Arcobacter butzleri is a gram-negative rod, with microaerobic growth at an optimal temperature of 37°C. It was reported to be the fourth most common Campylobacter-like organism isolated from patients with diarrhoea.
ObjectiveCharacterise a potential outbreak of A. butzleri detected in a short period of time in the University Hospital Marqués de Valdecilla.
MethodsEight strains of A. butzleri were detected in our hospital in only two months. Isolates were identified by MALDI-TOF MS system and 16S rDNA sequencing. Enterobacterial repetitive intergenic consensus-PCR (ERIC-PCR) and Pulsed Field Gel Electrophoresis (PFGE) were carried out to assess clonal relationship. Gradient strips (Etest) were used to determine susceptibility by agar diffusion.
ResultsERIC-PCR and PFGE confirmed the lack of clonal relationship between strains. Erythromycin or ciprofloxacin might be appropriate for antibiotic treatment of infections.
ConclusionsA. butzleri is an emerging pathogen with increasing incidence, and may be underestimated.
Arcobacter butzleri es un bacilo gramnegativo, con crecimiento microaerófilo a una temperatura óptima de 37°C. Ha sido descrito como el cuarto organismo asociado a Campylobacter más frecuentemente aislado de pacientes con diarrea.
ObjetivoCaracterizar un potencial brote de A. butzleri detectado en el Hospital Universitario Marqués de Valdecilla.
MétodosSe detectaron 8 cepas de A. butzleri en nuestro hospital en solo 2 meses. Los aislamientos fueron identificados con MALDI-TOF MS y secuenciación del ARNr 16S. Se llevó a cabo la PCR basada en secuencia de consenso intergénica repetitiva de enterobacterias(ERIC-PCR) y electroforesis en campo pulsado (PFGE) para asegurar la relación clonal. Para determinar la sensibilidad se usaron tiras de gradiente (Etest®) por difusión en agar.
ResultadosERIC-PCR y PFGE confirmaron la falta de relación clonal entre las cepas. Eritromicina o ciprofloxacino podrían ser apropiados para el tratamiento antibiótico de estas infecciones.
ConclusionesA. butzleri es un patógeno emergente con un aumento en la incidencia, y podría estar subestimado.
Arcobacter is a member of the Campylobacteriaceae family, closely related to the Campylobacter genus. The genus Arcobacter comprises 22 species and some of them – Arcobacter butzleri, A. cryaerophilus, A. skirrowii and A. thereius – have been isolated from human infectious diseases, mainly enteritis, being A. butzleri the most frequently isolated species, both from infectious processes and reservoirs, of environmental samples and food of animal origin.1
Arcobacter butzleri, currently called Aliarcobacter butzleri (Pérez-Cataluña et al., 2019), is a gram-negative rod oxidase positive, with microaerobic growth at an optimal temperature of 30°C, within a range from 15°C to 37°C,2 unlike the enteropathogenic species of the genus Campylobacter, which can grow at 42°C. It has been reported to be the fourth most common Campylobacter-like organism isolated from patients with diarrhoea. The transmission to humans by contaminated food and water can be manifested by acute persistent watery diarrhoea with abdominal pain, nausea, vomiting and sometimes fever with intestinal rhythm disturbances,3 which contrasts with bloody diarrhoea associated with Campylobacter jejuni. Although the mechanism by which diarrhoea is produced is not fully elucidated, adhesion is an important factor in bacterial pathogenicity since it is a required step for the establishment of the infection, which may be followed by colonisation of tissues and, in some cases, by bacterial invasion of host cells, intracellular multiplication, dissemination to other tissues, or persistence.4
Regarding antimicrobial susceptibility, A. butzleri is the only specie which is mostly discussed. Several antimicrobials have been suggested for the treatment of Arcobacter infections. Cephalosporins, alone or in combination with other antibiotics, is the first choice for bacteraemia treatment.5 However, regarding intestinal infections, quinolones, tetracyclines, macrolides or even a β-lactam antibiotic combined with a β-lactamase inhibitor have been used.6 Some authors have described the low resistance rate to fluoroquinolones so it is suggesting these for treating severe Arcobacter enteritis. Tetracyclines and aminoglycosides have also been proposed as a suitable antibiotic for treatment of Arcobacter spp.7
Available reports suggest that antimicrobial resistance is increasing against this emerging food-borne pathogen, leading to treatment failures with commonly used antimicrobials. A. butzleri is reported as comparatively more resistant than A. cryophilus and A. skirrowi.2,7
Although Arcobacter is not currently considered as a major public health concern, there is increasing evidence for its role in human illness as an emerging pathogen. Consistent with this, some authors have described a high presence of virulence-associated genes assuming the potential dangerous of these bacteria.
