China is the second high tuberculosis (TB) burden country in the world. This article was to determinate the molecular characteristic of drug resistance Mycobacterium tuberculosis (DRTB) strains from extra pulmonary tuberculosis (EPTB).
MethodsThe medical records of patients with EPTB were reviewed and collected from 2006 to 2016. The drug sensitivity of all samples was studied. All multiple drug resistance (MDR) and extensive drug resistance (XDR) strains were included. The detection of the deletion of region of difference 105 (RD105) and mycobacterial interspersed repetitive-unit variable-number tandem-repeat (MIRU-VNTR) were used to discriminate the molecular type of EPTB strains.
Results162 DRTB isolates were from patients with EPTB including 104 male and 58 female. Beijing genotype had a significant correlation with the patterns of DR (P<0.05), re-treatment patients (P<0.05) and gender (P<0.05). The history of treatment had a statistically significant correlation with patterns of DR (P<0.05) and gender (P<0.05). Patterns of DR had no correlation with gender (P>0.05). Of 162 strains Beijing family strains represented 91.4%. The cluster rate was 17.9% and clustering ratio was 11.1%. Beijing family genotype is predominant in the patients with EPTB. The cluster rate and clustering ratio was low.
ConclusionsBeijing family genotype is predominant and highly epidemic in the patients with drug resistance extra pulmonary tuberculosis (DR-EPTB). The cluster rate and clustering ratio was low. Genotype of re-treatment male patient with DR-EPTB is more likely Beijing family genotype.
China es el país con la segunda mayor carga de tuberculosis (TB) del mundo. Este artículo pretende determinar las características moleculares de cepas de Mycobacterium tuberculosis farmacorresistentes (MBFR) en la tuberculosis extrapulmonar (TBEP).
MétodosSe revisaron y recopilaron los registros médicos de pacientes con TBEP entre 2006 y 2016. Se estudió la sensibilidad farmacológica de todas las muestras. Se incluyeron todas las cepas con farmacorresistencia múltiple (FRM) y farmacorresistencia extensiva (FRE). Para discriminar el tipo molecular de las cepas de TBEP se utilizó la detección de la eliminación de la región de diferencia 105 (RD105) y las repeticiones en tándem con número variable de unidades repetitivas interespaciadas de micobacterias (MIRU-VNTR).
ResultadosCiento sesenta y dos aislados de pacientes con TBEP, de los cuales 104 eran varones y 58 mujeres. El genotipo de Beijing tuvo una correlación significativa con los patrones de FR (p<0,05), pacientes con retratamiento (p<0,05) y sexo (p<0,05). Los antecedentes de tratamiento tuvieron una correlación estadísticamente significativa con los patrones de FR (p<0,05) y sexo (p<0,05). Los patrones de FR no tuvieron correlación con el sexo (p<0,05). Del total de las 162 cepas, las cepas de Beijing representaron el 91,4%. La tasa de agrupamiento fue del 17,9% y la proporción de agrupamiento del 11,1%. El genotipo de Beijing es predominante en los pacientes con TBEP. La tasa de agrupamiento y la proporción de agrupamiento fueron bajas.
ConclusionesEl genotipo de Beijing es predominante y altamente epidémico en los pacientes con tuberculosis extrapulmonar farmacorresistente (TBEP-FR). La tasa de agrupamiento y la proporción de agrupamiento fueron bajas. El genotipo de paciente varón con TBEP-FR retratado es más probable en el genotipo de la familia de Beijing.
