Biofilm formation causes virulence and resistance in Candida albicans. However, little is known about breakthrough candidemia isolates. We evaluated the antifungal activity of fluconazole, anidulafungin, deoxycholate amphotericin B (dAMB), and amphotericin B lipid complex (ABLC) against biofilms of C. albicans isolated from patients with breakthrough candidemia.
MethodsThe present study used strains of C. albicans isolated from breakthrough and non-breakthrough candidemia patients (control group). The susceptibility of planktonic cells to amphotericin B, anidulafungin, and fluconazole was determined by broth microdilution. Antifungal activity in sessile cells was evaluated using the minimum biofilm eradication concentration (MBEC), metabolic activity was estimated by reducing MTT, and biomass was estimated using crystal violet retention.
ResultsThe planktonic strains were susceptible to amphotericin B, anidulafungin, and fluconazole, with minimum inhibitory concentrations of 1, ≤0.03, and 2mg/L, respectively. However, fluconazole and anidulafungin did not exert an antifungal effect on biofilms. Additionally, dAMB and ABCL reduced the metabolic activity and biomass. However, eradication was only achieved using 16mg/L dAMB. C. albicans isolates of breakthrough candidemia exhibited strong biofilm production, and the in vitro activity of available therapeutic options was poor.
ConclusionIn the present study, only dAMB and ABCL exhibited antibiofilm effects against sessile breakthrough candidemia isolates.
La formación de biofilm se asocia con la virulencia y la resistencia al tratamiento de Candida albicans (C. albicans) sin embargo, son poco conocidas las características de los aislamientos procedentes de pacientes con candidemias de brecha. Evaluamos la actividad antifúngica de fluconazol, anidulafungina, anfotericina B desoxicolato (dAMB) y el complejo lipídico de la anfotericina B (ABLC) frente a biofilms de C. albicans aisladas de pacientes con candidemia de brecha.
MétodosSe utilizaron cepas de C. albicans aisladas de candidemias de brecha y de otras candidemias (grupo control). La sensibilidad de las células planctónicas a la anfotericina B, la anidulafungina y el fluconazol se determinó mediante el método de microdilución en caldo. En células sésiles, la actividad antifúngica se evaluó mediante la concentración miníma de erradicación de biofilm (MBEC), la actividad metabólica se estimó mediante la reducción de MTT y la biomasa mediante la retención de cristal violeta.
ResultadosLas cepas en forma planctónica fueron sensibles a la anfotericina B, anidulafungina y fluconazol, con CMI de 1 mg/L, ≤ 0,03 y 2 mg/L, respectivamente; sin embargo, no se observó efecto antifúngico sobre los biofilms con fluconazol o anidulafungina. Con dAMB y ABCL se observó una reducción de la actividad metabólica y de la biomasa, pero la erradicación solo se consiguió con 16 mg/L de dAMB. Las cepas de C. albicans que causan candidemia de brecha producen abundante biofilm y las opciones terapéuticas disponibles no son activas in vitro frente a ellas.
ConclusiónSolo dAMB y ABCL exhibieron efecto antibiofilm frente a los aislamientos de C. albicans sésiles y planctónicos.
The incidence of invasive fungal diseases (IFDs) has increased in the last two decades being one the major causes of death in immunosuppressed patients. The incidence of candidemia follows the same tendency of other IFD. Among the complications of candidemia is the breakthrough candidemia. Breakthrough candidemia is characterized when Candida spp. is recovered in the blood culture after three days of an adequate systemic antifungal therapy.1
The occurrence of breakthrough candidemia contributes to unfavorable outcomes, which are aggravated by the ability of the microorganisms to form biofilms in medical devices. This interferes with appropriate infection management, reducing treatment efficacy and increasing mortality.2,3 The incidence of breakthrough candidemia ranges from 9% to 18% in large hospitals.2–4 However, the incidence can reach 52% in patients with severe hematological diseases.5
The pathogenicity of these microorganisms is measured by several virulence factors, including their ability to adhere to devices and biofilm formation.6 These mechanisms are complex and multifactorial. However, microorganisms use biofilms to survive and overcome hostile conditions in order to colonize and establish in new environments.7
The main groups of antifungal agents used in candidemia are azoles, echinocandins, and amphotericins. Among the azoles, fluconazole is the most used drug and is currently the second choice for the treatment of candidemia, after the discovery of echinocandins.8 Currently, echinocandins (micafungin, anidulafungin, and caspofungin) are the drugs of choice for the treatment of candidemia, and a study has demonstrated the clinical superiority of anidulafungin.9 Amphotericin is widely used in the treatment of severe candidemia. However, owing to nephrotoxicity, it has become an alternative drug to echinocandins and fluconazole. It is still indicated for use in refractory candidemia and resistance to azoles and echinocandins.10,11 To decrease the risk of nephrotoxicity, lipid formulations of amphotericin, including the lipid complex, have been developed and can be successfully used in candidemia.12 Therefore, these drugs were selected for the present study.
Several studies have assessed the risk factors associated with the development of breakthrough candidemia.13–15 However, little is known about the mechanism by which the microorganism achieves breakthrough from previous antifungal treatments. The present study investigated the hypothesis that strains of Candida albicans isolated from patients with breakthrough candidemia differ from those isolated from patients with non-breakthrough candidemia in terms of biofilm production capacity and response to antifungal agents, such as fluconazole, deoxycholate amphotericin B (dAMB), amphotericin B lipid complex (ABLC), and anidulafungin.