Eight strains of A. butzleri were isolated in two months in a tertiary hospital in Cantabria, Northern Spain. The aims of this study were: (i) to describe epidemiological and clinically the cases, (ii) to evaluate the participation of these strains in an outbreak, (iii) to analyse the antibiotic susceptibility and (iv) to evaluate the presence of several virulence genes in the strains.
MethodsBacterial strainsEight strains of A. butzleri were detected in only two months (15 October–15 December 2018) in the Microbiology Service of the University Hospital Marqués de Valdecilla, Santander, Spain. They were grown on Campylobacter selective agar plates (CCDA selective medium, Thermo Fisher Diagnostics, Hemel Hempstead, United Kingdom) in microaerophilia ambient at 37°C for 72h, following standard laboratory procedures.
Identification methodsProteomic methodIdentification of strains was obtained with the MALDI-TOF MS Vitek-MS™ system (bioMérieux, Marcy-I’Etoile, France), following manufacturer's recommendations. Analysis was performed with a Vitek-MS™ instrument supported by SARAMIS MS-IVD v3.2 database (Anagnos Tee GMBH, BioMérieux) in positive linear mode, with a mass range of 2–20kDa. Only hits within the spectra with scores of 99.9% were accepted.
Molecular methodThe 16S rRNA gene was amplified and sequenced for all A. butzleri strains using universal primers as previously described.5
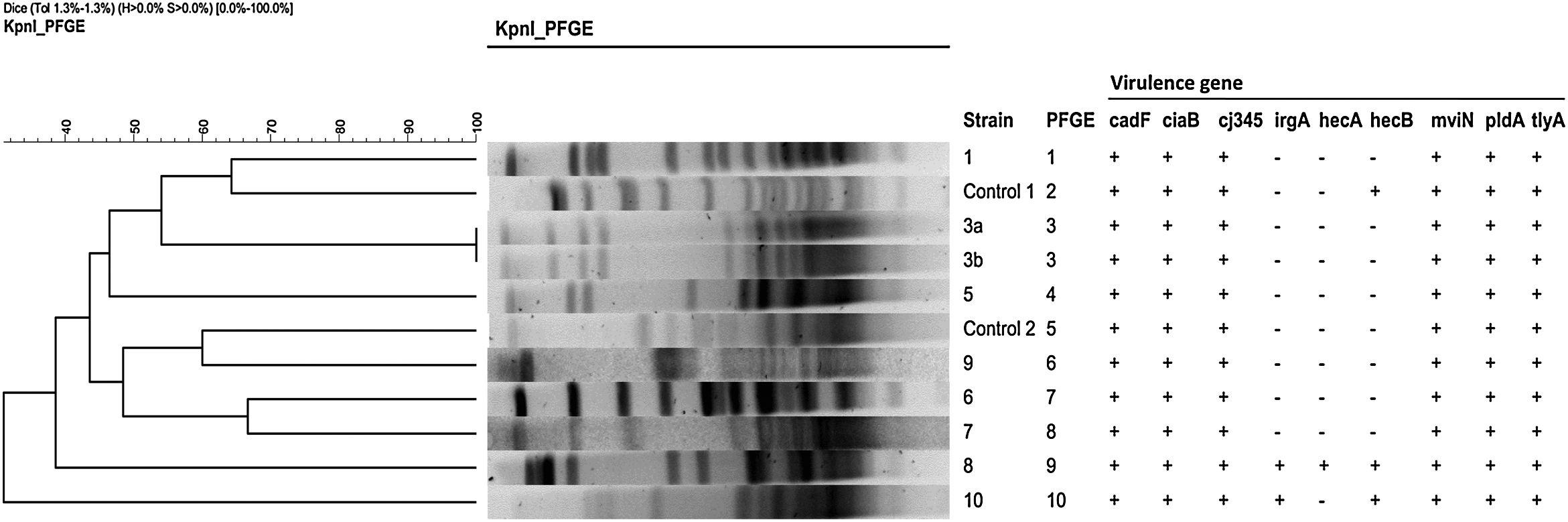
Typing methodsClonal relatedness among the strains was evaluated by enterobacterial repetitive intergenic consensus-PCR (ERIC-PCR)8 and Pulsed Field Gel Electrophoresis (PFGE) with KpnI, as previously reported.9
Antimicrobial susceptibility testMinimum inhibitory concentrations (MICs) for erythromycin (ERY), ciprofloxacin (CIP), imipenem (IPM), tetracycline (TET) and fixed 2:1 ratio amoxicillin–clavulanic acid (AMC) were performed using gradient strips (Etest®, bioMerieux) according to EUCAST (CIP, ERY, TET) and PK-PD EUCAST breakpoints (IPM, AMC) for C. jejuni (2019).