Mycobacterium tuberculosis (MTB) is still an international problem despite advances in the methods of diagnosis and treatment. China is the second high burden country of tuberculosis in the world. The latest reports were that the best estimate is that 10.0 million people (range, 9.0–11.1 million) developed TB disease in 2018. Globally, there were 1.2 million (range, 1.1–1.3 million) TB deaths among HIV-negative people in 2018 (a 27% reduction from 1.7 million in 2000) and an additional 251000 deaths (range, 223000–281000) among HIV-positive people (a 60% reduction from 620000 in 2000).1
The DRTB is of major interest and concern at global, regional and country levels. In 2018, there were approximately half a million (range, 417000–556000) new cases of rifampicin-resistant TB (RRTB), of which 78% had multidrug-resistant TB (MDR-TB). The three countries with the largest share of the global burden were India (27%), China (14%) and the Russian Federation (9%). Globally, 3.4% of new TB cases and 18% of previously treated cases had MDR/RRTB. In China the MDR incidence is 4.6/100000.1
EPTB is a common disease of TB and sites of disease include lymph node, genitourinary, central nerve system, gastrointestinal, pleura, bone and joints, skin and pericardial. According to the WHO Global Tuberculosis Report 2015, 32000 cases of EPTB were diagnosed in nearly 530000 new cases in China in 2014.2 EPTB is a growing public health concern in China, but data on drug resistance are limited, especially in Chongqing of southwest China. Although there were some researches about epidemiology of spinal tuberculosis,3 molecular characteristic of MTB4 and drug resistance MTB5 in Chongqing, there was little report about characteristics of drug resistance EPTB (DR-EPTB). In order to determine the characteristics of DR-EPTB in Chongqing, we reviewed and collected the medical records of consecutive patients with EPTB in Chongqing Public Health Medical Center that is major in treatment for infectious diseases from 2006 to 2016. Drug susceptibility of EPTB isolates was tested and they were genotyped to determine the genotypes of MTB isolates from EPTB in using RD105 and MIRU-VNTR analysis.
Materials and methodsEthical approvalThis research has been approved by the IRB of Chongqing Public Health Medical Center and Tianjin First Center Hospital.
PatientsThe medical records of consecutive patients admitted for EPTB to Chongqing Public Health Medical Center from 2006 to 2016 were collected. Diagnosis of EPTB was in accordance with WHO definition6 that is one culture-positive specimen, histological or strong clinical evidence consistent with active extra-pulmonary disease, followed by a decision by a clinician to treat with a full course of anti-tuberculous chemotherapy. In appropriate clinical contexts diagnoses made on molecular positive results was included.
Mycobacterial strainsSpecimens were collected during surgical procedures and stored at low temperature (4–8°C) and decontaminated using the N-acetyl-l-cysteine (NALC)–NaOH method. Various MTB culture and identification systems were used during the study period. The first was the BACTEC MGIT 960 system (Becton Dickinson, Sparks, Maryland, USA). Clinical specimens were processed, and the centrifuged sediment was inoculated onto Löwenstein–Jensen (LJ) medium (BBL; Becton Dickinson, Sparks, MD, USA) and Middlebrook 7H9 broth (BBL; Becton Dickinson). The cultures were incubated at 35°C in 5% carbon dioxide for up to 8 weeks. Identification of M. tuberculosis was based on colony morphology and biochemistry reactions (nitrate reduction and niacin test). Bacterial cells were isolated from LJ medium.
Drug susceptibility testingDrug susceptibility testing was performed using the proportion method in the Roche medium recommended by the World Health Organization,7 and the concentrations of drugs in media were as follows: isoniazid 0.2μg/ml, rifampicin 40μg/ml, ethambutol 2μg/ml, streptomycin 4μg/ml, rifapentine 40μg/ml, para-aminosalicylic acid 1.0μg/ml, amikacin 30μg/ml, capreomycin 40μg/ml, kanamycin 30μg/ml, levofloxacin 2μg/ml, protionamide 40μg/ml and dipasic 0.1μg/ml. A strain was declared resistant to a drug when the growth rate was >1% compared with the control. The non drug resistance strains were included.
Genomic DNA extractionMycobacterial genomic DNA was extracted from mycobacterial colonies growing on LJ medium by resuspending one loop of mycobacterial colonies in 200μl TE buffer (10mM Tris–HCl, 1mM EDTA) and was incubated at 85°C for 30min to obtain genomic DNA. After centrifugation of the suspension, the supernatant fluid containing DNA was removed and stored at −20°C until further use. Laboratory strain MTB H37Rv was used as a control for all microbiological and genetic procedures.