MethodsExperimental study designEight strains of C. albicans were studied from patients aged>18 years and admitted to the Hospital de Clínicas Complex of the Federal University of Paraná (CHC-UFPR) between January 2011 and December 2017 with acute myeloid leukemia. This study was approved by the ethics committee (under CAAE number 40592915.2.000.0096 of Universidade Federal do Paraná). Written informed consent was obtained from all patients.
The C. albicans strains were divided into two groups, i.e., (a) C. albicans from breakthrough candidemia (numbers 269, 307, 332, and 334) and (b) C. albicans isolated from blood culture patients that presented with non-breakthrough candidemia (identified by numbers 300, 328, 347, and 358). The identification of all isolates was confirmed by matrix-assisted laser desorption ionization/time-of-flight (MALDI-TOF) mass spectrometry (bioMérieux, Marcy l’Etoile, France) with a score value≥2.000.
Minimal inhibitory concentration (MIC) determinationFour antifungal drugs were used: dAMB (Sigma-Aldrich, St. Louis, MI), ABLC (Teva, São Paulo, Brazil), fluconazole (Sigma–Aldrich, St. Louis, MO), and anidulafungin (Wyeth-Pfizer, Kalamazoo, MI). The MIC was determined in accordance with the Clinical and Laboratory Standards Institute guidelines, using broth microdilution according to the protocol M27-S4.16
Biofilm productionA suspension of 1.5×106CFU/mL of each yeast was prepared using RPMI 1640, and 200μL was discharged into each well of a 96-well microplate (flat bottom). The microplate was then incubated for 24h at 36°C on a shaker at 120rpm after sealing with adhesive seal. The positive control (growth) was prepared using 200μL of each yeast suspension, whereas the negative control (sterility) was prepared using 200μL of RPMI 1640.17,18 Next, the biofilms of each strain were subjected to antifungal susceptibility tests.
After the biofilm formation step, the medium of each well was aspirated, and 200μL of RPMI 1640 was prepared at concentrations of 0.25–16, 0.25–64, 0.125–32, and 0.25–32mg/L of dAMB, ABCL, fluconazole, and anidulafungin, respectively. All antifungal solutions were prepared in RPMI 1640. The microplates containing the biofilm and each antifungal with respective concentrations were then incubated for 24h at 36°C on a shaker at 120rpm. Next, the medium was aspirated, and the resulting biofilm was washed with 200μL of PBS (pH 7.5) to remove planktonic/nonadherent cells.
Determination of minimum biofilm eradication concentration (MBEC)After the antifungal treatment and washing step (as mentioned above), 200μL of PBS (pH 7.5) was added to each well. The 96-well microplates were sealed with an adhesive seal and then sonicated in an ultrasonic bath for 5min at 37°C and 40kHz (Sanders Medical, Santa Rita do Sapucaí, Minas Gerais, Brazil) to disrupt the biofilm matrix cells. Serial dilutions were made using sterile saline, which was poured onto Sabouraud dextrose agar plates. The plates were then incubated for 24h at 36°C. The CFU count was determined and the results were calculated as CFU per area unit (log10 CFU/cm2), where MBEC is the minimum concentration in milligram per liter of each antifungal capable of reducing biofilm cells by 2 log10 per cm2.6
Estimation of metabolic activity by the MTT reduction assayThe biofilms were incubated in a 96-well microplate filled with 200μL MTT [3-(4,5-dimethylthiazol-2yl)-2,5-diphenyl tetrazolium bromide] (Sigma-Aldrich, St. Louis, MO, USA) at a concentration of 1mg/mL at 36°C for 2h. After incubation, MTT was aspirated, and the wells were washed thrice using PBS (pH 7.5). The resulting product (formazan) formed inside the cells was dissolved in 200μL of isopropanol and homogenized. Next, 100μL of this solution was transferred to a new 96-well microplate. Absorbance was measured using a Versa-Max microplate ELISA reader adjusted to a wavelength of 570nm.19 Biofilm classification was based on optical density (OD) according to a previous criteria.17 The results of the MTT assay were expressed as a percentage relative to the positive control (PC), which was considered 100%.
Estimation of biomass by crystal violet retentionBiofilms were fixed in a 96-well microplate using methanol (200μL) for 15min. Next, methanol was aspirated, and the wells were dried at room temperature. Thereafter, 200μL of 1% crystal violet was added to each well and the microplate was incubated at 36°C for 5min. The wells were carefully washed thrice with ultrapure water. Then, 200μL of 33% acetic acid was added to dissolve the stain, which was then homogenized. Next, 100μL of the solution was transferred to a new 96-well microplate and the total biomass was quantified using a Versa-Max microplate ELISA reader (Molecular Devices, Sunnyvale, CA) at a wavelength of 570nm. The results were stored as a percentage of biomass reduction.
