The most widely used marker for the diagnosis of invasive aspergillosis (IA) is the detection of galactomannan by ELISA. This study describes the evaluation of the results obtained by Euroimmun Aspergillus antigen ELISA (EIA-GM-E) in serum samples and bronchoalveolar lavage fluid (BAL) from patients at risk of IA, and compares these results with those obtained by Bio-Rad Galactomannan EIA (EIA-GM-BR).
MethodsAnonymous retrospective case–control comparative study in 64 serum samples and 28 BAL from 51 patients.
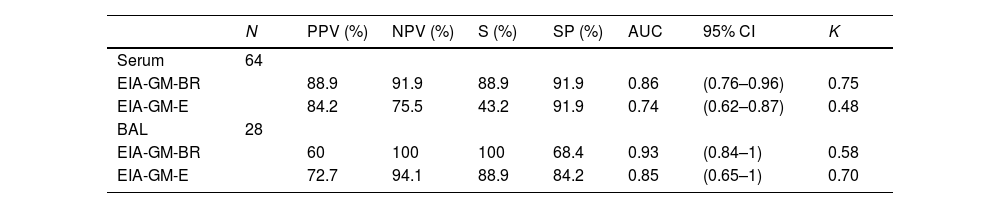
ResultsOverall agreement of the results of the two assays was observed in 72 of 92 samples (78.3%). The sensitivity of EIA-GM-BR and EIA-GM-E in serum samples was 88.9% and 43.2%, respectively, and 100% and 88.9% for BAL. The specificity of EIA-GM-BR and EIA-GM-E in serum samples was 91.9% for both assays, and 68.4% and 84.2% in BAL. There were no statistically significant differences in the results of both assays.
ConclusionsBoth methods show good results for the discrimination of patients with IA when BAL is tested, or serum in case of EIA-GM-BR.
El marcador más utilizado para el diagnóstico de aspergilosis invasora (AI) es la detección de galactomanano mediante la técnica de ELISA. Este estudio describe la evaluación de los resultados obtenidos por Euroimmun Aspergillus antigen ELISA (EIA-GM-E) en muestras de suero y lavado broncoalveolar (LBA) de pacientes con factores de riesgo de AI, y compara sus resultados con los obtenidos por Bio-Rad Galactomannan EIA (EIA-GM-BR).
MétodosEstudio comparativo caso-control retrospectivo anónimo en 64 muestras de suero y 28 de LBA de 51 pacientes.
ResultadosSe observó una concordancia global de resultados de los dos ensayos en 72 de las 92 muestras (78,3%). Los valores de sensibilidad de EIA-GM-BR y EIA-GM-E en suero fueron 88,9% y 43,2%, respectivamente, y para LBA 100% y 88,9%. La especificidad en suero de EIA-GM-BR y EIA-GM-E fue del 91,9% en ambos ensayos y para LBA 68,4% y 84,2%. No se observaron diferencias estadísticamente significativas en los resultados de ambos ensayos.
ConclusionesAmbos métodos demuestran buenos resultados para la discriminación de pacientes con AI cuando se emplea como muestra el LBA, o el suero en caso de EIA-GM-BR.
The detection of specific markers of Aspergillus spp. in clinical samples of patients subjected to microbiological surveillance, due to the presence of risk factors for developing invasive aspergillosis (IA), is of enormous importance due to the morbidity and mortality caused by this disease.1 Traditional risk factors have been associated with malignant hematological disease (MHD) in patients with prolonged neutropenia, however, the prevalence of this disease is increasing in non-neutropenic patients with other underlying diseases2 including the recently emerging COVID-19.3
The detection of galactomannan is included for the diagnosis of IA in the definition of invasive Aspergillus spp. disease of the European Organization for Research and Treatment of Cancer (EORTC)4 and is the most widely used marker for the diagnosis of IA by most microbiology laboratories.