GenotypingThe identification of genomic deletions in RD105 was performed by PCR to distinguish Beijing type from non-Beijing type. Briefly, each PCR mixture was prepared in a volume of 20μl containing 19μl RD105 PCR Mix and 1μl DNA template. The amplification cycle was 10min at 95°C followed by 25 cycles of 30sec at 94°C, 30sec at 68°C, and 3min at 72°C, with a final step for 7min at 72°C. The detection was performed using the kits (Beijing ComWin Biotech Co., Ltd., China).
Using 12 MIRU-VNTR genetic loci identify genotyping of M. tuberculosis: four original MIRU-VNTR loci: MIRU-10, MIRU-26, MIRU-31, MIRU-40; one locus of exact tandem repeats (ETRs): ETR- F; two Mtub loci: Mtub-04, Mtub-21; five Queen's University of Belfast (QUBs) loci: QUB-11b, -18, -26, -4156 and 1895. QUB-11b, QUB-18, QUB-26, QUB-4156, MIRU26, MIRU31, MIRU10, Mtub21 and Mtub04 locus of MTB isolates was amplified separately by PCR using specific primers. Briefly, 1μl of DNA was added to 19μl of reagent mix. The amplification parameters consisted of 10min at 95°C, followed by 30 cycles of 30s at 94°C, 30s at 58 °C, and 90s at 72°C, with a final extension at 72°C for 7min. QUB-1895, MIRU40 and ETR-F locus of MTB isolates was amplified separately by PCR using specific primers. Briefly, 1μl of DNA was added to 19μl of reagent mix. The amplification parameters consisted of 10min at 95°C, followed by 30 cycles of 30s at 94°C, 30s at 64 °C, and 90s at 72°C, with a final