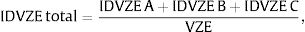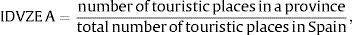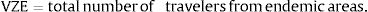The establishment of Aedes albopictus in new areas in Europe has changed the risk of local dengue transmission represented by imported human cases. The risk of transmission is determined by the distribution of travelers arriving from dengue-endemic areas and the distribution of Ae. albopictus as potential vectors of dengue in Spain.
MethodsEnvironmental, entomological, epidemiological, demographic, tourism and travel data were analyzed to produce a series of maps to represent: the distribution of Ae. albopictus across municipalities; the risk of expansion of Ae. albopictus based on a species distribution model; the calculated index of travelers from dengue-endemic areas (IDVZE) per province; the percentage contribution of each municipality to the total number of cases in Spain. The maps were then added using map algebra, to profile the spatial risk of autochthonous dengue in Spain at a municipal level from 2016 to 2018.
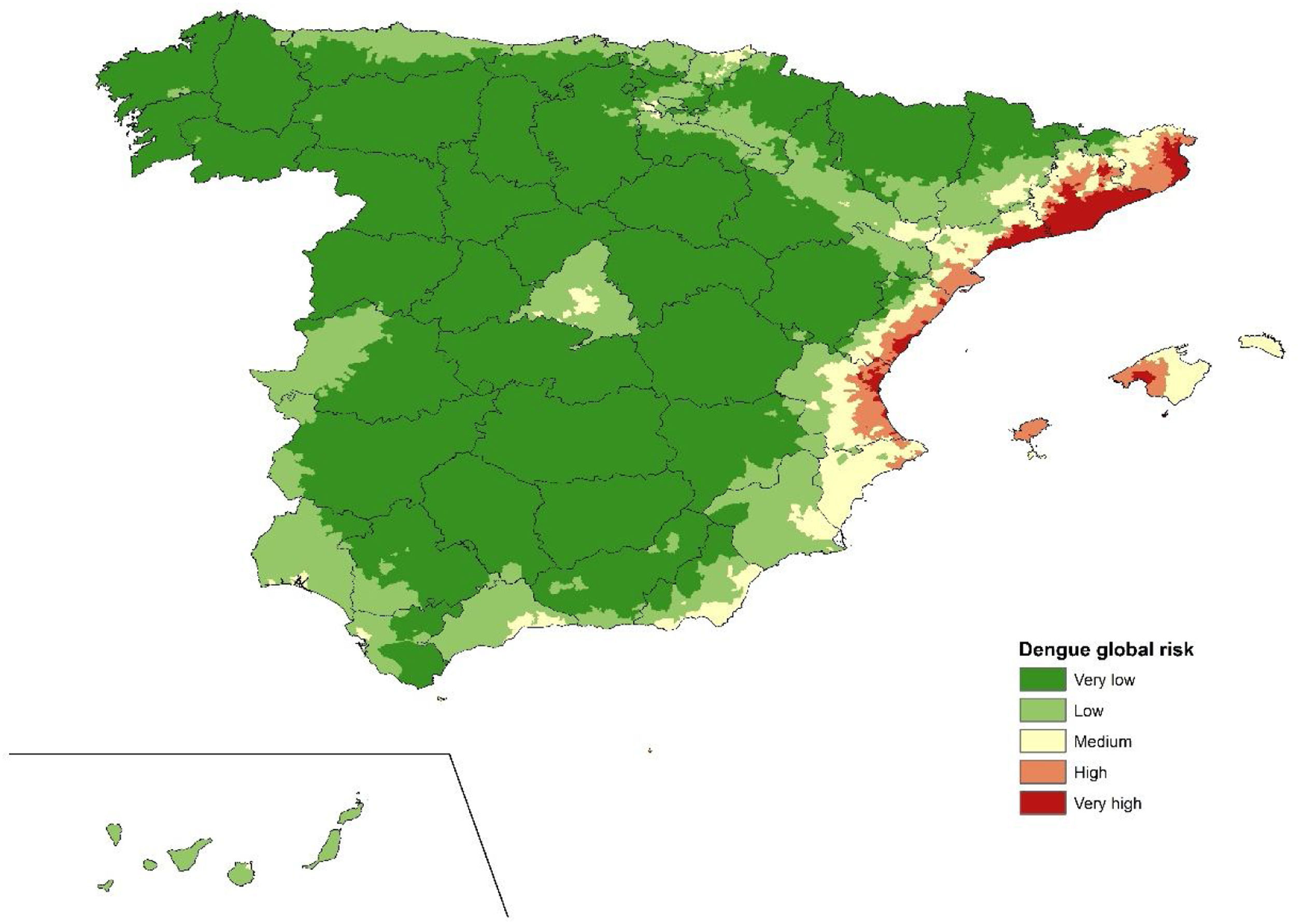
ResultsAe. albopictus was detected in 983 municipalities. The calculated IDVZE varied from 0.23 to 10.38, with the highest IDVZE observed in Madrid. The overall risk of autochthonous cases oscillated between 0.234 and 115, with the very high risk and high risk areas detected in the Mediterranean region, mainly in the Levantine coast and some parts of the Balearic Islands. Most of the interior of the peninsula was characterized as low risk.
ConclusionPrevention and control measures to mitigate the risk of autochthonous dengue should be prioritized for municipalities in the high risk areas integrating early detection of imported dengue cases and vector control.
