The increase in penicillin susceptibility among Staphylococcus aureus (SA-PenS) might have therapeutic relevance. We aimed to study the current situation in our environment.
Material and methodsOver a 2.5 years period, all SA isolates from bacteraemia were analysed. For all isolates, antimicrobial susceptibility profile, beta-lactam resistance genes (blaZ, mecA) and Panton-Valentine leucocidine encoding-genes were studied. For SA-PenS-blaZnegative isolates, spa-type, MLST and the presence of other resistance genes were studied.
ResultsAmong 84 patients with SA bacteraemia (35.7% MRSA and 64.3% MSSA), 77 were analysed; 22.2% of MSSA isolates were PenS and blaZnegative (Pen-MIC ≤0.03 µg/mL) corresponding to 14.3% of the total SA. In MSSA-PenS-blaZnegative isolates, eight spa-types corresponding to seven clonal complexes were detected.
ConclusionA high prevalence of MRSA/SA and MSSA-PenS-blaZnegative/MSSA was detected in blood cultures. Pen-MIC ≤0,3 µg/mL corresponded to MSSA-PenS-blaZnegative. This situation raises therapeutic options which should be further evaluated in larger studies and clinical trials.
El aumento de la sensibilidad a penicilina en Staphylococcus aureus (SA-PenS) podría tener relevancia terapéutica. Pretendemos conocer esta situación en nuestro medio.
Material y métodosSe analizaron bacteriemias por SA durante 2,5 años (2015–2017). Estudiamos la sensibilidad a antimicrobianos, genes de resistencia a beta-lactámicos (blaZ, mecA) y presencia de leucocidina de Panton-Valentine. En aislados SA-PenS-blaZnegativo se determinó el tipo de spa, MLST y genes de resistencia a antimicrobianos no-beta-lactámicos.
ResultadosHubo 84 pacientes con bacteriemia por SA (35,7% SARM y 64,3% SASM), se analizaron 77. El 22% de los SASM estudiados (n = 11) fueron PenS- blaZnegativo (CMI-Pen ≤0,3 µg/mL), correspondiendo a 14,3% del total de SA. En SASM-PenS-blaZnegativo se detectaron ocho tipos de spa asociados a siete complejos clonales.
ConclusiónDetectamos alta prevalencia de SARM/SA y de SASM-PenS-blaZnegativo/SASM en hemocultivos. Una CMI-Pen ≤0,3 µg/mL se correspondió con SASM-PenS-blaZnegativo. Esta situación plantea opciones terapéuticas que deberán reevaluarse con estudios más amplios y ensayos clínicos.
Staphylococcus aureus (SA) bacteraemia is an entity with special relevance due to its frequency, severity and high mortality. In the treatment of Methicillin-susceptible SA (MSSA) bacteraemia, the timeliness of suitable treatment is very important. In targeted therapy there is controversy about the use of different beta-lactams, with cloxacillin and cefazolin as first-line, and poorer results with second and third generation cephalosporins and beta-lactams with beta-lactamase inhibitors.1 Regarding penicillin, due to its high resistance, it is not generally used for these treatments, especially in bacteraemia. However, in addition to the vast experience with this antibiotic over decades, recent works indicate that it still has points in its favour for even standing as a treatment of choice for SA infections susceptible to this antibiotic (SA-PenS).2–4 These points include: 1) MIC values 10–50 times lower and a lower percentage of protein binding than cloxacillin; 2) the time above the MIC is longer than other antimicrobials, including cefazolin5; 3) narrower spectrum than other options, including first-generation cephalosporins (thus related to fewer infections by Clostridium difficile),6 and 4) less selection of mecA and mecC-mediated methicillin resistance.7
In various countries around the world there is a growing trend in penicillin-sensitive SA, especially in invasive infections like bacteraemia. Data from the USA,6,8–10 Canada,4 Europe (Sweden,5,11 Finland12 and Denmark2) or from Australia and New Zealand3 exemplify this phenomenon, including penicillin in a kind of "second chance".4
Penicillin resistance in mecA-negative SA strains is due to the production of beta-lactamases encoded by the blaZ gene. There are several phenotypic methods for its detection, such as the nitrocefin test or the reading of the halo edge of penicillin on disk-diffusion, but compared with PCR detection of the blaZ gene they have less sensitivity.7,9,11
The objective of this work was to understand the situation of penicillin sensitivity in cases of bacteriaemia caused by SA and its characteristics in our environment.