extension at 72°C for 7min. The PCR products were electrophoresed on a 1% agarose gel. The H37Rv strain was assayed in the same manner as a control. The detection was performed using the kits (Beijing ComWin Biotech Co., Ltd., China).
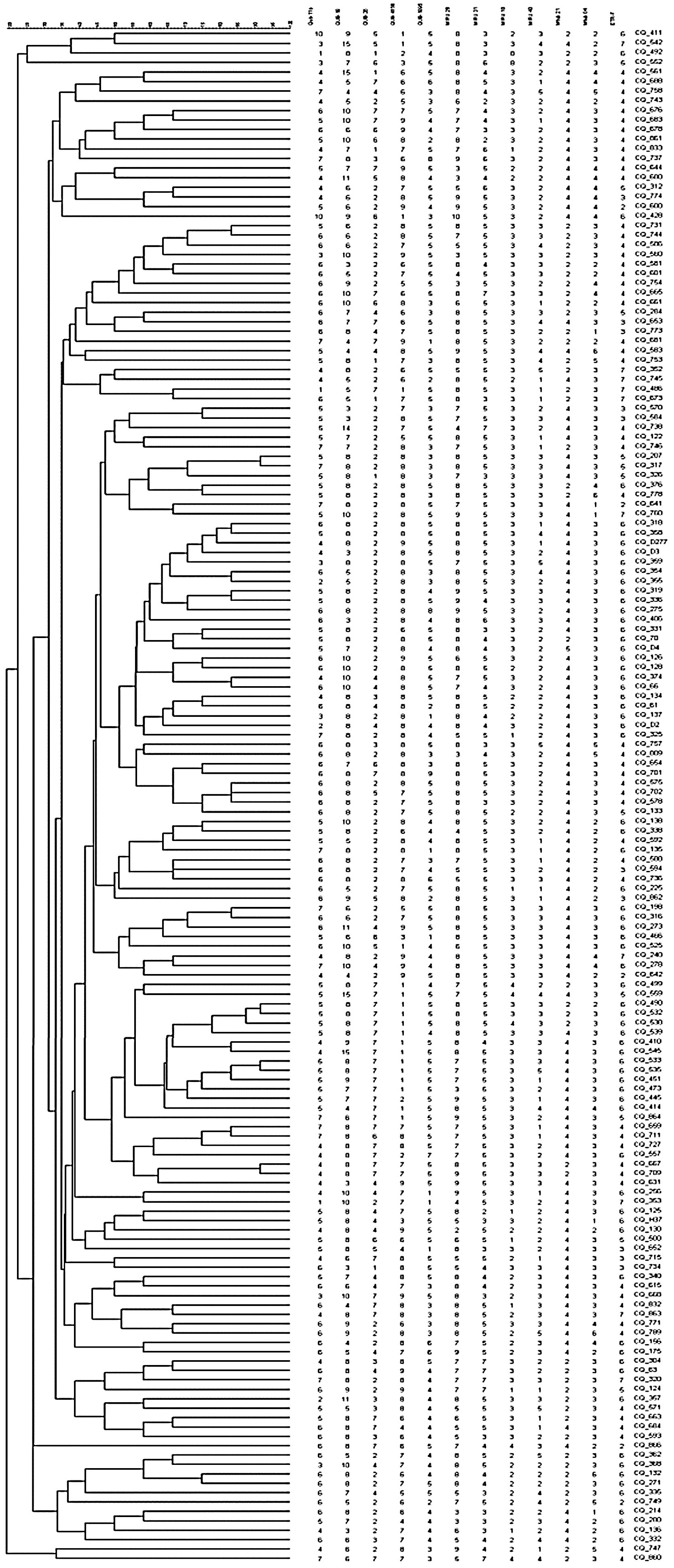
Statistical analysisAll data were presented as mean±standard deviation (SD) or frequency. Statistical analysis for possible significant association between the different characteristics and different genotypes was performed using Chi-square test. All tests were set as two sides and a P value of<0.05 was considered statistical significant. BioNumerics (version 5.0, Applied Maths, Sint-Martens-Latem, Belgium) was used to construct the Minimal Spanning Trees (MSTs) based on VNTR data. A dendrogram was constructed based on the unweighted-pair group method using average linkages and the software package MEGA (version 6.0).
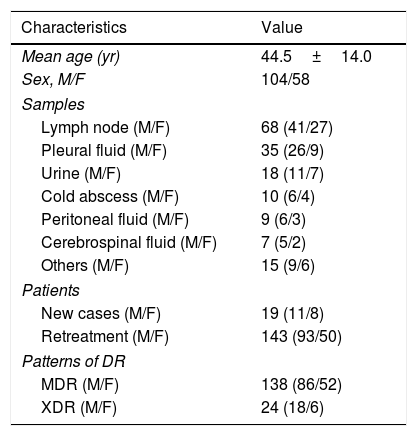
ResultsDemographyThere were 162 patients with EPTB enrolled in Chongqing Public Health Medical Center from 2006 to 2016. The demographic characteristics of 162 patients with EPTB display in Table 1. Male patients account for 64.2%. Of 162 samples lymph node account for 42.0% followed by the pleural fluid 21.6% and urine 11.1%. Re-treatment patients account for 88.3% in all patients. The proportions of MDR and XDR were 85.2% and 14.8% respectively.
Demographic characteristics of 162 patients with DR-EPTB.
