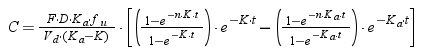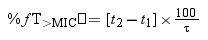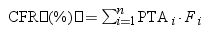The goal of this study is to assess, by means of pharmacokinetic/pharmacodynamic (PK/PD) analysis using the Monte Carlo simulation, the adequacy of oral cephalosporins cefuroxime axetil, cefixime and cefditoren at different dosing regimens as switch therapy after intravenous cephalosporin treatment in uncomplicated acute pyelonephritis.
MethodsThe methodology included: (i) dosing regimen selection and acquisition of pharmacokinetic data; (ii) microbiological data acquisition; and (iii) Monte Carlo simulation to estimate the PTA (probability of PK/PD target attainment) and CFR (cumulative fraction of response), as indicators of treatment success.
ResultsAt the current susceptibility breakpoints defined by EUCAST and CLSI for either cefuroxime axetil or cefixime, the probability of bactericidal target attainment is zero for the dosage regimens simulated. Considering the bactericidal target %fT>MIC>70%, the likelihood of the cefuroxime 500-mg q8h regimen or the cefixime 200-mg q12h regimen producing this exposure or achieving this target is only above 90% for organisms yielding MICs≤0.5mg/l and MICs≤0.25mg/l, respectively. Cefditoren pivoxil 400mg q12h provided probabilities of bactericidal target attainment of 80% or higher for MICs≤0.03mg/l, and ≤0.25mg/l if considering total instead of free drug concentrations.
ConclusionsThe results of the PK/PD target attainment analysis reveal that the likelihood of treatment success based upon the current breakpoints proposed by either EUCAST or CLSI is low. Of the three cephalosporins, cefixime 400mg q12h prove to be the best option in oral APN treatment, although this regimen is currently off label.
El objetivo de este estudio es evaluar, mediante el análisis farmacocinético/farmacodinámico (PK/PD) empleando la simulación de Montecarlo, la idoneidad de las cefalosporinas orales cefuroxima axetilo, cefixima y cefditoren en diferentes regímenes de dosificación, como terapia secuencial tras el tratamiento intravenoso con cefalosporinas, en pielonefritis aguda no complicada.
MétodosLa metodología incluyó: 1) selección del régimen de dosificación y adquisición de datos farmacocinéticos; 2) adquisición de datos microbiológicos; y 3) simulación de Montecarlo para estimar la probabilidad de alcanzar el objetivo (PTA) PK/PD y la fracción de respuesta acumulada (CFR), como indicadores del éxito del tratamiento.
ResultadosPara los puntos de corte de sensibilidad actuales definidos por EUCAST y CLSI para cefuroxima axetilo o cefixima, la probabilidad de alcanzar el objetivo bactericida es cero para los regímenes de dosificación simulados. Teniendo en cuenta el objetivo bactericida %fT>MIC>70%, la probabilidad de que el régimen de cefuroxima 500mg/cada 8h o el régimen de cefixima 200mg/cada 12h produzca esta exposición o alcance este objetivo es solo superior al 90% para los organismos que producen MIC≤0,5mg/l y MIC≤0,25mg/l, respectivamente. Cefditoren pivoxil 400mg/cada 12h proporcionó probabilidades de alcanzar el objetivo bactericida del 80% o más para MIC≤0,03mg/l, y ≤0,25mg/l si se considera el fármaco total en lugar de libre.
ConclusionesLos resultados del análisis PK/PD revelan que la probabilidad de éxito del tratamiento basado en los puntos de corte actuales propuestos por EUCAST o CLSI es baja. De las 3 cefalosporinas, la cefixima 400mg/cada 12h resultó ser la mejor opción en el tratamiento oral de pielonefritis aguda, aunque este régimen está actualmente fuera de ficha técnica.