Material and methodsDuring the study period (July 2015-December 2017) SA strains isolated from bacteraemia (both MSSA and methicillin-resistant Staphylococcus aureus [MRSA]) were analysed, in adult patients at the Hospital Royo Villanova [Royo Villanova Hospital], Zaragoza. The blood cultures (Bactec, Becton, Dickinson®) and the sensitivity study (MicroScan, Beckman® Combo 31 panel) were performed following the usual European Committe for Antimicrobial Susceptibility Testing protocols and cut-off points.
In all the SA strains (one per patient) the presence of the blaZ and mecAgenes was analysed, as well as the Panton-Valentine leukocidin (PVL) gene.13
The characterisation of the blaZ-negative (blaZnegative) isolates was obtained through: 1) spa typing by PCR and sequencing13; 2) determination of the sequence type (ST) and clonal complex (CC) by the multilocus-sequence typing (MLST) technique from a representative isolate of each of the different spa-types detected, assuming the ST for all the isolates of the samespa,13 and 3) PCR study of non-beta-lactam antibiotic resistance genes: macrolides (ermA/ermB/ermC/msrA/msrB y ermT), tobramycin (ant(4)’-Ia) or mupirocin (mupA).13
ResultsOver the 30 months of study there were 84 patients with SA bacteraemia (30 with MRSA [35.7%] and 54 with MSSA [64.3%]). Of the MSSA isolates, 12 were susceptible to penicillin (MSSA-PenS), representing 22.2% of MSSA and 14.3% of SA.
Of the total of 84 SA, 77 isolates were recovered for characterisation (27 MRSA and 50 MSSA). The 27 MRSA isolates were resistant to penicillin (MIC: >0.12mcg/m), carried the mecA and blaZ genes, and were negative for the PVL gene.
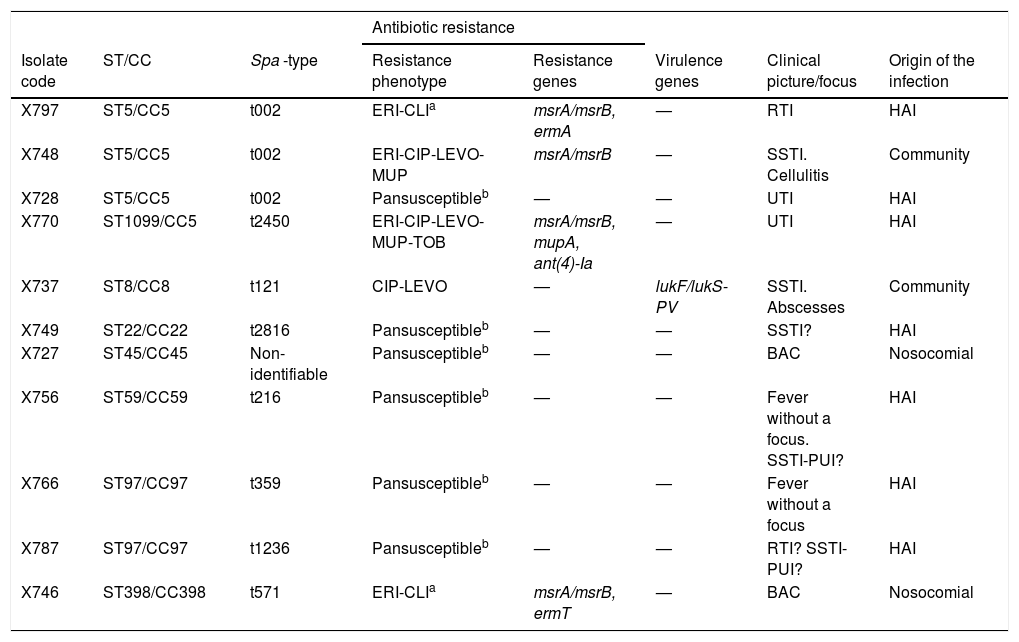
The 50 MSSA isolates lacked mecA, and in relation to penicillin: a) 39 of them showed phenotypic resistance to penicillin (MIC: >0.25 μg/mL) and contained the blaZ gene (MSSA-PenR-blaZpositive), and b) 11 were susceptible to penicillin and lacked the blaZ gene (MSSA-PenS-blaZnegative), all of them showing an MIC-Pen≤0.3 μg/mL (Table 1).