| Characteristics | Value |
|---|---|
| Mean age (yr) | 44.5±14.0 |
| Sex, M/F | 104/58 |
| Samples | |
| Lymph node (M/F) | 68 (41/27) |
| Pleural fluid (M/F) | 35 (26/9) |
| Urine (M/F) | 18 (11/7) |
| Cold abscess (M/F) | 10 (6/4) |
| Peritoneal fluid (M/F) | 9 (6/3) |
| Cerebrospinal fluid (M/F) | 7 (5/2) |
| Others (M/F) | 15 (9/6) |
| Patients | |
| New cases (M/F) | 19 (11/8) |
| Retreatment (M/F) | 143 (93/50) |
| Patterns of DR | |
| MDR (M/F) | 138 (86/52) |
| XDR (M/F) | 24 (18/6) |
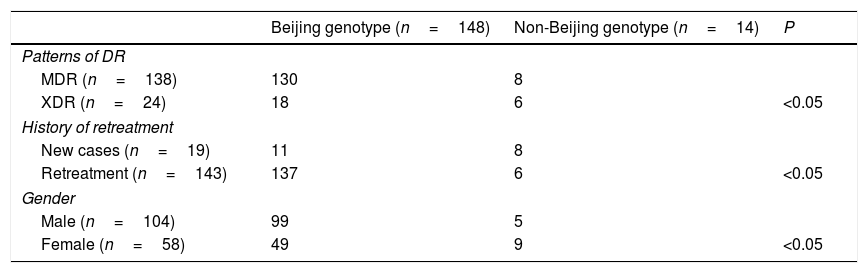
162 drug resistances M. tuberculosis isolates were analyzed by RD105 in this study. 148 (91.4%) belonged to the Beijing genotype, while 14 (8.6%) were from non-Beijing families.
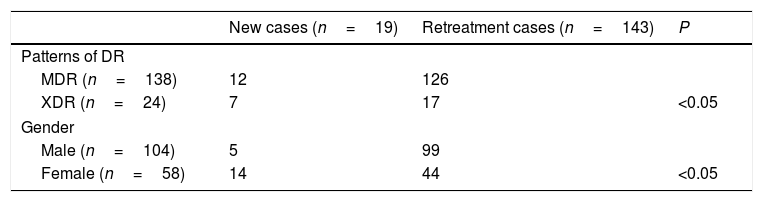
Correlations between genotypes and patterns of DR and patients, genderPatterns of DR, gender and treatment history were analyzed between the Beijing and non-Beijing family (Table 2). Beijing genotype had a significant correlation with the re-treatment patients (P<0.05) and gender (P<0.05). Also patterns of DR had a significant correlation with re-treatment (P<0.05) and gender (P<0.05) (Table 3). But patterns of DR had no correlation with gender (P>0.05) (Table 4).