Acute pyelonephritis (APN) is one of the most common community-onset infections. Escherichia coli is the microorganism most frequently involved in this type of infection, accounting for 75–95% of the cases.1 Intravenous cephalosporins are extensively used in hospitalized patients with APN with optimal results; however, there are still uncertainties about which oral antimicrobial agent should be prescribed once the criteria for switching to oral therapy are met. The IDSA 2011 guidelines for the treatment of uncomplicated pyelonephritis recommends a once-daily fluoroquinolone (ciprofloxacin or levofloxacin) or co-trimoxazole and states that oral β-lactams are less effective than the aforementioned drugs in the treatment of this disease.1 However, recent studies put under debate these recommendations, especially in areas with high fluoroquinolone resistance rates where alternative oral treatment options are seek.2 In fact, in Spain, the Spanish Society of Infectious Diseases and Clinical Microbiology (SEIMC) discourages the use of ampicillin, amoxicillin, amoxicillin-clavulanic acid, co-trimoxazole, fluoroquinolones, nitrofurantoin and fosfomycin-tromethamine for the empiric treatment of APN.3
The pharmacokinetic/pharmacodynamics (PK/PD) targets for optimal antimicrobial activity in patients with APN have not been studied specifically; nevertheless, experimental data derived from a model of ascending urinary tract infection in mice suggested that, in kidney infections, the plasma PK/PD indices of efficacy characteristic of the different antibiotic classes correlate with antibacterial activity in kidney tissue and in urine.4 At least in the case of β-lactams, it is clear that dosages that achieve effective concentrations in urine but not plasma are unable to reduce the bacterial burden in the kidneys.5 Multiple studies have shown that women with uncomplicated APN may be safely discharged and treated with an appropriate oral antibiotic after an observation period of up 24h and 1–2 doses of parenteral antibiotics.3,6,7
In sequential therapy, the dose and subsequent exposure to the active drug is considerably lower with oral betalactam antibiotics than with the previous parenteral treatment, with the available daily dose reduced by up to 80%.3 The adequacy of the proposed dosing regimen for pathogens with higher MIC values when switching to an oral formulation would be unacceptable.
The goal of the current study was to elucidate, by means of PK/PD analysis, the adequacy of oral cefuroxime axetil, cefixime and cefditoren pivoxil in sequential therapy in uncomplicated APN.
Materials and methodsThe methodology included the following steps: (i) dosing regimen selection and acquisition of pharmacokinetic data; (ii) microbiological data acquisition; and (iii) Monte Carlo simulation of the antibiotics studied in healthy adults. Monte Carlo simulation allowed us to estimate the probability of target attainment (PTA), defined as the probability that at least a specific value of a PK/PD index is achieved at a certain MIC, and to calculate the cumulative fraction of response (CFR), defined as the expected population PTA for a specific drug dose and a specific population of microorganisms.8
Dosing regimen selection and acquisition of pharmacokinetic dataCefuroxime axetil, cefixime and cefditoren pivoxil were chosen based on their use for the sequential therapy in uncomplicated APN in Spain.
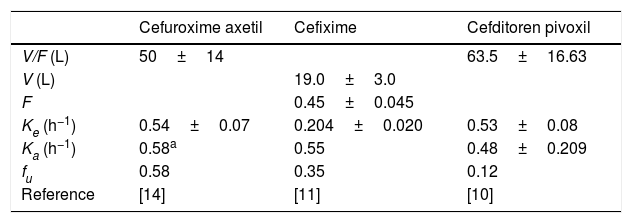
The following drug regimens were evaluated: (1) cefuroxime axetil: 500mg q12h and 500mg q8h; (2) cefixime: 200mg and 400mg q12h, and 400mg q24h; and (3) cefditoren pivoxil: 200mg and 400mg q12h. Pharmacokinetic parameters were obtained from published pharmacokinetic studies in healthy adults.9–14 All parameters were expressed as means and standard deviation (S.D.) (Table 1).
Pharmacokinetic parameters for each antimicrobial agent from published studies carried out in healthy adults (mean±standard deviation).
| Cefuroxime axetil | Cefixime | Cefditoren pivoxil | |
|---|---|---|---|
| V/F (L) | 50±14 | 63.5±16.63 | |
| V (L) | 19.0±3.0 | ||
| F | 0.45±0.045 | ||
| Ke (h−1) | 0.54±0.07 | 0.204±0.020 | 0.53±0.08 |
| Ka (h−1) | 0.58a | 0.55 | 0.48±0.209 |
| fu | 0.58 | 0.35 | 0.12 |
| Reference | [14] | [11] | [10] |
V: volume of distribution, F: bioavailability, Ke: elimination constant rate, Ka: absorption consant rate, fu: unbound fraction.