Until a few years ago, only Bio-Rad Galactomannan EIA was available on the market. Other assays, as lateral flow-based techniques, have recently appeared with promising results in serum samples, but mainly in bronchoalveolar lavage (BAL).5,6
More recently, another ELISA-based assay has been commercialized, the Aspergillus antigen ELISA, by Euroimmun (Germany), whose results in serum samples, in a comparative study with the Bio-Rad assay, have been described in a recent publication reporting good concordance between the two assays.7 Both ELISA assays are based on monoclonal antibodies with high specificity for Aspergillus antigens. The rat IgM antibody EB-A2, which is applied in the Bio-Rad assay, binds to the cell wall polysaccharide galactomannan. The IgG3 antibody JF5, which is applied in the Euroimmun assay, binds to a protein epitope localized in the hyphal cell wall. The protein-carbohydrate complex detected by the JF5 antibody was named galactomannoprotein by the manufacturer of the Euroimmun ELISA. The EB-A2 antibody used in the Bio-Rad ELISA was reported to cross-react with clinically important fungi. To date, no cross-reactivity was reported for JF5-derived tests.7
Serum and BAL are the most studied and significant samples for the detection of galactomannan. The diagnosis of IA is based on the presence of signs, symptoms and radiological and microbiological findings compatible with the infection in those patients with risk factors. It is important to choose the most suitable type of sample based on patient's underlying pathology and risk factors for developing IA. In patients who do not present severe neutropenia, the probability of pulmonary filamentous fungi angioinvasion is very remote8 so, the detection of galactomannan in serum is not profitable due to its low sensitivity, also altering the predictive values of the technique in this type of patients. The BAL would therefore be the recommended sample in these cases.
This study describes the first evaluation of the results obtained by Euroimmun Aspergillus antigen ELISA in serum and BAL samples simultaneously from patients subjected to microbiological surveillance for having risk factors for IA or diagnosed with IA, and compares these results with those obtained by the Bio-Rad Galactomannan EIA.
Material and methodsA comparative anonymous retrospective case-control study was carried out for the evaluation of two diagnostic ELISA assays for IA diagnosis, the Platelia™ Aspergillus antigen (Bio-Rad, Marnes-la-Cocquette, France) (EIA-GM-BR) for the detection of galactomannan and the Aspergillus antigen ELISA (Euroimmun Medizinische Labordiagnostika AG, Lubeck, Germany) (EIA-GM-E), a new assay that detects a protein-carbohydrate complex named galactomannoprotein by the manufacturers.
Routine workflow for the diagnosis of IA. Until July 2020, our laboratory performed the prospective detection of galactomannan in fresh samples with EIA-GM-BR. As of that date, the EIA-GM-E test was introduced. Following the indications of both manufacturers, a positive threshold index (GMI)≥0.5 was used for serum. For BAL samples, GMI≥1 was applied. The clinical diagnosis of IFI was considered according to EORTC criteria.4 For patients not included in that criteria, IA diagnosis was considered when mechanical ventilation was applied and clinical, radiological and microbiological findings were present. After performing the test, an aliquot from the primary tube was kept frozen at −80°C until a second processing for the purpose of conducting the present study.