Correlations between genotypes and patterns of DR, history of treatment and gender (n=162).
| Beijing genotype (n=148) | Non-Beijing genotype (n=14) | P | |
|---|---|---|---|
| Patterns of DR | |||
| MDR (n=138) | 130 | 8 | |
| XDR (n=24) | 18 | 6 | <0.05 |
| History of retreatment | |||
| New cases (n=19) | 11 | 8 | |
| Retreatment (n=143) | 137 | 6 | <0.05 |
| Gender | |||
| Male (n=104) | 99 | 5 | |
| Female (n=58) | 49 | 9 | <0.05 |
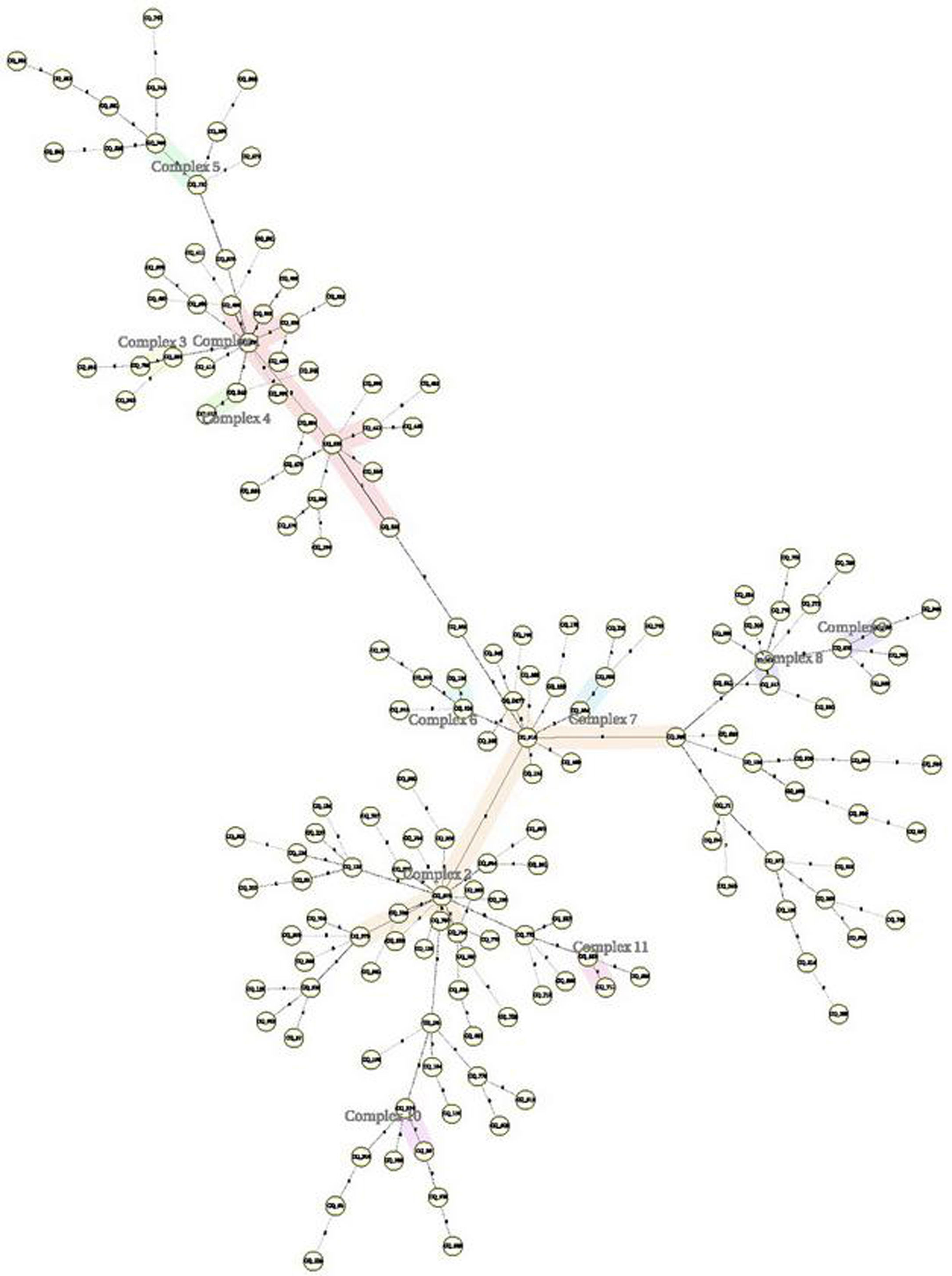
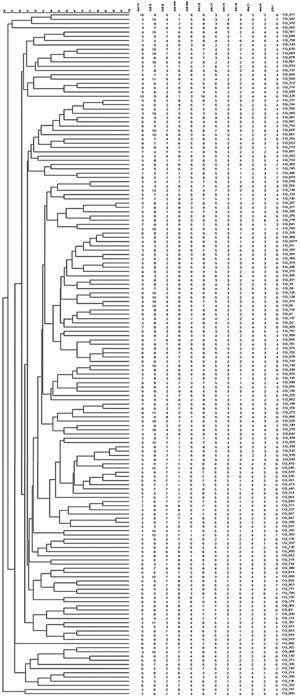
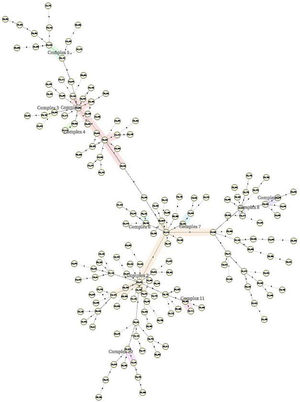
The 162 M. tuberculosis strains were genotyped by 12 MIRU-VNTR loci combinations. The MIRU-VNTR genotyping results showed that the 134 isolates had unique patterns. A dendrogram was constructed based on the genotypes of 162 isolates using 12 loci (Fig. 1). There were 11 obvious clusters (Fig. 2). The cluster rate was 17.9% and clustering ratio was 11.1%.