Estimated from the concentration–time data11 according to one-compartment model by using the Phoenix®WinNonlin® software.
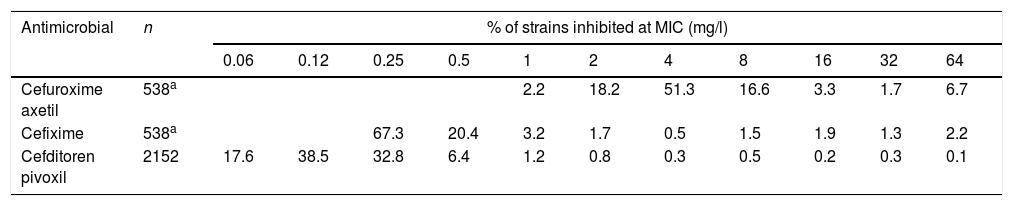
Susceptibility data of clinical isolates to each antibiotic were obtained from two recently published studies15,16 (Table 2). Both studies evaluated Enterobacterales isolates recovered from urine samples from patients with community-acquired uncomplicated UTI (CA-UTI). Isolates were recovered in Spain, Belgium and Germany. For cefixime and cefuroxime axetil only E. coli isolates were considered for this study since this pathogen is the most common causative agent in UTIs; a total of 538 strains were evaluated. Regarding cefditoren, a total of 2229 strains were included, of which 81.8% were E. coli, 9.4%, Klebsiella spp., 5.2% Proteus mirabilis and 3.6% other Enterobacteriaceae species.
Activity of the antibiotics studied against Enterobacterales isolates from community-acquired urinary tract infections.15,16
A 5000 subject Monte Carlo simulation was conducted for each antibiotic agent using Oracle® Crystal Ball Fusion Edition v.11.1.1.1.00 (Oracle USA Inc., Redwood City, CA). As β-lactam antibiotics show time-dependent antimicrobial activity, and the PK/PD index related to its activity is the percentage of time that free drug concentration remains over de MIC (%fT>MIC). A target %fT>MIC of 70% has been identified for near maximal bactericidal activity of cephalosporins, and a target of 40% is required for bacteriostasis.17,18
%fT>MIC was calculated for over an MIC range of serial twofold dilutions from 0.015mg/l to 64mg/l. We assumed a one-compartment pharmacokinetic model and, according to statistical criteria, logarithmic transformation was applied to the mean and S.D. of the pharmacokinetic parameters to normalize their distributions.
For cefuroxime axetil, cefixime and cefditoren pivoxil, which are administered by oral route, the following equation was used:
where F is the drug bioavailability, Ka is the absorption rate constant, K is the elimination rate constant, fu is the unbound fraction, τ is dosing interval, and n is the number of administered doses that ensures that the steady state is reached (10 doses was always selected).Using Oracle® Crystal Ball, the values of time at which concentration equals the MIC values were calculated and used to estimate %fT>MIC as follows:
where t1 and t2 corresponds to the time at which the drug concentration reaches the MIC in the ascendant and in the elimination phase of the plasma concentration–time curve, respectively.The PK/PD targets to estimate the %fT>MIC values were 40% and 70% of the dosing interval. For cefditoren pivoxil, the percentage of time that total drug concentration remains over de MIC (%T>MIC) was also calculated.