Phenotypic and genetic characteristics of 11 strains of Staphylococcus aureus Pen−S-blaZnegative isolated from blood cultures.
| Antibiotic resistance | |||||||
|---|---|---|---|---|---|---|---|
| Isolate code | ST/CC | Spa -type | Resistance phenotype | Resistance genes | Virulence genes | Clinical picture/focus | Origin of the infection |
| X797 | ST5/CC5 | t002 | ERI-CLIa | msrA/msrB, ermA | — | RTI | HAI |
| X748 | ST5/CC5 | t002 | ERI-CIP-LEVO-MUP | msrA/msrB | — | SSTI. Cellulitis | Community |
| X728 | ST5/CC5 | t002 | Pansusceptibleb | — | — | UTI | HAI |
| X770 | ST1099/CC5 | t2450 | ERI-CIP-LEVO-MUP-TOB | msrA/msrB, mupA, ant(4́)-Ia | — | UTI | HAI |
| X737 | ST8/CC8 | t121 | CIP-LEVO | — | lukF/lukS-PV | SSTI. Abscesses | Community |
| X749 | ST22/CC22 | t2816 | Pansusceptibleb | — | — | SSTI? | HAI |
| X727 | ST45/CC45 | Non-identifiable | Pansusceptibleb | — | — | BAC | Nosocomial |
| X756 | ST59/CC59 | t216 | Pansusceptibleb | — | — | Fever without a focus. SSTI-PUI? | HAI |
| X766 | ST97/CC97 | t359 | Pansusceptibleb | — | — | Fever without a focus | HAI |
| X787 | ST97/CC97 | t1236 | Pansusceptibleb | — | — | RTI? SSTI-PUI? | HAI |
| X746 | ST398/CC398 | t571 | ERI-CLIa | msrA/msrB, ermT | — | BAC | Nosocomial |
?: probable, unproven infection; BAC: catheter-related bacteriaemia; CIP: ciprofloxacin; CLI: clindamycin; ERI: erythromycin; SSTI: skin and soft tissue infections; HAI: health care-associated infections; RTI: respiratory tract infection; UTI: urinary tract infection; LEVO: levofloxacin; MUP: mupirocin; TOB: tobramycin; PUI: pressure ulcer infection.
The 11 MSSA-PenS-blaZnegative isolates were assigned to 8 different spa -types (t002 the most frequent), associated with 8 STs and 7 CCs (CC5, CC8, CC22, CC45, CC59, CC97 y CC398). Six of the 11 MSSA-PenS-blaZnegative isolates were susceptible to all the antibiotics tested and the remaining 5 presented resistance to macrolides, lincosamides, tobramycin, mupirocin and/or fluoroquinolones (genes ermA, ermT, msrA, msrB, ant(4)’-Ia or mupAbeing detected). Lastly, one of the MSSA-PenS-blaZnegative strains was a carrier of the PVL gene (t121-ST8/CC8), and only resistant to fluoroquinolones.
DiscussionIn this study of SA bacteraemia, a MRSA prevalence of 35.7% was detected with 22.2% susceptibility to penicillin among MSSA isolates (14.3% of total SA). The percentage of MRSA is higher than that of other European countries and that of most Spanish hospitals (https://ecdc.europa.eu/en/about-us/partnerships-and-networks/disease-and-laboratory-networks/ears-net).