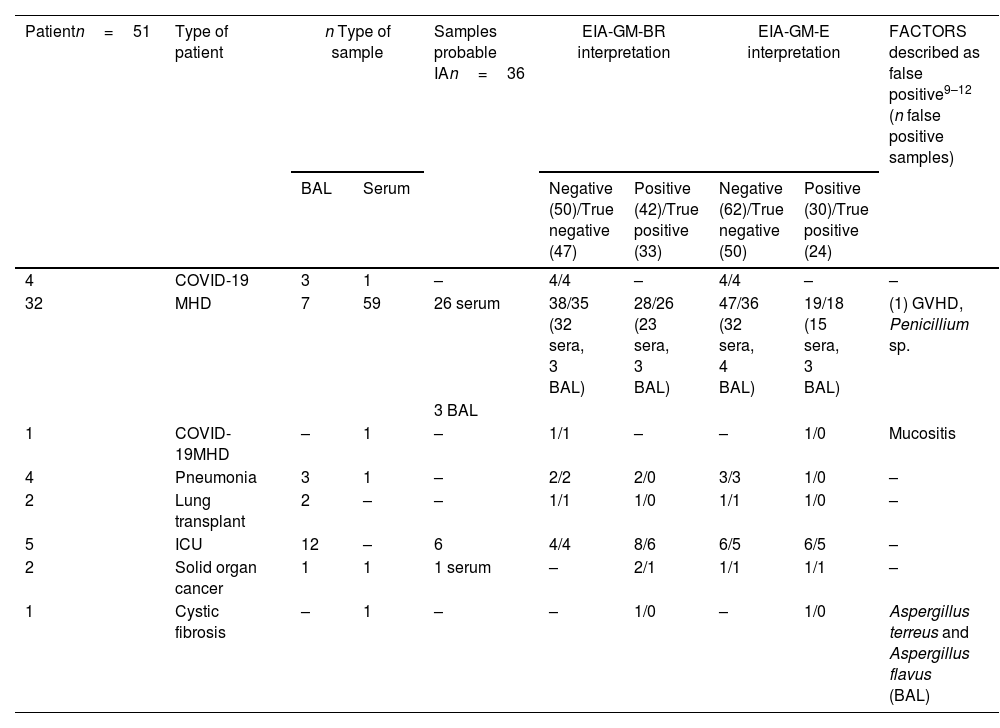
Patient and sample types. To carry out the study, a total of 92 frozen samples (64 sera and 28 BAL) received during 2019 and 2020 from 51 patients, were randomly selected. Sixty-one samples had been previously run freshly with EIA-GM-BR and 31 with EIA-GM-E as part of routine workflow (41 yielded GMI<0.5 and 51 with GMI≥0.5). Clinical data and type of sample were subsequently consulted in the records included in the laboratory's computer system, following the confidentiality protocols established by our center. Patient/sample types were divided into: 32 MHD patients (59 sera and 7 BAL), 1 MHD/COVID-19 patient (1 serum), 4 COVID-19 patients (1 serum and 3 BAL), 5 patients from the Intensive Care Unit (12 BAL), 2 solid organ cancer patients (1 serum and 1 BAL) and 7 patients with pneumonia with other risk factors for IA (2 sera and 5 BAL). Thirty-eight positive samples were from 13 patients with IFI: 34 samples from 9 probable IA MHD patients, 1 from 1 possible IA MHD patient, 1 from 1 probed candidemia MHD patient, 1 from 1 probable IA Intensive Care Unit patient and 1 from 1 probable IA solid organ cancer patient.
Performance of the EIA-GM-BR and EIA-GM-E assays. Taking into account that the 92 samples had been analyzed with one or the other assay in the routine prospective analysis, all samples were re-processed with both assays manually after thawing, on the same day and by the same laboratory technician following the instructions of both manufacturers. The same scrubber and spectrophotometer were used to ensure the same processing conditions for both tests.
Statistical study. For the statistical analysis, the SPSS program (20.0) was used. Sensitivity, specificity and positive and negative predictive values was carried out by constructing 2×2 tables. To determine the significance of the differences between the different values, the 95% confidence interval (95% CI) was calculated. The Kappa index was applied for the concordance study.
Cases were defined as both serum and BAL samples from patients diagnosed with probable IA or proven IA during the routine workflow. In our series, all BAL samples with Aspergillus spp. were from patients diagnosed of probable IA. One sample from a patient diagnosed of proven invasive fungal infection (IFI) due to Candida krusei candidemia was excluded for the purpose of this study, since there was no evidence of infection by Aspergillus spp. Another case with possible IFI was also not included since, by definition, it does not have microbiological criteria of IA. The samples from these 2 patients were included in the control group.
Both, serum and BAL samples from not diagnosed patients with IA, were considered as control cases. Both, serum and BAL positive samples from patients diagnosed with IA, were considered as true positive. Those positive samples that did not meet the above criteria were considered false positive. In these cases, it was studied whether they had other causes described in the literature that could justify these false positive results.
Negative samples from patients diagnosed with IA and those BAL in which Aspergillus spp. was isolated were considered false negative. Negative samples from patients without a diagnosis of IA or without Aspergillus spp. in BAL cultures, were considered true negative.
ResultsTables 1 and 2 show the comparative results obtained with both assays.
Comparative results obtained with the EIA-GM-BR and the EIA-GM-E assays in sera and BAL samples.