Estimation of cumulative fraction of response (CFR)The CFR, understood as the expected probability of success of a dosing regimen against bacteria in the absence of the specific value of MIC, was also calculated. It results from the total sum of the products of the PTA at a certain MIC times the frequency of isolates of microorganism exhibiting that MIC over the range of susceptibility, according to the following equation.19
where i indicates the MIC category, PTAi is the PTA of each MIC category, and Fi is the fraction of microorganisms population in each MIC category.The dosing regimens were considered optimal if the PTA or CFR were ≥90%, whereas a CFR or PTA ≥80% but <90% were associated with moderate probabilities of success.20,21
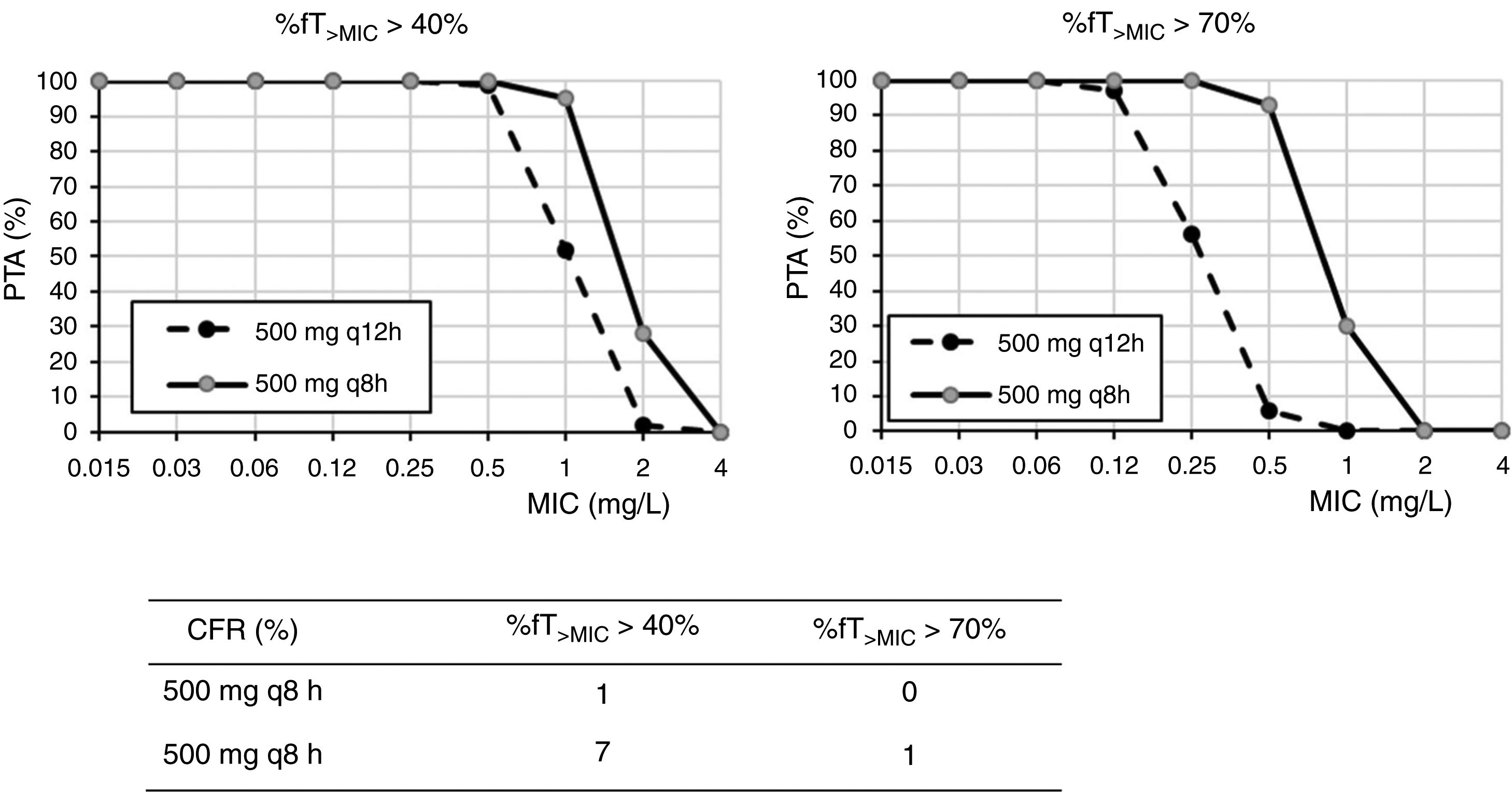
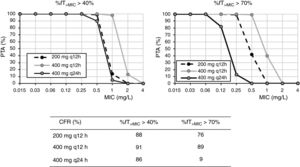
ResultsFig. 1 shows the PTA and CFR values calculated for the different dosing regimens of cefuroxime axetil. At the susceptibility breakpoints of EUCAST (MIC≤8mg/l) or CLSI (MIC≤4mg/l), the PTA of cefuroxime axetil 500mg q12h or 500mg q8h is invariably zero. Considering the bactericidal target (%fT>MIC>70%), 500mg q12h and 500mg q8h provided PTA values higher than 90% for strains with MIC≤0.12mg/l and 0.5mg/l, respectively. Nevertheless, according to the microbiological profiles (Table 2), all the strains yielded cefuroxime MICs>0.5mg/l. CFR values of above 90% are not reached for any of the simulated regimens regardless of the target.