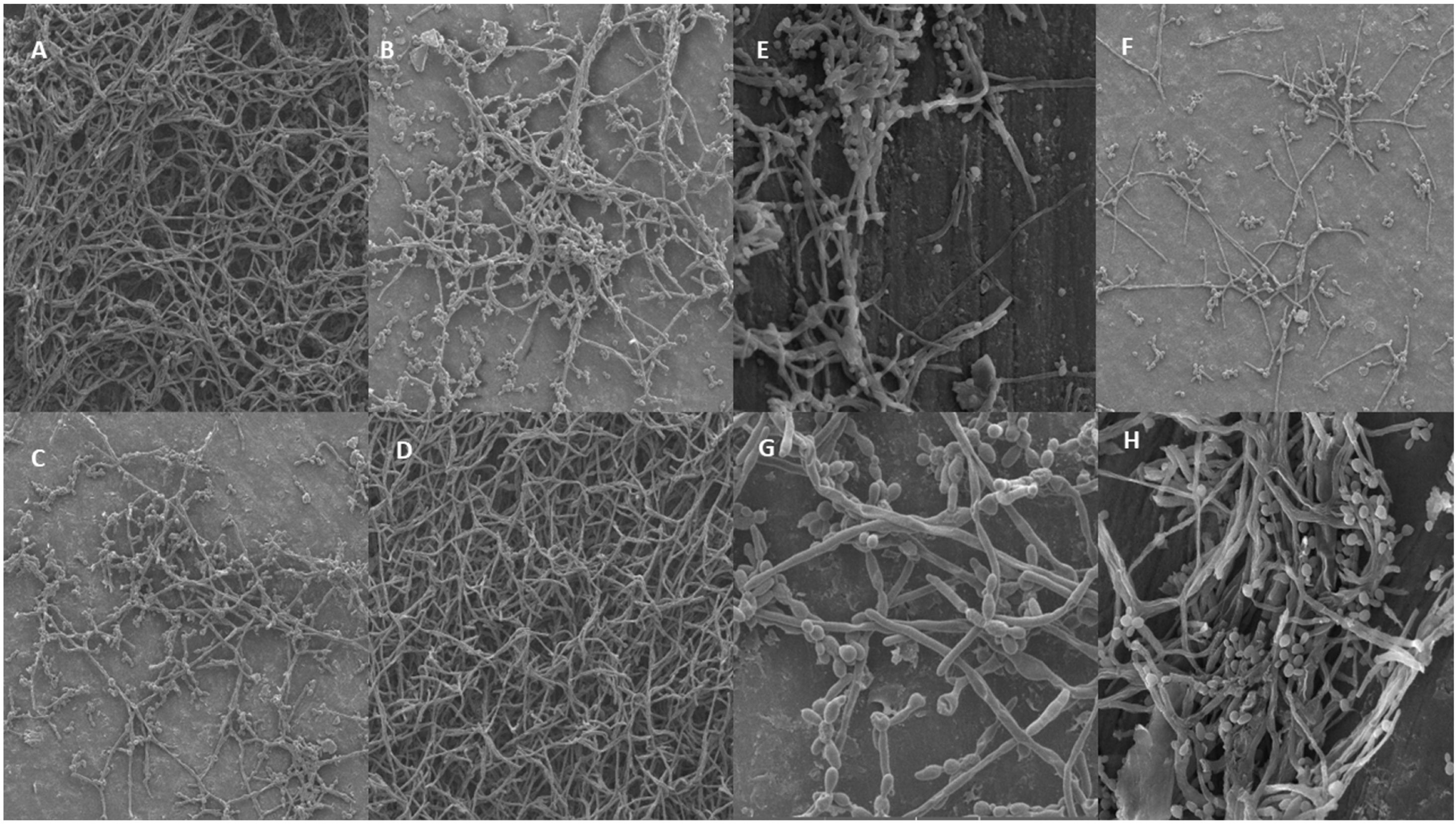
Biofilm analysis using scanning electronic microscopy (SEM)To evaluate the structure and biofilm adherence in vitro, SEM was performed using polystyrene discs (16mm×2mm). This method allows the capture of high-resolution images of a sample's surface and is largely used to observe the biofilm structure. Images were obtained at magnification of 1000× for each strain of each group.
Statistical analysisAll tests were performed in triplicate. Qualitative data are described as percentages, and quantitative data are expressed as medians with 10% and 90% percentiles. To compare the results between isolated samples from patients with breakthrough candidemia and non-breakthrough candidemia, the Mann–Whitney U test was used to compare medians. Statistical significance was set at p<0.05. The Shapiro–Wilk normality test showed a non-normal distribution of data, justifying the usage of a non-parametric test.
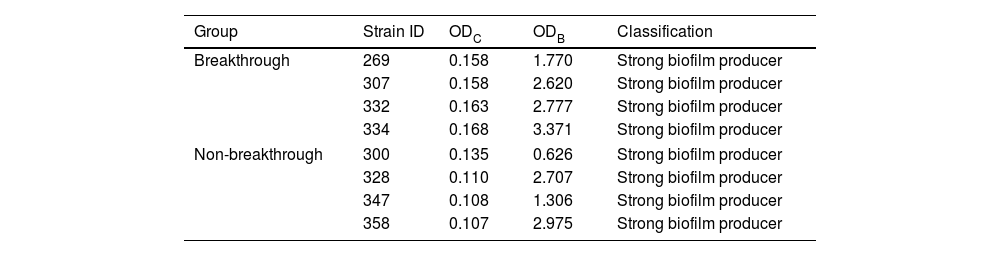
ResultsIdentification of C. albicans strains and biofilm characterizationThe ability to adhere, grow, and produce biofilms was observed for all isolates from the two groups. Biofilm production in isolates from breakthrough candidemia was four times higher than in isolates from non-breakthrough candidemia using violet crystal (measured in optical density). Therefore, all isolates were classified as strong biofilm producers (Table 1).
