weeks; 85.2% on benznidazole 150mg for 4 weeks in combination with E1224; and 82.8% on benznidazole 300mg weekly in combination with E1224, compared to 3.3% for placebo. Safety results suggested high adherence to treatment in all groups. Six patients (20%) discontinued treatment on benznidazole 300mg for 8 weeks; 1 (3.3%) for 4 weeks; none for 2 weeks; 1 (3.3%) in benznidazole 150mg for 4 weeks; 3 (10%) in benznidazole 150mg for 4 weeks in combination with E1224; and 4 (13.3%) in benznidazole 300mg weekly in combination with E1224. Most adverse events were mild to moderate, with only 6 patients presenting with serious events (SAE). No SAE were reported in benznidazole 300mg for 2 weeks and 150mg for 4 weeks.111
The MULTIBENZ study is a phase-II randomized, multi-centre, international study conducted in Brazil, Argentina, Colombia, and Spain, assessing benznidazole at lower doses compared to the standard scheme, in patients with chronic Chagas disease. A total of 240 patients was randomized to receive benznidazole 300mg daily for 60 days, benznidazole 400mg daily for 15 days, and 150mg daily for 60 days, with 80 patients in each group and 60 patients per country.
Two observational studies have been published with schemes using a lower overall dose of benznidazole. Viotti et al. analyzed the efficacy measured through the seroconversion of patients who had to interrupt treatment due to adverse events.112 Eighty-one adult patients with Chagas disease previously treated with benznidazole for a median of 10 days were followed. Twenty percent of these patients (16/81) were considered cured. In the same group, a new scheme of benznidazole was assessed with intermittent doses of benznidazole at 5mg/kg/day in 2 daily doses every 5 days for a total of 60 days among 20 patients in the disease's chronic phase. The endpoint of the study was treatment failure assessed by PCR and the number of treatment suspensions, as well as a reduction in the severity of adverse effects. The adverse events ratio was similar to that previously reported in the literature (50%) but with a lower discontinuation rate. (Only 1 patient suspended treatment.)113
Another approach explored to reduce the adverse event rates and, therefore, the treatment accomplishment, has been to initially administer benznidazole in an increasing dose. Almost 500 patients received a non-randomly standard dose scheme versus an escalating scheme lasting for 5 days up to a maximum of 300mg/day. The new scheme did not significantly improve drug tolerability or the treatment discontinuation rates during the first 30 days of treatment.87
New strategies with nifurtimox have also been explored. The CHICAMOCHA-3 trial (ClinicalTrials.gov Identifier: NCT02369978) is intended to evaluate the efficacy of both nifurtimox and benznidazole using a conventional dose for 60 days or a half-dose for 120 days.
A novel clinical trial has recently started its recruitment phase. The TESEO trial (ClinicalTrials.gov Identifier: NCT03981523) will evaluate the efficacy and tolerability of both benznidazole and nifurtimox by lowering the dose and the treatment duration.
Challenges in the treatment of T. cruzi infectionSurrogate markers for a cureChagas disease therapeutic response is currently based on a reversion to negative results in conventional serological tests, which may take 10–20 years in chronic infections.33 Parasitological methods including the PCR have low sensitivity and have not demonstrated a correlation with clinical outcomes after treatment in the chronic phase.45 Therefore, long-term follow-up is mandatory for patients treated in the chronic phase with its related costs. Another important derived issue is the lack of short-term markers of clinical response with which to evaluate the effectiveness of therapies in clinical trials.
Several studies have recently tried to identify surrogate biomarkers of a cure with different approximations. Vallejo et al. evaluated the adaptative immune response in treated patients compared to control patients showing significantly lower immune activation and lower regulatory T-cell counts, with an increased Th17 and Th1 response.114 Other studies have also described a change in the regulatory pattern of interferon-γ and cytokines.115 In addition, hypercoagulability has been studied in Chagas disease patients, with Pinazo et al. proposing haemostasis parameters as an early surrogate biomarker for an early cure.116 An upregulation of metabolic biomarkers such as A-I (APOA1) and specific fragments of human fibronectin (FN1) have also been described. Santamaría et al. published its normalization after 3 years in half of treated patients.117 One specific antibody response (antibody 3, Ab3) was also evaluated in 1635 chronic patients of the SamiTrop cohort. It showed a strong correlation with T. cruzi parasite persistence as determined by a T. cruzi PCR-positive result with a significant decline in its signal after trypanocidal treatment.118 Finally, various T. cruzi antigens and their sera reactivity have been evaluated, though the results are still far from ideal.119 Hence, the research of new reliable biomarkers, although prolific, has produced limited results. Further long-term evaluation must be done to prove the usefulness of biomarkers in clinical practice.
Development of new trypanocidal drugsRecent advances both in knowledge about the parasite and in the drug discovery process have led to the identification of new targets and new compounds. Several families have been tested: inhibitors of trypanothione metabolism, inhibitors of cysteine proteases, lysophospholipid analogues, modern ergosterol biosynthesis inhibitors, and bis-triazole derivatives, among others. Unfortunately, the results obtained in the animal model have stopped its posterior clinical development, for either lack of efficacy (a non-sterile cure) or its toxicity.
However, likely, among these new compounds, oxaborole derivates have shown the most promising results in preclinical studies.120 Moreover, SCYX-7158, an orally active benzoxaborole, has been tested against human African trypanosomiasis (ClinicalTrials.gov Identifier: NCT03087955). The results have not yet been published. This is reminiscent of the same clinical strategy used with fexinidazole. What makes that new family remarkable is its potential activity among other kinetoplastids such as leishmania.121
Access to treatmentAccess to both benznidazole and nifurtimox represents a challenge in most non-endemic countries, as well as for patients living in poor rural areas of endemic countries, due to restricted access to health care systems and limited provider awareness, among other causes.122–124 Even though benznidazole was recently approved by the FDA,41 many barriers to accessing this treatment remain for Chagas disease patients in the United States.122 Additionally, in Latin America (at least in 14 counties), an important gap exists between the current demand for drugs and the prevalence rate estimations of the disease, proving that much work remains to be done in terms of diagnosing and treating Chagas disease globally.125
A shortage of benznidazole occurred in 2011, leaving thousands of patients worldwide without access to this first option for treatment. During that period, in Spain (the European country with the highest prevalence of Chagas disease), a lack of access to benznidazole lasted over a year, forcing a prioritizing of the treatment for acute infection, neonates, and immunosuppressed patients.126 Nowadays, benznidazole is available in Spain and, depending on the region in which the patient lives, can be obtained through the hospital pharmacy or the regional health service of the Foreign Drugs Supply Department, with a different cost depending on the regional policy.127 Even though the WHO announced the availability of benznidazole free of charge for patients younger than 18 years,35 to date, this has not been implemented in Spain.
While investment in Chagas disease has increased throughout the years, and while institutions and non-profit organizations are working to facilitate the diagnosis and access to health care of patients, there is still much room for improvement.128 More effective, better-tolerated, and low-cost drugs are urgently needed.
FundingFunding was provided by ISCIII (Instituto de Salud Carlos III), project RD16/0027/0020. Red de Enfermedades Tropicales, Subprograma RETICS (Redes Temáticas de Investigación Cooperativa en Salud), and FEDER (Fondo Europeo de Desarrollo Regional).