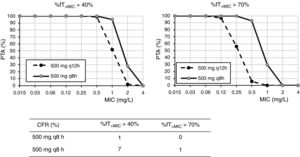
For cefixime (Fig. 2), at the current EUCAST and CLSI susceptibility breakpoint of 1mg/l, the PTA for bactericidal effect (%fT>MIC>70%) is 0 or 40%, depending on the dose level. PTA values higher than 90% are achieved with 200mg q12h for MICs ≤0.25mg/l and with 400mg q24h, for MICs≤0.06mg/l. With 400mg q12h, off-label dosage regimen, PTA≥90% is achieved for MICs≤0.5mg/l. For bacteriostatic activity (%fT>MIC>40%), the three dosage regimens provided CFR values >80%, although only with 400mg q12h if bactericidal activity is considered.
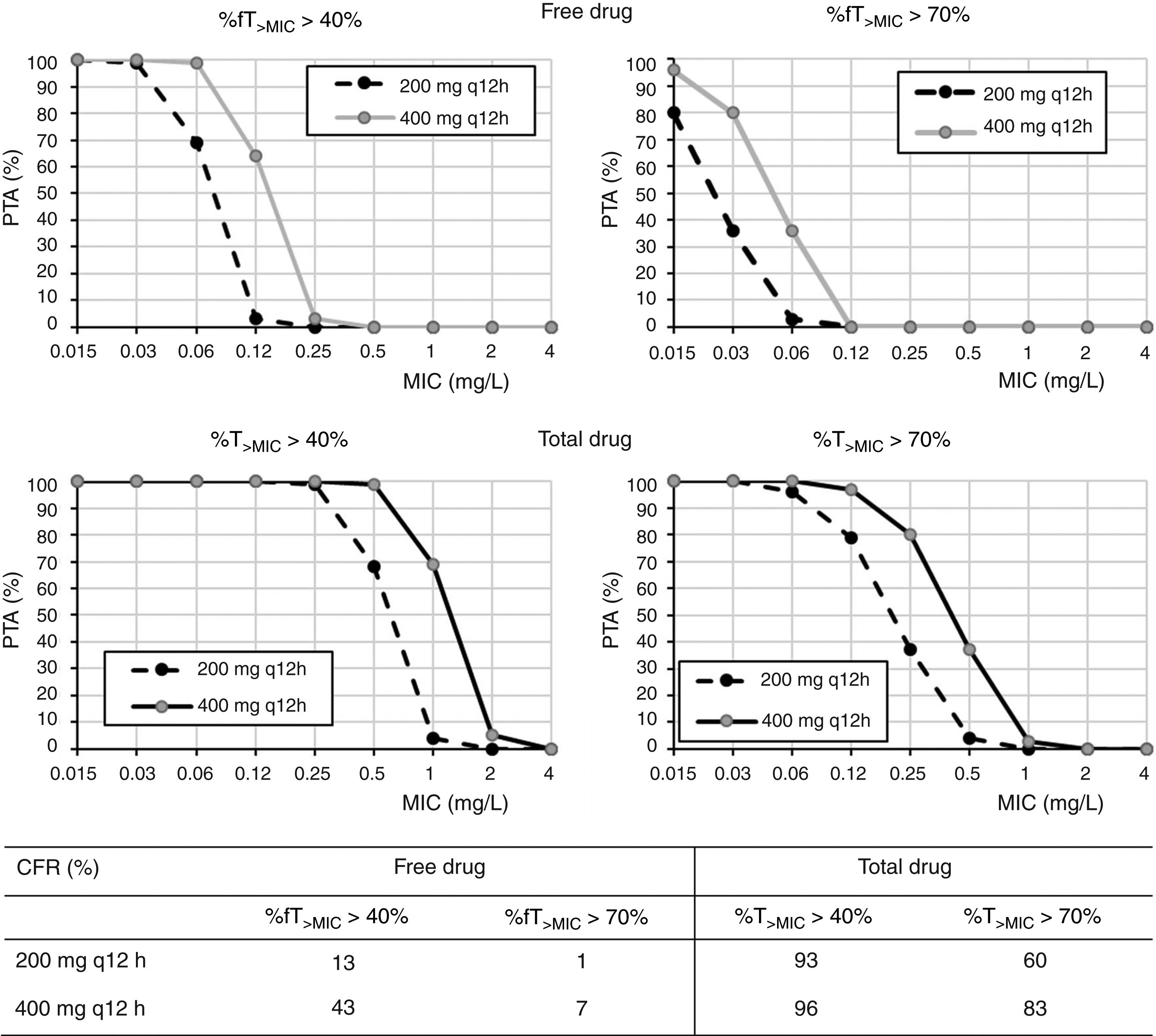
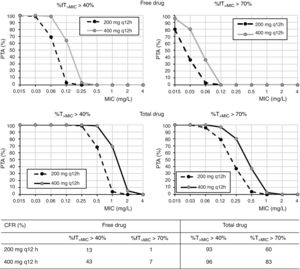
Regarding cefditoren pivoxil (Fig. 3), considering free drug and for a MIC of 1mg/l, PTA values are always zero irrespective of the dosing regimen and target; moreover, CFR values are by far lower than 90%. Nevertheless, for total drug and the bacteriostatic target attainment (%fT>MIC>40%), 400mg q12h and 400mg q24h provide PTA≥80 for strains with MIC values of ≤0.25mg/l and ≤0.06mg/l, respectively. Regarding CFR, values ≥90% were only obtained for bacteriostatic effect and taking into account total drug.
DiscussionThe objective of this study was to evaluate, by PK/PD analysis and Monte Carlo simulation, if the administration of the oral cephalosporins cefuroxime axetil, cefixime and cefditoren pivoxil at different dosing regimens is suitable for the treatment of uncomplicated APN, as switch therapy after intravenous treatment.
The PK/PD analyses carried out was used to evaluate current in vitro susceptibility test interpretative criteria decisions and to evaluate the adequacy of oral cephalosporins in sequential IV/oral antibiotic for APN after IV treatment. PK/PD modelling is a useful tool to achieve an optimal clinical and microbiological response while minimizing the probability of exposure-related toxicity. Once the exposure targets for optimal clinical response are known, Monte Carlo simulation is a useful tool to combine pharmacokinetic, pharmacodynamic and microbiological data in order to predict an antibiotic dosing regimen's probability of achieving the targeted pharmacodynamic exposure. For the antimicrobials under study, the %fT>MIC of 60–70% has been identified for near maximal bactericidal activity whereas a target of 40% has been proposed to be required for achieving bacteriostasis. In the specific case of UTIs, bactericidal effect should be pursued in order to achieve the sterilization of the urinary tract.3