El establecimiento de Aedes albopictus en nuevas zonas de Europa ha cambiado el riesgo de transmisión local del dengue. El riesgo de transmisión está determinado por la distribución de viajeros que llegan de áreas endémicas de dengue y la distribución de Ae. albopictus como vectores potenciales del dengue en España.
MétodosSe analizaron datos ambientales, entomológicos, epidemiológicos, demográficos, de turismo y de viajes para producir mapas: la distribución de Ae. albopictus a nivel municipal; el riesgo de expansión del mosquito basado en un modelo de distribución de especies; el índice calculado de viajeros de zonas endémicas de dengue (IVZED); la contribución porcentual de cada municipio al total de casos en España. Los mapas se agregaron utilizando álgebra de mapas para perfilar el riesgo espacial del dengue autóctono en España a nivel municipal de 2016 a 2018.
ResultadosAe. albopictus se detectó en 983 municipios. El IVZED calculado varió de 0,23 a 10,38, observándose más alto en Madrid. El riesgo global de casos autóctonos osciló entre 0,234 y 115, detectándose las zonas de muy alto y alto riesgo en la región mediterránea, principalmente en la costa levantina y algunas zonas de Baleares. La mayor parte del interior de la península se caracterizó de bajo riesgo.
ConclusiónLas medidas de prevención y de control para mitigar el riesgo de dengue autóctono deben priorizarse para los municipios de las zonas de alto riesgo que integren la detección temprana de casos de dengue importado y el control de vectores.
Dengue is an Aedes-borne viral disease, endemic in South America, Central America and the Caribbean, Southeast Asia, the Western Pacific and Sub-Saharan Africa.1 Globally, there are an estimated 100–400 million infections per year and almost half the world population is at risk of infection by DENV.1,2 The incidence has increased dramatically over the last years, with the highest number of dengue cases recorded in 2019.1
Dengue virus is transmitted primarily via the bite of female mosquitoes of Aedes aegypti and Aedes albopictus, which are also vectors of chikungunya, yellow fever, West Nile and Zika viruses,1,3,4 and of a total of 26 viruses – at least, experimentally.5Ae. albopictus is a highly invasive vector which has managed to spread throughout the world, beyond tropical and subtropical zones through international travel and trade and is now present in many areas in southern Europe, including Spain.6
Ae. albopictus can resist over winter,7,8 although its period of vector activity ranges from spring to autumn. The optimal climatic conditions for its establishment and expansion include temperatures>0°C, with annual averages>11°C and 500mm of precipitation, and summers with 25–30°C and rain. Ae. albopictus was first detected in Spain in 20049 and it is established in the regions of Catalonia, Valencia, Murcia, Andalusia, the Balearic Islands, Aragon and the Basque Country and more recently in Madrid and Extremadura.10
Many travelers return with viraemic infections to Europe and consequently transmission is possible when Ae. albopictus is present in their areas or residence. In the case of Spain viremic travel-related cases are reported every year and their number is projected to continue increasing in the near future.11
Profiling the spatial risk of autochthonous dengue is necessary in identifying areas with populations at risk and guiding the geographic strategy for prevention and control measures.12–14 Risk maps can combine information on the distribution of the vector, its potential expansion, as well as the occurrence of the disease to predict the risk of infection.2,14 Therefore, the aim of this work was to map the risk of autochthonous dengue cases in Spain based on the potential expansion of Ae. albopictus, the number of travelers from endemic countries and the number of cases declared to the Spanish National Network of Epidemiological Surveillance (RENAVE) in order to guide preparedness and response plans for detection and control of dengue in Spain.
Materials and methodsData sourcesAe. albopictus trap-based dataUnder the national plan for preparedness and response against vector–borne diseases, entomological surveillance is conducted in different areas of the country to identify and control competent vectors.15 Active entomological surveillance includes annual sampling during the activity season of Ae. albopictus to detect its occurrence into new areas.
We listed the municipalities with positive traps i.e. traps where Ae. albopictus had been detected anytime during the period 2016–2018, and calculated their centroids.
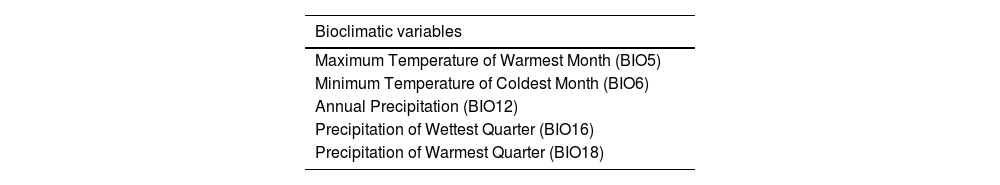
Environmental dataA recent literature review identified the climatic variables that have been associated to the distribution of mosquitoes in previous studies.16Table 1 shows the variables for the distribution model of Ae. albopictus, as identified in the literature.
Selected bioclimatic and ecological variables that influence the distribution of Ae. albopictus according to Brugueras et al. (2020) literature review.
| Bioclimatic variables |
|---|
| Maximum Temperature of Warmest Month (BIO5) |
| Minimum Temperature of Coldest Month (BIO6) |
| Annual Precipitation (BIO12) |
| Precipitation of Wettest Quarter (BIO16) |
| Precipitation of Warmest Quarter (BIO18) |
| Ecological |
|---|
| Digital elevation model |
| Population density |
| Corine Land Cover |
Correspondence to Worldclim database variables is indicated in brackets.