Classification of biofilm production of Candida albicans isolates using crystal violet measured by optical density (OD) from breakthrough (ODB) and non-breakthrough (ODC) candidemia.
| Group | Strain ID | ODC | ODB | Classification |
|---|---|---|---|---|
| Breakthrough | 269 | 0.158 | 1.770 | Strong biofilm producer |
| 307 | 0.158 | 2.620 | Strong biofilm producer | |
| 332 | 0.163 | 2.777 | Strong biofilm producer | |
| 334 | 0.168 | 3.371 | Strong biofilm producer | |
| Non-breakthrough | 300 | 0.135 | 0.626 | Strong biofilm producer |
| 328 | 0.110 | 2.707 | Strong biofilm producer | |
| 347 | 0.108 | 1.306 | Strong biofilm producer | |
| 358 | 0.107 | 2.975 | Strong biofilm producer | |
In addition to this classification, the architectural structure was observed through SEM, confirming that all strains of C. albicans produced mature and structurally organized biofilms after 48h on the discs (Fig. 1). At 1000× magnification, the biofilm was found to be adhered to the flat and smooth surfaces of the polystyrene discs in the presence of blastoconids and interlaced hyphae. Fig. 1A–D represents C. albicans from breakthrough candidemia (numbers 269, 307, 332, and 334, respectively) and 1E–H represent C. albicans isolated from blood culture patients that presented with non-breakthrough candidemia (identified by numbers 300, 328, 347, and 358, respectively).
Images obtained through scanning electronic microscopy (SEM) of biofilms formed by Candida albicans isolated from breakthrough and non-breakthrough candidemia strains on polystyrene discs, at a 1000× magnification. Images A: strain 269, B: strain 307, C: strain 332, and D: strain 334, all representing breakthrough strains obtained from a location closer to the edges of the polystyrene disk, where the biofilm was denser and it is possible to observe the predominance of intertwined pseudohyphae and/or blastoconids. Images E: strain 300, F: strain 328, G: strain 347, and H: strain 358, all representing non-breakthrough candidemia.
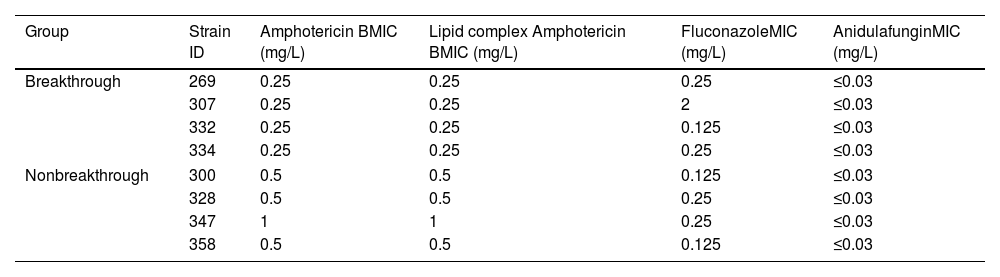
The MICs are presented in Table 2. All the drugs tested showed good activity against planktonic/free cells. For dAMB and ABLC, MICs varied between 0.25 and 1mg/L. MICs varied from 0.125 to 2mg/L for fluconazole (all susceptible to fluconazole). For anidulafungin, all MICs were ≤0.03mg/L.
MICs (mg/L) of antifungals against planktonic cells from breakthrough and non-breakthrough candidemia strains.
| Group | Strain ID | Amphotericin BMIC (mg/L) | Lipid complex Amphotericin BMIC (mg/L) | FluconazoleMIC (mg/L) | AnidulafunginMIC (mg/L) |
|---|---|---|---|---|---|
| Breakthrough | 269 | 0.25 | 0.25 | 0.25 | ≤0.03 |
| 307 | 0.25 | 0.25 | 2 | ≤0.03 | |
| 332 | 0.25 | 0.25 | 0.125 | ≤0.03 | |
| 334 | 0.25 | 0.25 | 0.25 | ≤0.03 | |
| Nonbreakthrough | 300 | 0.5 | 0.5 | 0.125 | ≤0.03 |
| 328 | 0.5 | 0.5 | 0.25 | ≤0.03 | |
| 347 | 1 | 1 | 0.25 | ≤0.03 | |
| 358 | 0.5 | 0.5 | 0.125 | ≤0.03 | |
After biofilm production, three complementary techniques were used to evaluate the susceptibility to antifungals: MBEC, estimating metabolic activity by reducing MTT, and evaluating biomass by crystal violet retention. All detailed data of MBEC, MTT and biomass are included in the supplement material (S1).