PCR-based assays to detect virulence genesA PCR designed by Douidah et al.10 was applied with primers based on the A. butzleri ATCC 49616 genome sequence for cadF, ciaB, cj1349, irgA, hecA, hecB, mviN, pldA0, and tlyA virulence genes. Also three virulence associated genes (act, alt, ast) responsible for different toxins with haemolytic, cytotoxic and enterotoxic activity in Aeromonas, were studied by PCR using primers described by Hoel et al.11
ResultsDuring a two month period, 328 stool samples, belonging to different patients, were collected and the following microorganisms were recovered: C. jejuni (n=34), Salmonella enterica (n=28), A. butzleri (n=8), Aeromonas spp. (n=7), Campylobacter coli (n=6) and Yersinia enterocolitica (n=1). A. butzleri reached 9.5% of all positive stool cultures in that period, which encouraged us to study that series of cases.
The eight patients, from which A. butzleri was isolated, were classified into three groups, according to the epidemiological and clinical data: Four of them were over 72 years of age, with persistent diarrhoea, between 8 and 20 days of evolution, that did not require antibiotics, only supportive treatment. A second group of two middle-aged patients (26 and 47 years old) shared a history of travel abroad (Thailand and Peru) with persistent diarrhoea and nausea, one of them having been treated with antibiotic (AMC). Finally, a third group showed severe underlying pathology, as leukemia, ranch worker, morbid obesity or sepsis, with acute diarrhoea that was treated with different antibiotics (meropenem, and meropenem with clindamicin and linezolid). All the cases had a good outcome even the one in intensive care unit.
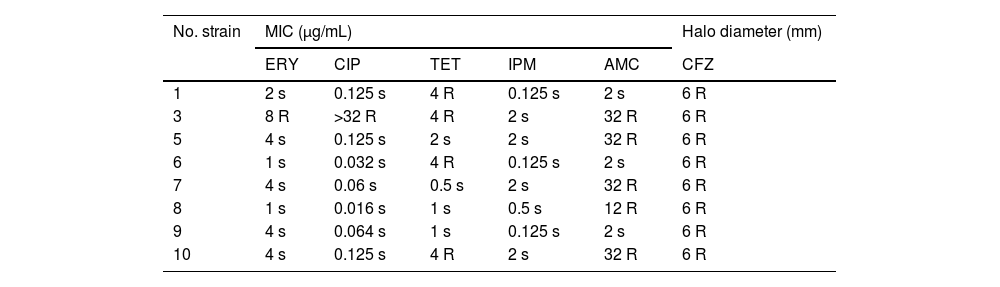
All strains were sensitive to IPM. Only one was resistant to ERY and CIP. Four strains were TET resistant and 5 strains were AMC resistant. Antimicrobial susceptibility is shown in Table 1.