Spatial bioclimatic data were then extracted from the Worldclim databases, whit spatial resolution of approximately 1km2.
A digital elevation model and Corine Land Cover were obtained from the National Geographic Institute of Spain.
Disease dataSince 2015, probable and confirmed dengue cases are notified to the National Surveillance Network (RENAVE in Spanish). The number of notified dengue cases per municipality for the years 2016–2018 was obtained. If the municipality of residence of the dengue case was missing, the municipality of declaration was used instead.
Demographic dataPopulation density per km2 was obtained from Gridded Population of the World https://sedac.ciesin.columbia.edu/.
The population and the number of residents from dengue-endemic countries per province were obtained from Statistics National Institute (INE in Spanish).
Travel and tourism dataWe obtained the number of passengers arriving in Spain from the Spanish Airports and Air Navigation (AENA)17 and the World Tourism Organization (UNWTO),18 for 2014–2017. The annual mean number of passengers for each year was calculated.
Furthermore, we used the estimated number of bed-places at provincial and national level from INE, to calculate the annual mean number of touristic places in each province.
Data analysisDistribution of Ae. albopictus and Ae. albopictus suitability mapThe centroids of the municipalities with positive Ae. albopictus traps were used to plot the distribution of the positive traps of Ae. albopictus in Spain. The coordinates of these centroids were also used in species distribution model (SDM) of Ae. albopictus.
To create the Ae. albopictus suitability map we used a SDM, taking into account current climatic conditions. In our study, we used the maximum entropy approach, implemented in the software Maxent (v. 3.3.3k). This approach is based on the optimal environmental conditions for which the vector can be established by looking for pixels with characteristics similar to those areas where the vector is present.
A cartographic process was performed using a Geographic Information System (GIS) to join, prepare and cut spatial data at the same resolution and spatial framework.
Eighty percent of the data from Ae. albopictus positive traps were used to train the model and the remaining 20% to test the model's performance (robustness and predictive power). This was measured by the area under the receiver operating characteristic (ROC) curve or AUC: a plot of sensitivity against specificity which measures the ability of the model to discriminate between sites where a species is present (y=1) against where it is absent (y=0).19 Five hundred iterations were performed.
Calculation of the endemic zone traveler index (IDVZE)An index was developed to account for the risk of introduction of the pathogen.
The country of origin of residents was classified as dengue-endemic according to the evidence consensus of the International Research Consortium on Dengue Risk Assessment, Management and Surveillance published in 2012 by Brady et al.20
Arrivals from those countries considered as endemic for dengue were classified into three traveler groups: (A) non-residents who came to Spain for tourism; (B) non-residents who came to visit family and friends; and (C) residents returning from a trip abroad.17 Weights were used to calculate the distribution of travelers throughout Spain. These weights were based on (a) the number of touristic places of each province with respect to the total number of touristic places in Spain, for non-residents for tourism (group (A)), (b) the number of residents originating from dengue-endemic zones in each province compared to the total number of residents from these countries in Spain, for non-residents visiting family and friends (group (B)), and (c) the provincial population with respect to the nationwide population for residents returning from a trip (group C). Thus, we obtained three components for each traveler profile, respectively: IDVZE A–C.
IDVZE was calculated using the following formula:
whereThe calculated IDVZE per province were plotted in a map.
Dengue case mapThe percentage contribution of each municipality to the national dengue cases was calculated by dividing the total number of dengue cases in each municipality of residence reported from 2016 to 2018, over the total number of cases in Spain for this 3-year period. A map was produced to show the distribution.
Risk map for autochthonous dengue casesThe aforementioned maps for the distribution of the (i) suitability of Ae. albopictus, (ii) endemic zone traveler index, and (iii) percentage contribution of dengue cases per municipality were standardized. The three indexes were aggregated at each geographical unit by adding up their values by municipalities and then stablishing five categories (quintiles) of the results using the QGIS Algebra mapping tool. The resulting map depicts the risk of autochthonous dengue at the municipal level classified in five categories (quintiles): very low; low; medium; high and very high.