Regarding cefuroxime axetil and according to the PK/PD analysis, standard dosages of 500mg q12h or q24h do not reach the recommended target. The current susceptibility breakpoint of cefuroxime axetil for Enterobacterales is fixed at ≤8mg/l by EUCAST and at ≤4mg/l by CLSI. EUCAST specifies that this breakpoint should only be followed in uncomplicated UTI. Since its introduction in the late 1970s, the second-generation cephalosporin cefuroxime axetil has been widely used to treat UTIs caused by Enterobacterales. In a recent study, intravenous cefuroxime was found to be as effective as cefotaxime in the initial empirical treatment of community-acquired nonobstructive APN.22 In our setting and in line with other studies,3 most of the strains (90%) recovered from urine samples of patients with APN yielded MIC values ≤8mg/l, categorized as susceptible by the two main committees (CLSI and EUCAST). However, the PK/PD analysis carried out suggests that current in vitro susceptibility test interpretative criteria may lead to therapeutic failure in strains with MICs within the susceptibility range. Actually, in other study, clinical failure in form of relapses was reported in critically ill patients when the strain, despite being susceptible, yields MICs close to the clinical breakpoint, generally above 4mg/l.23
Cefixime is a third generation cephalosporin frequently used in the outpatient management of UTI of young children since it has shown to be a safe and effective treatment option in such infections.24 The susceptibility breakpoint is fixed by both EUCAST and CLSI in MIC ≤1mg/l. Nevertheless, according to our results, in strains yielding MICs of 1mg/l, bactericidal PTA is under 80%, and thus, suboptimal. Dose regimens of 200mg q12h, 400mg q12h and 400mg q24h seem to be adequate for MIC≤0.25, 0.5, and 0.06mg/l, respectively. For empirical treatment, only 400mg q12h provides high probability of treatment success; however, this dosage regimen is currently off-label and not supported by the manufacturer. In any case, this high dose may not be necessary if the patient is not an overweighed woman.
In what cefditoren pivoxil is concerned, although there are currently no established susceptibility breakpoints for this antimicrobial, some authors consider isolates with MICs ≤1mg/l as susceptible.15,25 According to the PK/PD analysis and the PTA values obtained by considering the free drug, none of the dosing regimens studied provided high probabilities of treatment success for this MIC value. Although it is well known that only the free drug fraction is active, Sevillano et al.26 reported that for cefditoren, extrapolation of active drug from total drug by using the protein-binding rate seems inadequate to study the antibacterial activity and to interpret cefditoren pharmacodynamics, and, as for other antibiotics, fraught with underestimations of antimicrobial activity.27 For instance, tigecycline