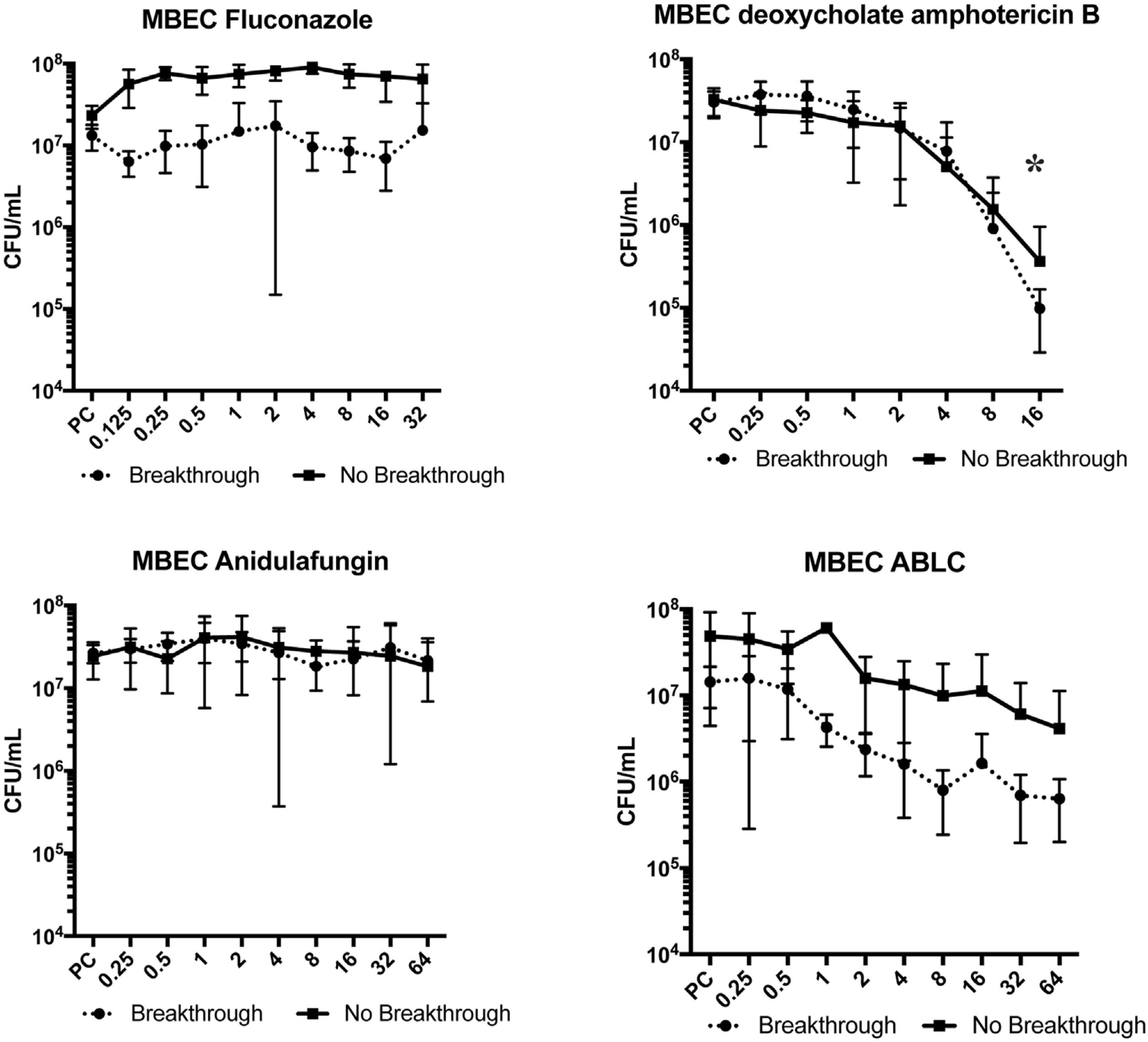
Fluconazole and anidulafungin were unable to eradicate the biofilm (p>0.05). ABLC decreased the cell concentration by 1 log10 for breakthrough candidemia isolates (p=0.09), but not for non-breakthrough candidemia isolates. When dAMB was evaluated, a 2 log10 reduction was observed in the biofilm cells at 16mg/L of this antifungal (p<0.05 for breakthrough candidemia isolates as well as for non-breakthrough candidemia isolates) (Fig. 2).
Minimal biofilm eradication concentration (MBEC) for fluconazole, deoxycholate amphotericin B, anidulafungin, and amphotericin B lipid complex (ABLC) of Candida albicans isolates from blood culture in patients with breakthrough (n=4) and non-breakthrough (n=4) candidemia. The X axis is the antifungal concentration (mg/L). * p<0.05 in comparison with control.