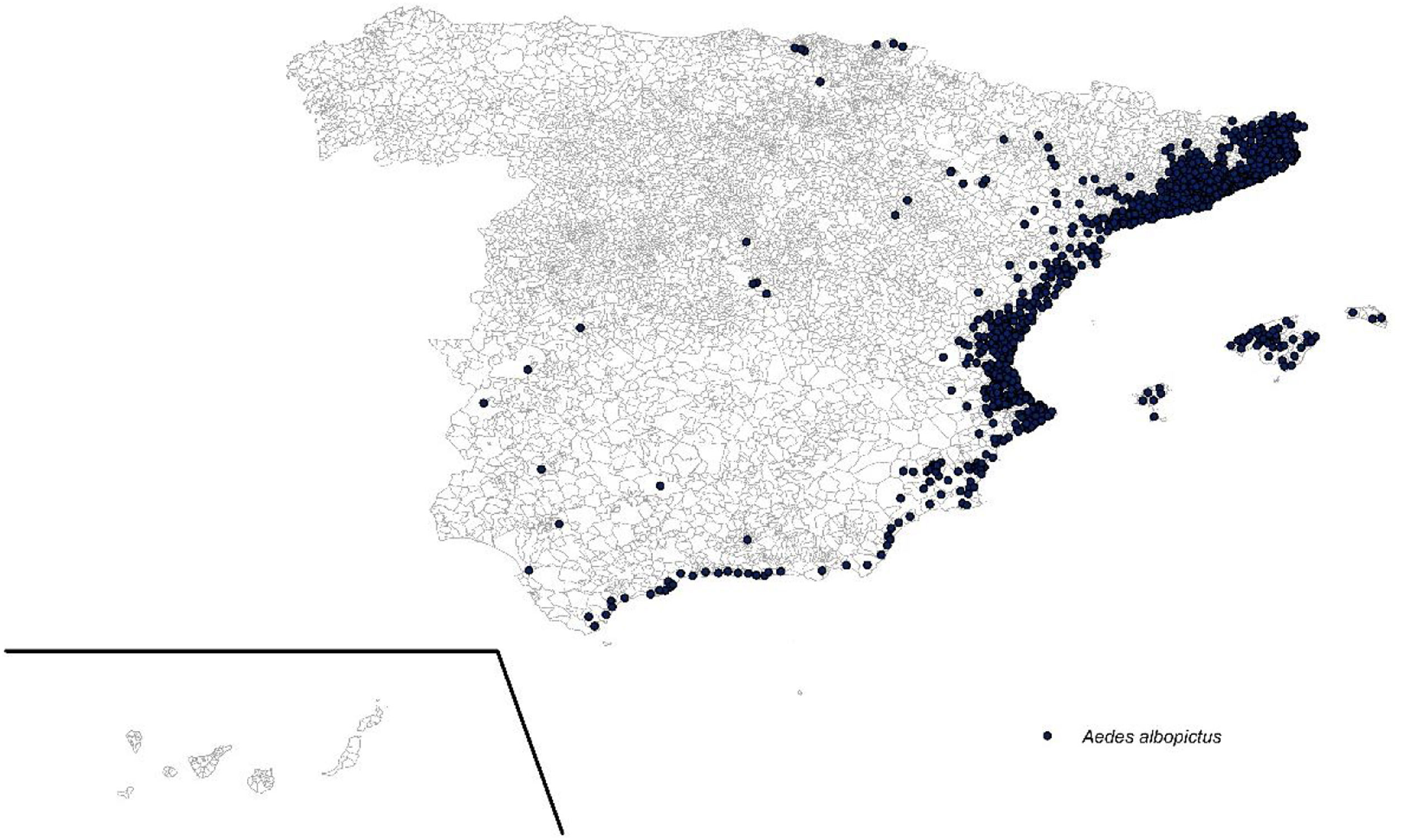
ResultsAe. albopictus presence mapIn 2016–2018, Ae. albopictus was detected in traps in 983 municipalities, distributed mostly in the Mediterranean area, including the Balearic Islands and a few sporadic in the interior of the peninsula (Fig. 1).
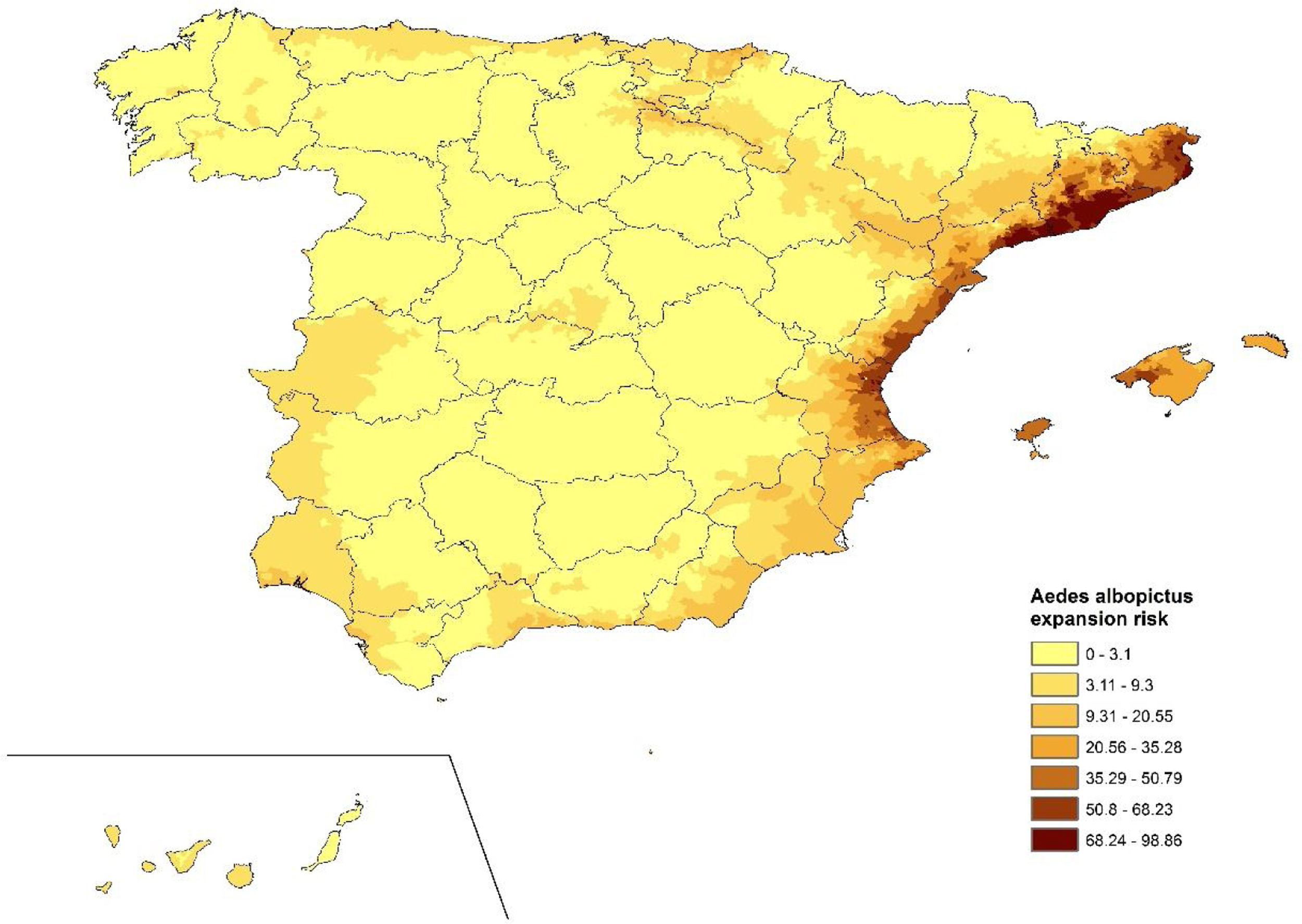
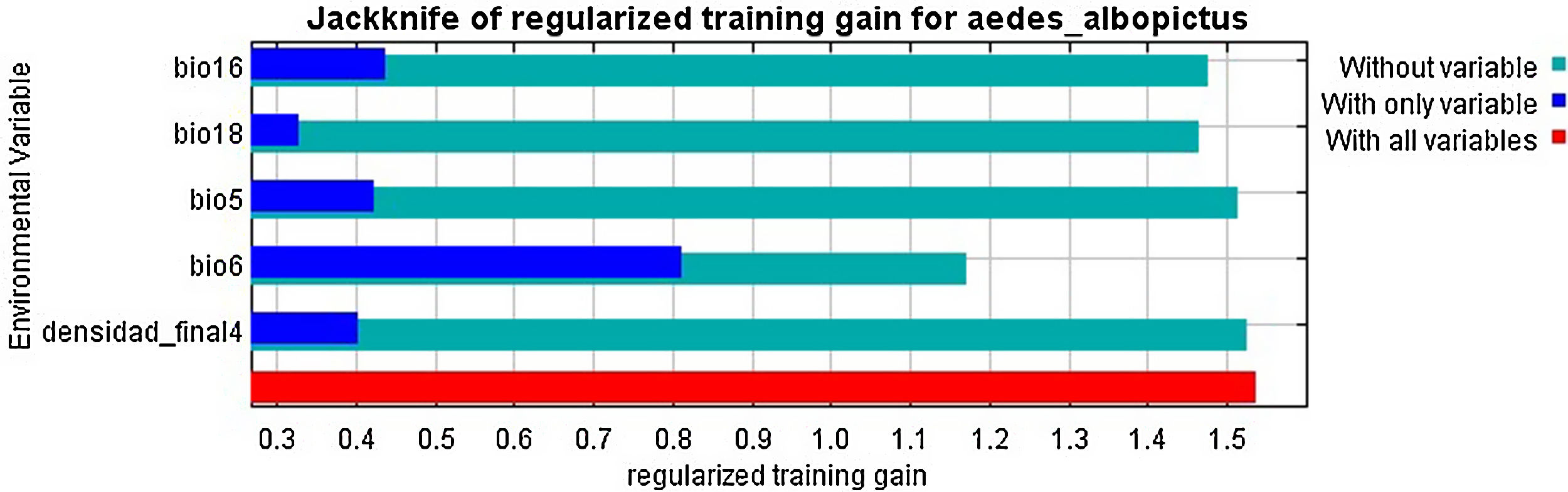
Ae. albopictus environmental suitability mapFour variables were used in the final species distribution model: the minimum temperature of the coldest month (BIO6), maximum temperature of the warmest month (BIO5), precipitation of the wettest quarter (BIO16), precipitation of the warmest quarter (BIO18) and population density. The variable that contributed the most to the model was the minimum temperature of the coldest month while the variable that contributed the least was precipitation in the warmest quarter (Supplementary Fig. 1). The AUC for model performance was 0.928.
Fig. 2 indicates the suitable areas for the expansion of Ae. albopictus in Spain according to the results of SDM. There is spatial variability in the potential distribution of Ae. albopictus across municipalities. At the country level different suitability areas can be distinguished; they were classified in three levels: (i) areas with high suitability which are those in which the vector has already been established, such as the Mediterranean zone; (ii) areas where the vector was recently introduced, such as Madrid and some areas in Extremadura; and (iii) areas where it could be introduced but has not been detected yet, such as the Cantabrian coast or the Ebro basin.