| Patientn=51 | Type of patient | n Type of sample | Samples probable IAn=36 | EIA-GM-BR interpretation | EIA-GM-E interpretation | FACTORS described as false positive9–12 (n false positive samples) | |||
|---|---|---|---|---|---|---|---|---|---|
| BAL | Serum | Negative (50)/True negative (47) | Positive (42)/True positive (33) | Negative (62)/True negative (50) | Positive (30)/True positive (24) | ||||
| 4 | COVID-19 | 3 | 1 | – | 4/4 | – | 4/4 | – | – |
| 32 | MHD | 7 | 59 | 26 serum | 38/35 (32 sera, 3 BAL) | 28/26 (23 sera, 3 BAL) | 47/36 (32 sera, 4 BAL) | 19/18 (15 sera, 3 BAL) | (1) GVHD, Penicillium sp. |
| 3 BAL | |||||||||
| 1 | COVID-19MHD | – | 1 | – | 1/1 | – | – | 1/0 | Mucositis |
| 4 | Pneumonia | 3 | 1 | – | 2/2 | 2/0 | 3/3 | 1/0 | – |
| 2 | Lung transplant | 2 | – | – | 1/1 | 1/0 | 1/1 | 1/0 | – |
| 5 | ICU | 12 | – | 6 | 4/4 | 8/6 | 6/5 | 6/5 | – |
| 2 | Solid organ cancer | 1 | 1 | 1 serum | – | 2/1 | 1/1 | 1/1 | – |
| 1 | Cystic fibrosis | – | 1 | – | – | 1/0 | – | 1/0 | Aspergillus terreus and Aspergillus flavus (BAL) |
Samples probable IA: samples from patients with a diagnosis of probable invasive aspergillosis; COVID-19: infectious disease caused by SARS-CoV-2; ICU: intensive care unit; MHD: malignant hematological disease; GVHD: graft versus host disease; BAL: bronchoalveolar lavage; n: number.
Results of the statistical study.
| N | PPV (%) | NPV (%) | S (%) | SP (%) | AUC | 95% CI | K | |
|---|---|---|---|---|---|---|---|---|
| Serum | 64 | |||||||
| EIA-GM-BR | 88.9 | 91.9 | 88.9 | 91.9 | 0.86 | (0.76–0.96) | 0.75 | |
| EIA-GM-E | 84.2 | 75.5 | 43.2 | 91.9 | 0.74 | (0.62–0.87) | 0.48 | |
| BAL | 28 | |||||||
| EIA-GM-BR | 60 | 100 | 100 | 68.4 | 0.93 | (0.84–1) | 0.58 | |
| EIA-GM-E | 72.7 | 94.1 | 88.9 | 84.2 | 0.85 | (0.65–1) | 0.70 |
| Data regardless of sample type | ||||||||
|---|---|---|---|---|---|---|---|---|
| N | PPV (%) | NPV (%) | S (%) | SP (%) | AUC | 95% CI | K | |
| Samples | 92 | |||||||
| EIA-GM-BR | 78.6 | 94 | 91.7 | 83.9 | 0.86 | (0.77–0.95) | 0.69 | |
| EIA-GM-E | 80 | 80.6 | 66.6 | 89.3 | 0.79 | (0.69–0.90) | 0.54 | |
EIA-GM-BR: Biorad assay; EIA-GM-E: Euroimmun assay; BAL: bronchoalveolar lavage; N: number of cases; PPV: positive predictive value; NPV: negative predictive value; S: sensitivity; SP: specificity; AUC: area under the curve; CI: confidence interval; K: Cohen kappa Index.
Positive samples. Forty-two of the 92 samples tested (45.7%) were positive (27 sera and 15 BAL) of which 33 (78.6%) were true positive (24 sera and 9 BAL) and 9 (21.4%) false positive (3 sera and 6 BAL).
Negative samples. Fifty of the 92 samples tested (54.3%) were negative (37 sera and 13 BAL) of which 47 (94%) were true negative (34 sera and 13 BAL) and 3 (6%) were false negative (3 serum samples).
EIA-GM-E assayPositive samples. Thirty of the 92 samples (32.6%) were positive (19 sera and 11 BAL) of which 24 (80%) were true positive (16 sera and 8 BAL) and 6 (20%) were false positive (3 sera and 3 BAL).
Negative samples. Sixty-two of the 92 samples (67.4%) were negative (45 sera and 17 BAL) of which 50 (80.6%) were true negative (34 sera and 16 BAL) and 12 (19.3%) were false negative (11 sera and 1 BAL).
Concordance resultsOverall agreement from the two trials was observed in 72 of the 92 samples (78.3%); by sample type, in 48 of 64 sera (75%) and in 24 of 28 BAL (85%). Regarding the type of test result, there was agreement in 26 positive samples (distributed in 11 BAL and 15 sera) and 46 negative samples (13 BAL and 33 sera).