antibacterial activity has shown to be greater than that suggested by the free fraction of the drug.28 Therefore, we should expect higher probabilities of treatment success than that predicted by our results. For this reason, we also calculated the PTA and CFR values for total drug, being much more favourable, although undoubtedly overestimated. According to our results for total drug, 400mg q12h would be useful for empirical treatment, with a probability of treatment success (CFR) higher than 80%. Although these results must be taken with caution, they are consistent with previous studies that propose cefditoren pivoxil as an alternative antimicrobial for the treatment of UTIs, showing superior in vitro activity compared to other oral drugs such as cefuroxime, ciprofloxacin or co-trimoxazole.25,29,30 Actually, it has been recommended as empirical treatment of UTI in outpatients.25
This study has some limitations. First, in vitro antimicrobial susceptibility data were collected from two different studies carried out according to their correspondent protocols and during different periods of time: 2010 in Spain and 2016 in Germany. Second, although PK/PD Monte Carlo simulations offer support in the selection of optimal antibiotic and dosing regimens, these simulations are based on a number of assumptions. The limitations are widely explained by Frei et al.31 in a publication about PK/PD analysis with Monte Carlo simulation. Third, data used in our study included isolates from uncomplicated UTI, and therefore, the MIC values could be overestimated (for instance, isolates from non-recurrent cystitis and others that not require microbiological analysis are not included). Therefore, the probability of treatment success may be underestimated. Fourth, the effect of the previous intravenous treatment was not evaluated. Thus, the probabilities of success may be greater than those predicted by the PK/PD analysis due to the reduction of bacterial load achieved after the previous intravenous administration of the antibiotic.
ConclusionsIn summary, our results reveal that oral cephalosporin exposure may be insufficient at current or proposed clinical breakpoints. This may be a limitation in clinical routine, since most microbiology laboratories analyze the in vitro susceptibility with automated systems, which use a straight range of concentration, around the clinical breakpoint. Our study also shows that out the three oral cephalosporins studied, the better option for empirical treatment resulted to be cefixime at the dose of 400mg q12h, although this regimen is currently off label.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interestThe authors declare no conflict of interest.