Antimicrobial susceptibility testing for the eight strains of Arcobacter butzleri.
| No. strain | MIC (μg/mL) | Halo diameter (mm) | ||||
|---|---|---|---|---|---|---|
| ERY | CIP | TET | IPM | AMC | CFZ | |
| 1 | 2 s | 0.125 s | 4 R | 0.125 s | 2 s | 6 R |
| 3 | 8 R | >32 R | 4 R | 2 s | 32 R | 6 R |
| 5 | 4 s | 0.125 s | 2 s | 2 s | 32 R | 6 R |
| 6 | 1 s | 0.032 s | 4 R | 0.125 s | 2 s | 6 R |
| 7 | 4 s | 0.06 s | 0.5 s | 2 s | 32 R | 6 R |
| 8 | 1 s | 0.016 s | 1 s | 0.5 s | 12 R | 6 R |
| 9 | 4 s | 0.064 s | 1 s | 0.125 s | 2 s | 6 R |
| 10 | 4 s | 0.125 s | 4 R | 2 s | 32 R | 6 R |
ERY: erythromycin, CFZ: cefazolin, CIP: ciprofloxacin, AMC: amoxicilin–clavulanic acid, IPM: imipenem, TET: tetracycline.
The first analysis of ERIC-PCR gave no clustering but some similar patterns moved us to make subsequent typing with PFGE, as is recommended in some publications.12 In the PFGE analysis (Fig. 1) 10 pulsotypes were identified, thus confirming the lack of clonal relationship between strains. A cluster (#3, red box) was identified in two isolates from the same patient, and used as internal control (Fig. 1).
All the strains carried cadF, ciaB, cj1349, mviN, pldA, and tlyA virulence genes. two strains also possessed irgA and hecA genes, being hecB also detected in one of them. These last two, more virulent strains, owned to the group of over 72 years with persistent diarrhoea, but only one of these matched one of the four most resistant strains. None of the Aeromonas genes (act, alt and ast) were detected in our strains.
DiscussionEight A. butzleri isolates were collected in our hospital during a 2-month period. The type of affected patient fits with other published elsewhere before: cattle farming relationship, underlying pathology or a recent trip.3 However the type of diarrhoea, slightly differs from what is described in the literature: persistent rather than acute3 and with abdominal pain or nausea, besides that none of our patients were children.
A. butzleri is an emerging pathogen with increasing incidence, and may be underestimated mainly because of the only growth temperature at 42°C of most of laboratory at the time to isolate Campylobacter spp., but also because the new molecular gastrointestinal panels do not include this bacterium.13
Only one of our strains possessed all the virulence genes investigated (9.5%) which is in consonance with the 14.8% of Douidah et al.,10 but it seems that this is not so much related to acute diarrhoea but rather to more chronic symptoms, taking into account the small number of cases in our series.
Contrasting to other studies14 that report increasing resistance to CIP, only one of our strains was resistant to it. Also we do not recommend TET, as other authors do,15 because of the low susceptibility to this antibiotic in our strains (50%). IPM could be an alternative treatment for multidrug resistant Arcobacter.
In the series presented, we have shown, by various methods, that the cases were not related, but as this organism is contracted by food, like other enteropathogens, outbreaks in which it would be necessary to identify the focus should not be ruled out.
Finally, A. butzleri is an emerging enteropathogen that can go unnoticed in microbiology laboratories. In most cases it does not produce severe symptomatology but in certain patients it could compromise their life. ERY or CIP might be appropriate for antibiotic treatment of infections and IPM as alternative.
FundingThis study was supported by Plan Nacional de I+D+i 2013–2016, Instituto de Salud Carlos III, Subdirección General de Redes y Centros de Investigación Cooperativa, Ministerio de Economía y Competitividad, Spanish Network for Research in Infectious Diseases (REIPI RD16/0016/0007).
Transparency declarationsAll authors have nothing to declare. This study has not been financially supported by any Diagnostic/Pharmaceutical company.
Conflict of interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.