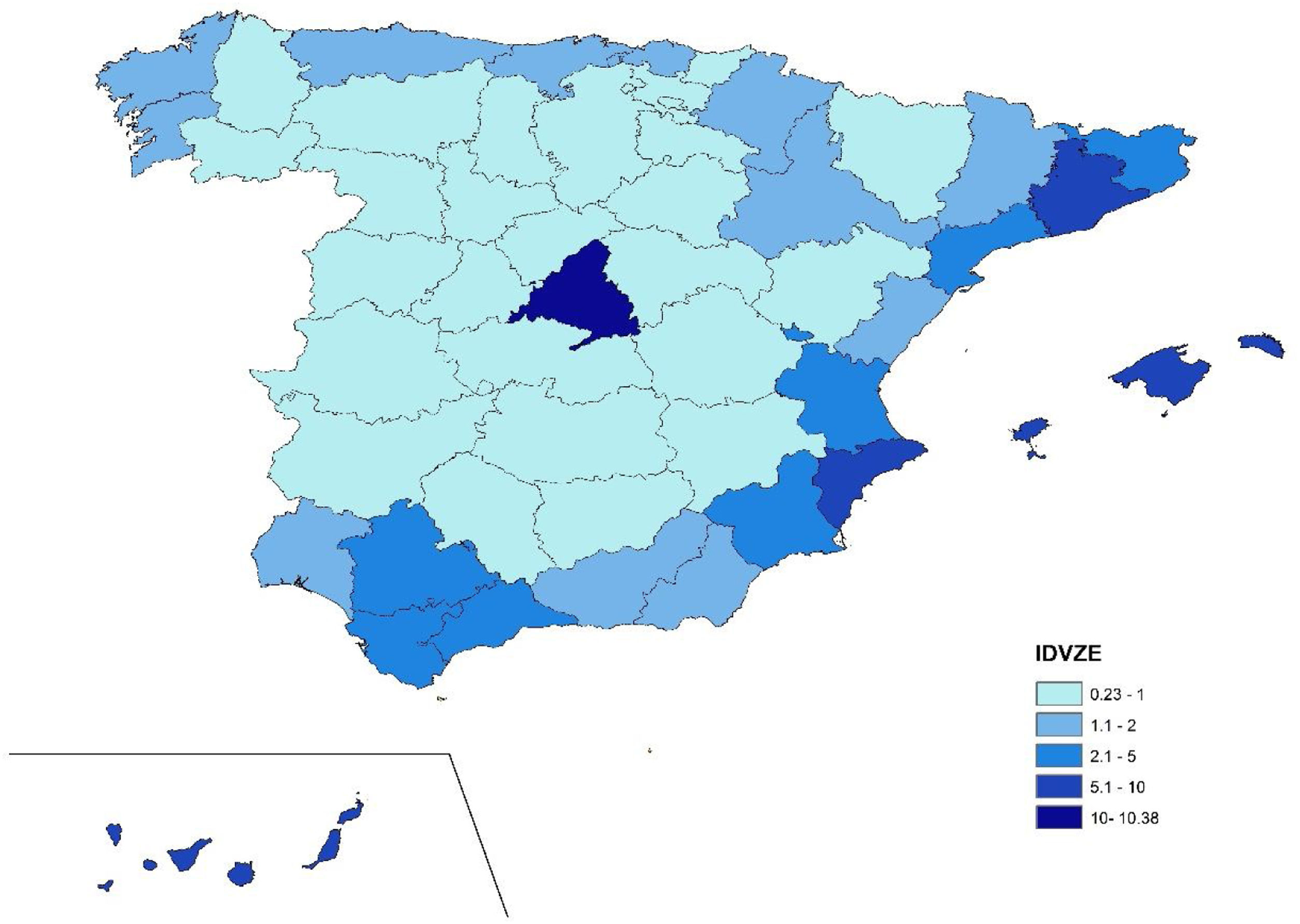
Calculation of the endemic area traveler index (IDVZE)The annual average number of arrivals according to AENA was 773,442,780 travelers from 175 countries, out of which 128 were endemic for dengue. The largest number of travelers came from the United States of America (1,601,460), followed by Colombia (503,530), Brazil (491,020), Argentina (465,582), Peru (310,488), the Dominican Republic (283,511), Cuba (228,173), Ecuador (144,706) and Venezuela (121,798). More than three quarters (77.5%) of the travelers were non-residents, of which 79.6% were visiting Spain for tourism and 20.4% to visit friends and relatives. The remaining 22.5% were residents returning to Spain.
Overall, the IDVZE depicted in Fig. 3 summarizes the weighted probability that a random international traveler has a province as final destination (the highest the index, the more likely). The IDVZE varied from 0.23 to 10.38, being the highest observed in Madrid, followed by Barcelona, Alicante and the Canary and Balearic Islands. On the other hand, the lower IDVZE with values less than 1.00 were recorded in the interior of Spain.
Dengue human cases mapThere were 595 imported and six autochthonous dengue cases.