Regarding the MSSA-PenSisolates, published figures are between 20 and 33% in northern European countries,5,11 USA9,14 or Canada.4,10 In Spain we have not found any recent data, but in a multicentre study15 with 20-year evolution data (1986–2006) the results of 6 prevalence studies in Spanish hospitals were analysed and it was observed that there was an increase in global susceptibility to penicillin in SA isolates from different clinical samples, with the lowest figures in 1991 (2.9%) and a sustained increase up to 2006 (11.0%). The data of our series of 14.3% global and 22.2% in MSSA would be in line with an increase in global susceptibility to penicillin in SA.
Of all the PenS strains characterised, (n = 11), all presented MIC-Pen≤0.3 μg/mL values and lacked the blaZ gene. This observation contributes to simplifying the microbiological diagnosis of penicillin susceptibility determination, since PCR detection of the blaZ gene is not available in most laboratories, the interpretation of susceptibility by disk-diffusion is not very objective and other tests such as nitrocefin are not recommended. Our data coincide with other authors that demonstrate that in strains with high susceptibility to penicillin (<0.03 μg/mL) the presence of beta-lactamases mediated by blaZ1,9 can be reliably ruled out. Clonal complexes CC5, CC8, CC45 y CC22 associated with our MSSA-PenS-blaZnegative were also detected in a similar study in Sweden, with CC5-t00211 also predominant.
An explanation for the increased susceptibility to penicillin in SA could be attributed to a lower selective pressure due to its lower use. In fact, there is a trend in this regard in the first years of the 21 st century compared to previous years, which is especially marked in the USA and Canada.6,10 The only country reporting a downward trend is Sweden,5 a country with an antibiotic policy that has prioritised narrow-spectrum antibiotics for years, which went from 57% susceptibility in 2008−09 to 29% in 2014−15. Perhaps it could be interpreted as an increasing phenomenon in countries where penicillin is not consumed and decreasing in those where it is used, with both situations coinciding with figures of 20-30% at the present time. Another possible explanation is that new, more epidemiologically successful SA clones are coming in to replace the previous ones, and among these some lack blaZ. This aspect should be monitored in the future.
One of the limitations of our study is that of being single-centre. It would be interesting to see if this prevalence of penicillin susceptibility (with absence of blaZ) in SA exists in other hospitals in our country.
There are no randomised studies comparing benzylpenicillin with other antistaphylococcal penicillins (cloxacillin) or with cefazolin, and its use has been so low since the end of the last century (due to increased resistance) that sufficient data has not been collected in retrospective studies. In some studies that analyse the use of penicillin, a higher mortality has not been observed compared with comparators such as dicloxacillin.2,6 In one study,6 the use of penicillin went from 0 to 50% of susceptible cases, with good results. Another recent article3 shows a slightly higher mortality of flucloxacillin compared to benzylpenicillin, although with methodological limitations.
Pencillin susceptibility in MSSA expands targeted treatment options. The limited data on its usefulness is favourable, but randomised studies are needed to determine the most appropriate treatment. The narrowest of spectrums is one of the aspects that must take precedence in the treatment of infections, without ruling out other aspects such as administration dosage difficulties.
FundingThis work was funded in part by the Agencia Estatal de Investigación (AEI) [State Bureau of Investigation] of Spain and the EU European Regional Development FundSAF2016-76571-R project.
O.M. Mama has a predoctoral scholarship from Mujeres por África [Women for Africa]-Universidad de La Rioja [La Rioja University] and L. Ruiz-Ripa has a predoctoral scholarship from the Universidad de La Rioja.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Aspiroz C, Mama OM, Martínez-Álvarez RM, Ruiz-Ripa L, Ceballos S, Torres C. Bacteriemia por Staphylococcus aureus sensible a penicilina. Importancia epidemiológica, clínica y posibles implicaciones terapéuticas. Enferm Infecc Microbiol Clin. 2020;38:434–437.