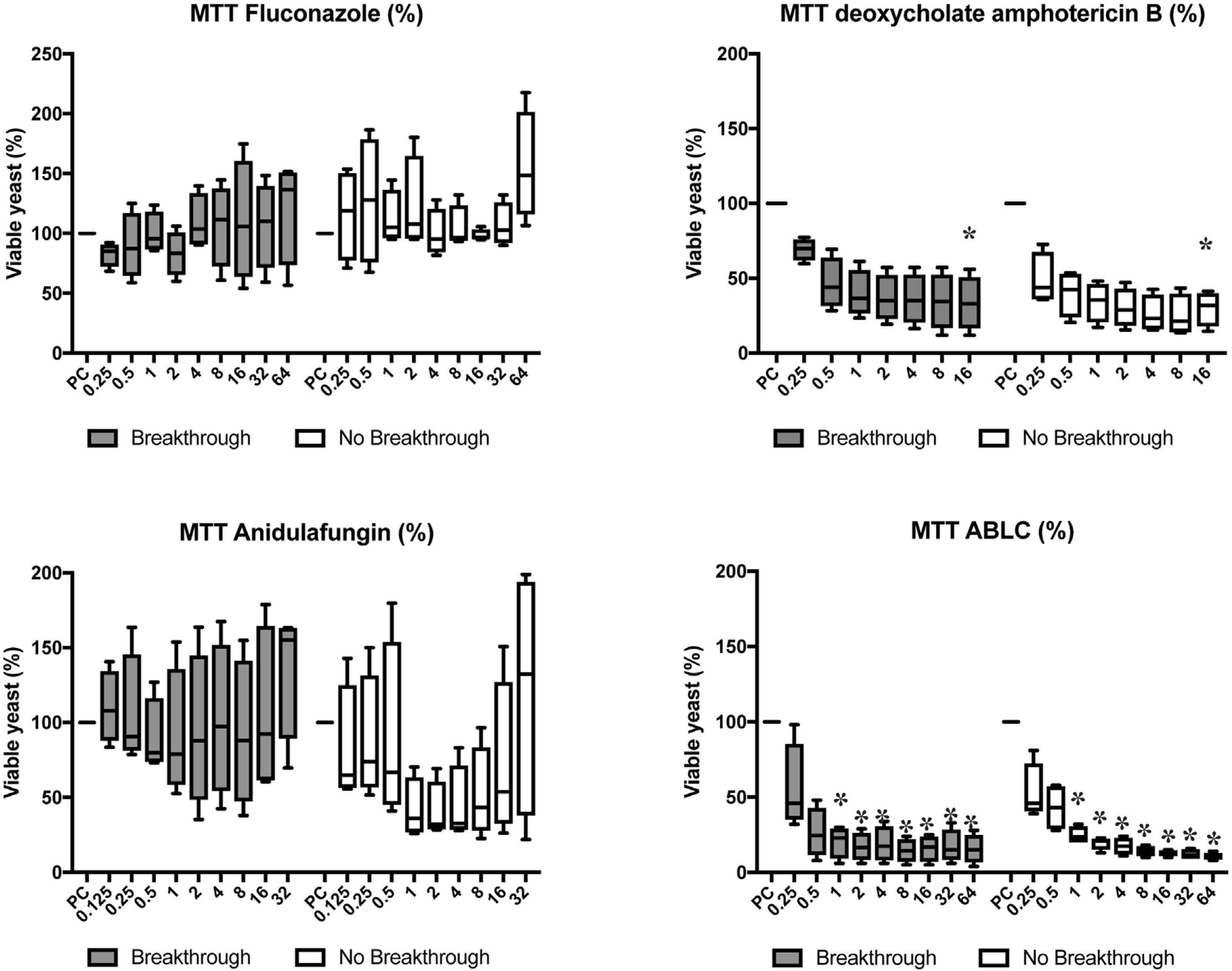
Fluconazole and anidulafungin did not reduce metabolic activity in cells (p>0.05). Only dAMB and ABLC showed antifungal activity against the biofilms. dAMB reduced the metabolic activity by approximately 50% at 0.5mg/L of the drug, and 75% at 16mg/L (p<0.05). ABLC reduced metabolic activity by approximately 50% at 0.25mg/L, the lowest dose tested, and by 75% at 1mg/L (p<0.05) (Fig. 3).
Candida albicans biofilm cell viability using MTT method for fluconazole, deoxycholate amphotericin B, anidulafungin, and amphotericin B lipid complex (ABLC) in breakthrough (n=4) and non-breakthrough (n=4) candidemia. The box plot represents min, max, and interquartile range at 25% and 75%. The values are expressed as percentage in relation to positive control (PC). The X axis is the antifungal concentration (mg/L). * p<0.05 in comparison with control.
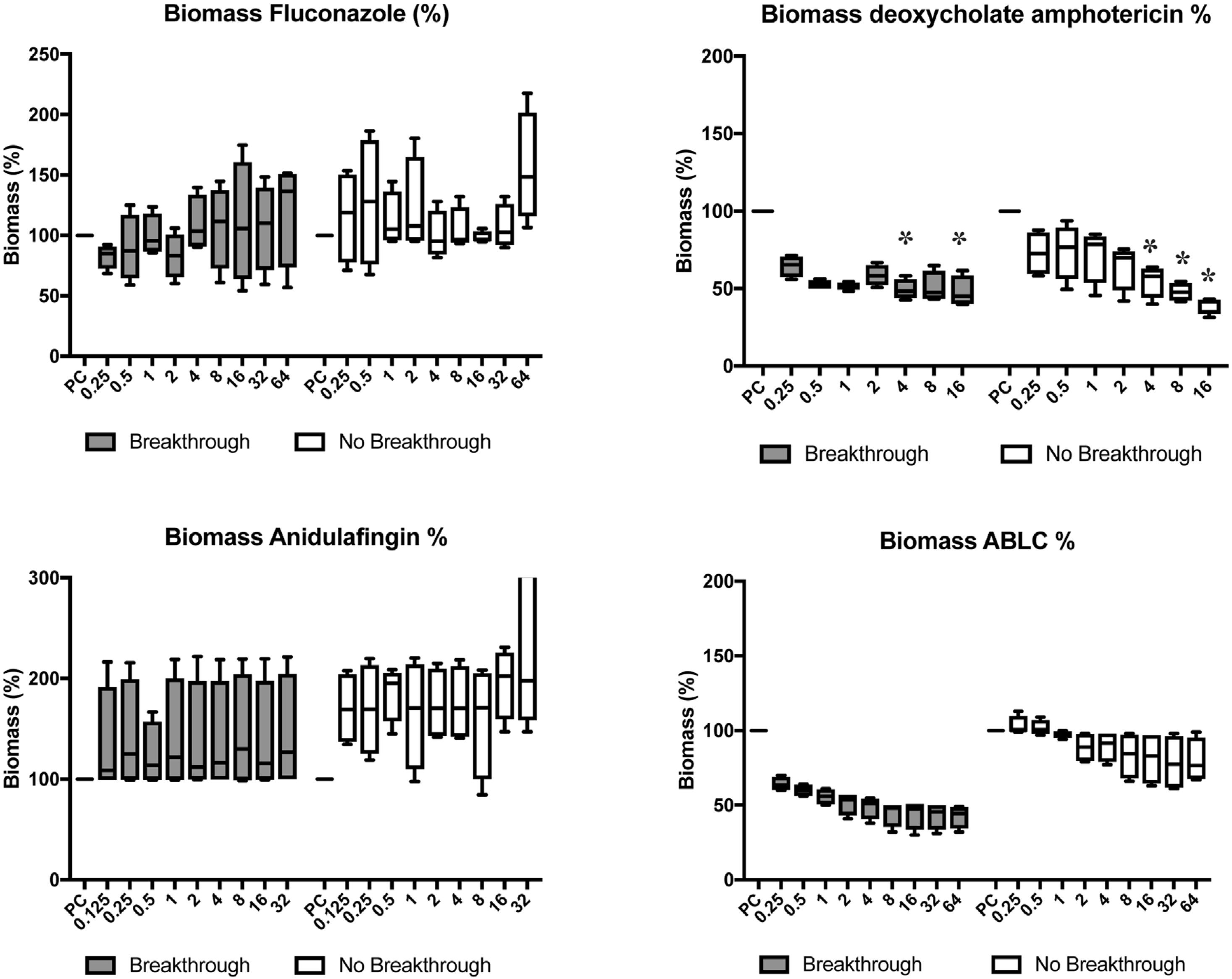
No significant reduction was observed in biomass (p>0.05) when testing fluconazole and anidulafungin. dAMB exhibited better antifungal activity toward the biofilm, thus reducing 52% of the biomass at a concentration of 0.5mg/L in the strains from breakthrough candidemia (p<0.05) at 4 and 16mg/L concentrations of the antifungal agent. For non-breakthrough candidemia isolates, a 54% reduction was observed in biomass at 4mg/L, with p<0.05, for concentrations of 8 and 16mg/L. Using ABCL, a 50% reduction was observed at 2mg/L in the breakthrough candidemia group. In comparison with the non-breakthrough candidemia group, a maximum reduction of approximately 25% was observed at concentrations of 32 and 64mg/L (Fig. 4).
Candida albicans biomass after progressive concentrations of fluconazole, deoxycholate amphotericin B, anidulafungin, and amphotericin B lipid complex (ABLC) in breakthrough (n=4) and non-breakthrough (n=4) candidemia. The box plot represents min, max, and interquartile range at 25% and 75%. The values are expressed as percentage in relation to positive control (PC). The X axis is the antifungal concentration (mg/L). * p<0.05 in comparison with control.