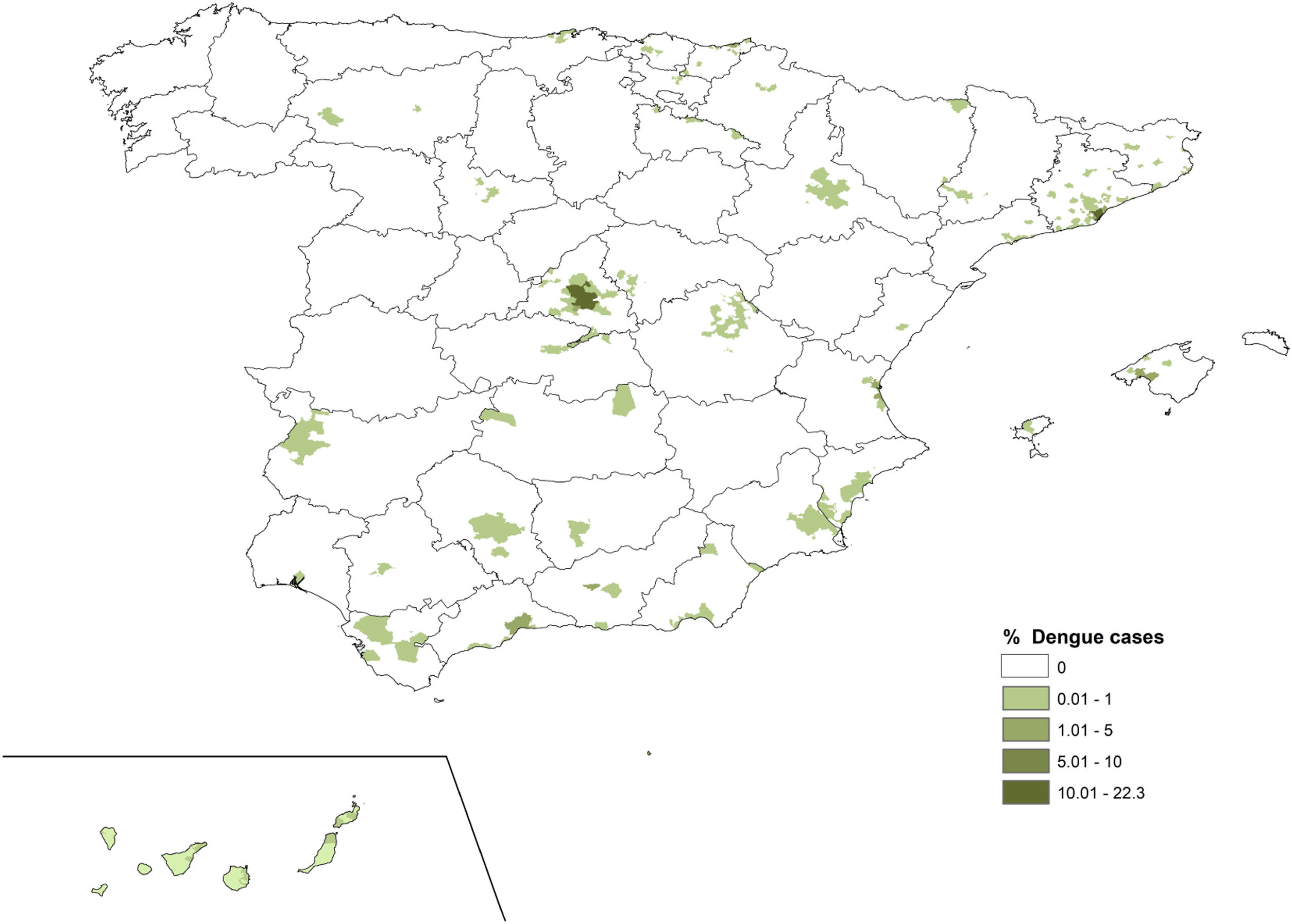
The percentage contribution of dengue cases of each municipality varied between 0 and 22 (Fig. 4). The cities of Madrid and Barcelona contributed the most to the total number of dengue cases notified in Spain.
Autochthonous dengue risk mapFig. 5 shows the spatial distribution of the risk of dengue in municipalities in Spain, resulting from the addition of risk indexes derived from Ae. Albopictus suitability, IDVZE and previous reported dengue cases per municipality (Figs. 2–4). The highest risk would then correspond to locations where previous dengue cases, environmental vector suitability and international travelers from endemic countries are present. The risk varied between 0.234 and 115, with the “very high risk” and “high risk” areas detected in Mediterranean regions, mainly in the Levantine coast and some parts of the Balearic Islands. Around these “very high risk” and “high risk” zones, there was a “medium risk” belt. “Medium risk” municipalities were also observed in the city and the region of Madrid, south coastal areas, Basque Country and on the banks of the Ebro river. The rest of the country was described as “low risk” or “very low risk”, especially in the interior of the peninsula as well as the Canary Islands.
DiscussionIn this work a risk assessment of autochthonous dengue occurrence in Spain, by municipality, has been carried out, taking into account environmental factors for the expansion of Ae. albopictus, the volume of travelers from endemic areas and the number of notified dengue cases.
The meteorological conditions make Spain and especially the eastern areas, a favorable area for the introduction, establishment and expansion of the vector. Different studies carried out in Europe indicate that the Mediterranean area as well as other locations in the north of our country are suitable for the mosquito.14,21,22 In fact, since the first detection of Ae. albopictus in Spain, occurred in 2004 in Catalonia,9 the vector has colonized other neighboring areas and is currently present in most locations in the Levantine coast, Balearic Islands and even in inland and northern areas.
Ae. albopictus species was usually associated with sporadic dengue cases or self-limited outbreaks, due to its limited competence related to its feeding behavior and the relative recent adaptation of flaviviruses to the vector (including dengue fever virus).23 However, since 2010, a few autochthonous outbreaks of dengue caused by Ae. albopictus occurred in Europe.24 In addition to the 2018 and 2019 outbreaks in Spain, outbreaks were also detected in France and Croatia. Nevertheless, it is important to highlight that, although the presence of the vector is necessary it is not sufficient for the occurrence of autochthonous cases. Other factors such as vector density, population density, environmental conditions and the regular presence of viremic people are necessary for such outbreaks to occur and for sustained transmission over time.