Statistical analysisSensitivity, specificity, predictive values, Kappa index, area under the curve (AUC) and 95% confidence interval (CI) are shown in Table 2.
Reproducibility of results between fresh and frozen conditionsEIA-GM-BR test. Concordant results between fresh and thawed samples were observed in 40 of the 41 negative samples (97.6%) and in 19 of the 20 positive samples (95%). None of the patients had lost the IA diagnosis after the study performance.
EIA-GM-E test. Concordant results between fresh and thawed samples were observed in 17 of the 31 positive samples (54.8%). Five samples previously positive when tested with fresh sera showed negative in the thawed samples, and belonged to 1 probable IA MHD patient.
DiscussionAt present, several tests for the detection of IA markers are available on the market. In our study, we have analyzed the results of the ELISA format from two different commercial manufacturers and whose results do not seem to show statistically significant differences (Fisher's test p=0.32 and p=0.13 in serum and BAL samples respectively).
Regarding false positive results, the data obtained agree with those previously published in the scientific literature,9–12 although more false positive cases in absence of any of the described sources of false positive results were observed in BAL samples than in serum. One explanation could be the presence of other microorganisms in the sample, since BAL may contain oropharyngeal microbiota, the presence of other microorganisms that cause true infections or antibiotic treatments. Among the 6 BAL and 3 false positive sera found with EIA-GM-BR, 1 BAL had a positive culture with Penicillium spp. and 1 serum was from a patient with cystic fibrosis who had been a carrier of Aspergillus terreus and Aspergillus flavus in samples from the lower respiratory tract for years. Among the 3 false positive sera found with EIA-GM-E, 1 serum was from the patient with cystic fibrosis already mentioned, and another serum from a hematological patient with grade 3 mucositis. No cross-reactivity with Penicillium spp. was detected in EIA-GM-E. This data is in accordance with previous reports.7
Another data of interest, are the concordance results of the two assays. In our study, 25% of the sera and 15% of the BAL showed discrepant results, data to take into account when making the diagnosis of probable IA. It would therefore be desirable to carry out not only comparative studies between different assays but also reproducibility studies with EIA-GM-E with a larger number of samples as previously reported with EIA-GM-BR.13 Our reproducibility results are scarce because of the low number of samples and did not show significant clinical results. For both assays, the repetition of the test with another serum sample should continue to be recommended in case of obtaining a positive result as well as the need to indicate and interpret the results taking into account the type of patient, type of sample, suitable moment within the evolution of the disease and the results of microbiological diagnostic tests and radiological findings.
In our study, both assays showed good results for the discrimination of patients with IA when BAL samples were tested (sensitivity>80%) or in serum samples in the case of EIA-GM-BR, while for the discrimination of non-IA patients, serum samples showed good results with both assays. Positive predictive value in serum and negative predictive value in BAL showed good results to confirm and dismiss IA diagnosis, respectively. Taking into account the AUC analysis, both methods demonstrated good diagnostic performance (AUC>80%). Regarding the kappa index, a good agreement was observed with the results obtained in serum with EIA-GM-BR (K=0.75) and in BAL with EIA-GM-E (K=0.70). Nonetheless, the low values obtained by EIA-GM-BR in BAL (K<60) as well as EIA-GM-E in serum, indicate that their use must be discouraged.
To date, we have not found any study in the literature evaluating the performance of both ELISA assays in serum and BAL samples simultaneously. The data obtained in our study indicate that both methods are similar for the diagnosis of IA if the appropriate samples are used for each method, being BAL the best sample for EIA-GM-E, since it shows better results and concordance index, and serum if EIA-GM-BR is used. Our study has the limitation of being retrospective and with a low number of samples analyzed in a single center, nevertheless, it has the strength of analyzing samples from a homogeneous patient population where cases and controls come from patients with IA risk factors, the population for which galactomannan biomarker detection is indicated. This would explain the relative high sensitivity and specificity data obtained in comparison to the high variability found in many previously published studies, and enables reliability in the interpretation of the predictive values of the technique.
Nonetheless, prospective and multicenter studies with a larger number of samples (serum and BAL) would be needed to better assess the performance, reproducibility and possible causes of false positive results of the EIA-GM-E assay.
FundingThis research has not received specific aid from public sector agencies, commercial sector or non-profit entities.
Conflict of interestThe authors declare no conflict of interest.