When analyzing the groups by evaluating the techniques and antifungals separately, regardless of the concentration of the tested drug, no statistical difference was observed between C. albicans isolated from breakthrough and non-breakthrough candidemia in the tests: MBEC technique using dAMB (p=0.312), ABLC (p=0.112), and anidulafungin (p=0.911); MTT reduction using dAMB (p=0.203), ABLC (p=0.103), and fluconazole (p=0.197), and biomass analysis when using dAMB (p=0.459), ABLC (p=0.245), and fluconazole (p=0.154). A statistically significant difference was observed between the groups in the MBEC technique using fluconazole (p<0.001) and MTT and biomass when tested with anidulafungin (p=0.004 and p=0.001, respectively).
DiscussionThe insertion of a catheter is a risk factor for developing candidemia, as the manipulation of these devices contributes to contamination and development of biofilms caused by yeasts such as Candida spp.20 Our results corroborate previous findings that described the biofilm produced by C. albicans as having a heterogeneous architecture comprising multilayered yeasts and hyphae.21 These structures formed by C. albicans are difficult to eradicate and have greater stability and resistance to most antifungals.22 In addition, candidemia due to biofilm-producing strains has been associated with increased patient mortality.23 We found that biofilm production in breakthrough candidemia isolates was higher than in those isolates from non-breakthrough candidemia. Biofilm producing is an independent virulence factor in Candida spp.24 Furthermore, clinical studies have shown that biofilm-producers isolates increase the mortality rate.25
All isolates from the present study were susceptible to the antifungals tested, but not biofilms. For dAMB, planktonic cells obtained a maximum MIC of 1mg/L, whereas under sessile conditions, 16mg/L was required to eradicate the biofilm in both groups. Other studies have demonstrated a high prevalence of antifungal resistance in biofilms formed by C. albicans.26 This resistance can be attributed to several mechanisms, such as ineffective penetration of the drug through the extracellular matrix of the biofilm, decreased growth rate of C. albicans, cell persistence, and expression of resistance genes induced by the community.
Azoles have been shown to have poor activity against Candida spp.27 Choi et al. (2007) observed a reduction in cellular metabolism and reported that biofilms were resistant to fluconazole at concentrations>1024mg/L.28 Melo et al., in accordance with the present study, reported that the isolates were susceptible to fluconazole in planktonic form, but resistant in their sessile form.27 Time-dependent resistance was associated with the expression of genes related to efflux pumps after drug induction.