DiscussionThe Beijing genotype of Mycobacterium tuberculosis was firstly found by Soolingen in 1995.8 There were some reports about Beijing genotype was the main genotype among strains isolated from patients with pulmonary tuberculosis.9–11 Especially in China, many researches about molecular epidemiology of M. tuberculosis in different provinces were reported.12–20 So the molecular characteristic of pulmonary tuberculosis was Beijing family genotype in China, but not on extra-pulmonary TB, especially in Chongqing of southwest China that is a high incidence area on MTB. In this study, we found Beijing genotype was also main strains among the patients with DR-EPTB. There were some different characteristics of DR-EPTB in different countries and areas of all over the world. According to report in Saudi Arabia, lymph node, bone and joints and gastrointestinal system were three common sites affected by MTB and female gender is a predisposing risk factor for the development of EPTB as opposed to PTB.21 While in Australia, lymph node, gastrointestinal system and central nervous system were three common sites.22 In our study lymph node, pleura and urinary system were three common sites of DR-EPTB and male patients were more than female. In addition there was no correlation between the patterns of DR and gender in this study.
Genotype of pulmonary tuberculosis (PTB) always constituted transmission clusters and showed the population diversity. There were a lot of researches about genotype characteristics of PTB in China.4,13–20,23 But there were little reports about molecular characteristics of EPTB in China, especially in Chongqing. In all above reports Beijing family genotype was the predominant in the patients with MTB or DR-PTB. According to our study the 148 isolates from DR-EPTB were Beijing family genotype. The 162 strains showed 11 clusters including 32 strains and 130 isolates that were single genotype. The cluster rate was 17.9% and clustering ratio was 11.1%. The reason why Beijing family genotype was predominant in the patients with DR-EPTB is treatment failure in the patients with Beijing family genotype24 and EPTB occur secondary to the primary infection site and spread occurs either via the arterial or the venous route.
In addition, there are many ways to detect genetic types of MTB including spoligotyping, detection of the deletion of region of difference 105 (RD105), mycobacterial interspersed repetitive-unit variable-number tandem-repeat (MIRU-VNTR) and restriction fragment length poly-morphism (RFLP) analysis. Especially IS6110-RFLP is considered a “gold standard” for DNA fingerprinting of MTB that was not widely used due to time-consuming and technically demanding, as well as requiring large quantities of DNA. During the studies on detecting the genetic types of MTB those different ways were used.17–19 According to comparison results of different ways, spoligotyping or RD105 was a reliable standard for identifying strains as belonging to the Beijing family because it is simple, highly reproducible and applicable to a digital format and MIRU was the most reliable method for the genetic differentiation of MTB isolates because the discriminatory power of this method may be comparable to that of IS6110 typing.19,20,25
ConclusionIn conclusion Beijing family genotype is predominant and highly epidemic in the patients with drug resistance extra pulmonary tuberculosis (DR-EPTB). The cluster rate and clustering ratio was low. Genotype of re-treatment male patient with DR-EPTB is more likely Beijing family genotype.
Authors’ contributionsConceived and designed the study: TS and TXL. Collected the data: FC, YS and WXJ. Analyzed the data: TXL, TS and YKC. Wrote the paper: TS. Interpreted the results: TXL and TS. Funding acquire: TXL. All authors have read and approved the final manuscript.
FundingThe Medical Scientific Research Major Project No. 2012-1-085, Chongqing Municipal Health Bureau of China.
Conflict of interestsThe authors declare that they have no competing interests.