Globalization and international travel and trade, have increased the risk of introduction of imported diseases.6 As reported by the World Tourism Organization, Europe receives the highest number of visitors with more than 580 million visits per year with an average increase of 2.8% over the last 10 years.25 According to the statistics on border movements (FRONTUR) from the National Institute of Statistics, Spain received in 2018 more than 82 million of tourists, with almost 20% from countries outside Europe.26 Considering AENA's arrivals by plane, the annual average from 2014 to 2017 of travelers from endemic countries was 5.3 million. Based on the number of travelers from endemic countries arriving to Europe,6 indicated that southern Europe is a zone at risk of autochthonous dengue outbreaks, as it has been probed with the above mentioned outbreaks.
Other studies have also investigated the spatial distribution of Ae. albopictus and dengue in Europe and worldwide. Cunze et al. presented risk maps for the expansion of Ae. albopictus in Europe based on the suitability of the habitat and the environmental limiting factor for its establishment. Using a maximum entropy model with environmental conditions such as the ones used in our work, they found that Spain and in particular the Levantine coast had high suitability for the expansion of Ae. albopictus.21 Leta et al. employed species distribution models with environmental conditions and disease distribution to produce worldwide maps indicating the territories suitable for Ae. albopictus and Ae. aegypti. In these maps, Spain showed a relative low suitability for both species in a worldwide context.14 Liebig et al. a global model was built to predict the risk of indigenous dengue cases based on the arrivals of travelers from endemic countries. According to the findings of this study, Spain appears among the countries with high numbers of estimated imported cases, although below Italy and France.27
The first autochthonous cases of dengue in our country occurred in 2018 and were attributed to Ae. albopictus. The autochthonous cases had been in Andalusia, Murcia and Catalonia at the estimated time of transmission.28 In 2019, another autochthonous case was declared in Catalonia.29 These events seem to be consistent with our findings; the dengue risk map showed a medium risk in Murcia while municipalities in Catalonia characterized as high risk or very high risk. Early identification of areas at risk for the appearance of dengue cases can alert local authorities to stablish prevention and control measures, but also clinicians and public health authorities related to surveillance and alert and respond systems to develop more targeted plans by stratifying risk areas and prioritize actions. Among them, surveillance, prevention and control of vectors should be included as an important node on health interventions in the integrated management of vector–borne diseases. Such plans would not only mitigate the potential risk of dengue but also of other severe diseases transmitted by this invasive mosquito.
The findings of this study are subject to some limitations. Firstly, the centroid of the municipality was used instead of the exact coordinates of the mosquito trap. This may have affected the Ae. albopictus risk distribution risk model since environmental conditions can vary even within a municipality. Although this could be the case, the analysis and production of maps was conducted at the municipal level, minimizing possible environmental differences. The model used for the Ae. albopictus expansion risk map was based on current environmental conditions and did not take into account climate change which may affect both meteorological conditions and vector distribution. Mosquitoes have already been detected in areas in which they were not previously found. Other limitations concern the IDVZE calculation. The travelers’ information was analyzed at the provincial level, since the municipality was unknown and countries were defined as endemic as a whole, i.e. the entire territory was considered endemic without distinguishing endemic zones in the country and grading the risk accordingly.
The results of this analysis can guide public health measures for the surveillance and control of dengue and the proper allocation of resources, to prevent autochthonous dengue cases and outbreaks in our country, once travel restrictions will be eased.
ConclusionThis is the first work that provides a dengue risk assessment tool for Spain, based on a spatial approach. Several areas in the Mediterranean coast of Spain are at a higher risk of autochthonous dengue, based on environmental conditions, the expansion of Ae. albopictus, the number of imported cases of dengue and the number of travelers coming from endemic countries. The risk map can inform public health and local authorities in order to stratify risk and planning actions aimed at controlling the disease and guide the proper allocation of resources. Measures to mitigate the risk of local dengue transmission and prevent future dengue outbreaks should be prioritized for municipalities in these areas.
Authors’ contributionsBeatriz Fernandez-Martinez: conceptualization; data curation; formal analysis; methodology; writing – review & editing.
Despina Pampaka: writing – original draft; writing – review & editing.
Pablo Suarez-Sanchez: formal analysis; methodology.
Josué Martinez de la Puente: writing – review & editing.
Jordi Figuerola: writing – review & editing.
Maria Jose Sierra: writing – review & editing.
Inmaculada León-Gomez: formal analysis; methodology; writing – review & editing.
Javu¡ier del Aguila: review & editing.
Diana Gómez Barroso: conceptualization; methodology; project administration; resources; supervision; writing – original draft; writing – review & editing.
FundingThis study was supported by project PI18/00850, funded by Instituto de Salud Carlos III and cofunded by European Union (ERDF7ESF, “Investing in your future”).
Conflicts of interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
We would like to thank all professionals involved in data collection and manage, on which this study relies, particularly from RENAVE (clinicians, coders, administrative staff, computer scientists, epidemiologists, microbiologists) and the Ministry of Health and entomologists supporting data on vectors.
Supplementary material associated with this article can be found in the online version available at doi:10.1016/j.eimce.2023.06.010.