Of the three antifungal classes currently used to treat candidemia, only polyenes and echinocandins have shown consistent in vitro activity against biofilms of C. albicans.29
We observed unexpected results regarding the use of anidulafungin. Other studies have reported that echinocandins have a good effect on C. albicans biofilms in vitro and in vivo and significantly reduce the number of cells at higher doses than those found for planktonic cells.30,31 Simultaneously, Rosato et al. (2013) described that anidulafungin has a paradoxical growth effect (PGE) on C. albicans.32 These studies reported reduced microbial growth at low concentrations of the antifungal agent and a resurgence of growth at concentrations above the MIC. To date, the clinical significance of the PGE remains unclear. One explanation for PGE includes involvement of the calcineurin pathway and upregulation of the protein kinase-C cell wall integrity pathway.33 Even though PGE has been described with fluconazole, this phenomenon is more common with echinocandins.34
In comparison, amphotericin B, with or without the lipid complex, showed greater antifungal activity against the biofilms. A 50% reduction in biomass was achieved in the breakthrough candidemia group at 0.5 and 2mg/L for dAMB and ABLC, respectively. Several studies have reported that a 50% reduction is used to determine an important antifungal effect on biofilms.29 However, biofilm eradication was only achieved using 16mg/L of dAMB. The MTT assay was used to evaluate cell viability in biofilms, and crystal violet was used to evaluate biomass. These are classical tests for analyzing biofilms. It is important to discriminate between the two methods when evaluating the anti-biofilm activity of antifungal drugs. In the present work, we propose the use of the tetrazolium salt assay (MTT assay), which is a frequent procedure for determining the number of eukaryotic cells in studies where the viability of cells in culture is essential. Mitochondrial enzymes cleave the yellow tetrazolium salt (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, MTT) to formazan, and the resulting product forms a purple solution when dissolved in the appropriate reagents.35 The MTT assay has been successfully applied to evaluate the size of microbial populations growing in biofilms. Formazan formation by biofilms is a manifestation of respiratory enzyme activity in the cell walls of bacteria and/or dehydrogenase activity in filamentous fungi and yeast.36
dAMB was once considered a gold standard among antifungals due to its broader antifungal spectrum. However, lipid formulations have been developed in an attempt to reduce its toxicity.6 Kuhn et al. (2002) determined that the exposure of planktonic cells to subinhibitory concentrations of ABLC inhibited subsequent biofilm formation.29 In addition, the authors reported that despite the large size of the ABLC molecule, it penetrates the extracellular matrix to reach the fungal cells within the biofilm, although its dispersion in phospholipids can facilitate passage through the polysaccharide.
Although dAMB eradicated the biofilm at 16mg/L, the mean peak plasma concentrations ranged from 0.5 to 2mg/L in adults receiving repeated doses of approximately 0.5mg/kg/day. In osteomyelitis, it may be exposed to a higher concentration of amphotericin B, thereby presenting a greater antifungal effect on the biofilm.37 It has been shown that dAMB presents high activity against C. albicans biofilm in an in vitro model.38 Moreover, the use of dAMB in silicon catheters demonstrated complete growth and biofilm formation inhibition.39 Other studies have pointed out that cases of catheter-related C. albicans infections could be treated with dAMB at 2–2.5mg/L, allowing to save the catheter and a patient treated with a concentration of 5mg/L could be cured.40–42
The techniques applied for evaluation of biofilms may pose challenges, as subtle changes such as temperature, rotation, pipetting, and washing processes can interfere with the amount of biofilm produced and analyzed.43 Furthermore, in vitro and in vivo methods can produce different results due to a less controlled environment and interference with host immunity.44 Nevertheless, in other study, the formation of the in vitro biofilm of C. albicans correlated well with the biofilm models in vivo, obtaining similar time in the growth phases as well as the architectural structure of the biofilms recovered from patients with infection.45
The major limitation of this study was the number of isolates included. However, the anti-biofilm activity was evaluated by three methods with three antifungal classes. A higher concentration of echinocandin and fluconazole could be tested, however, the values would not be useful in clinical conditions, unless if used as lock-therapy.
In conclusion, isolates from breakthrough candidemia produced more biofilm than controls from non-breakthrough candidemia. Only the dAMB and ABLC formulations exhibited antifungal effects in sessile cells. For a broader understanding of the hypothesis that breakthrough candidemia isolates could differ from non-breakthrough candidemia isolates in terms of their ability to produce biofilms and their susceptibility to antifungals, a greater number of isolates should be studied.
Authors’ contributionConceptualization: L. Kraft, F.F. Tuon.
Methodology: L. Kraft, V.S.T. Ribeiro, G.A. Gonçalves, P.H. Suss.
Formal analysis: L. Kraft, F.F. Tuon.
Writing: Original draft preparation – L. Kraft, F.F. Tuon.
Writing: Review and editing – L. Kraft, V.S.T. Ribeiro, G.A. Gonçalves, P.H. Suss.
Supervision: F.F. Tuon.
Ethical statementThis study was approved by the ethics committee (CAAE number 40592915.2.000.0096 of Universidade Federal do Paraná).
Data availabilityAll data have been used in this paper, and raw data are available upon request.
FundingNone.
Conflicts of interestF.F. Tuon is a CNPq researcher. The other authors declare no conflicts of interest.
None.