We analysed the changes in the susceptibility of Pseudomonas aeruginosa to antimicrobials over an 18-year period (2000-2017) in order to evaluate the adequacy of the antimicrobial therapy against this organism in patients admitted in a tertiary Spanish hospital (excluding the intensive care unit). In addition, the antimicrobial activity was evaluated using pharmacokinetic/pharmacodynamic (PK/PD) criteria as a microbiological surveillance tool.
MethodsSusceptibility was studied according to the Clinical and Laboratory Standards Institute breakpoints. Monte Carlo simulations were conducted to calculate the cumulative fraction of response (CFR). Linear regression analysis was applied to determine the trends in susceptibility and in the CFR.
ResultsIn 2017, susceptibility rates were: amikacin, penicillins and cephalosporins ≥85%, tobramycin 76%, meropenem 75% and gentamicin, imipenem and fluoroquinolones <70%. PK/PD analyses was able to identify changes in antimicrobial activity not detected by only assessing MICs; meropenem administered in extended infusion attained a CFR >90%, ceftazidime, piperacillin/tazobactam and imipenem provided CFRs between 80-90%, all of them administered at the highest doses.
ConclusionsAnalysis of susceptibility and PK/PD modelling, should be considered together to select the most appropriate antimicrobial drug and dosage regimen. Empirical antipseudomonal therapy would vary considerably if both microbiological surveillance tools were considered. In this study, the PK/PD analysis made it possible to preserve the therapeutic value of antimicrobials with low susceptibility rates, such as carbapenems, and the selection of the most effective antimicrobials among those with high rates of susceptibility.
Para evaluar la terapia antimicrobiana frente a Pseudomonas aeruginosa (P. aeruginosa) en pacientes ingresados en un hospital terciario español (excluida Unidad de Cuidados Intensivos), se analizaron los cambios en la sensibilidad a los antimicrobianos durante 18 años (2000-2017). También se evaluó la actividad antimicrobiana utilizando criterios farmacocinéticos/farmacodinámicos (PK/PD) como herramienta de vigilancia microbiológica.
MétodosLa sensibilidad se estudió utilizando los puntos de corte del CLSI. Se realizaron simulaciones de Monte Carlo para calcular la fracción de respuesta acumulada (CFR). Se llevó a cabo un análisis de tendencia de sensibilidad y CFR mediante regresión lineal.
ResultadosEn 2017, la sensibilidad a amikacina, penicilinas y cefalosporinas fue ≥ 85%; tobramicina 76%, meropenem 75% y para gentamicina, imipenem y fluoroquinolonas <70%. El análisis PK/PD fue capaz de identificar cambios en la actividad antimicrobiana no detectados mediante la evaluación únicamente de las concentraciones mínimas inhibitorias; meropenem administrado en forma de infusión extendida alcanzó una CFR> 90%, ceftazidima, piperacilina/tazobactam e imipenem proporcionaron CFR entre 80 y 90%, todos ellos administrados a las dosis más altas.
ConclusiónLa evaluación de la sensibilidad y el análisis PK/PD deben considerarse conjuntamente para seleccionar el tratamiento antimicrobiano más apropiado: fármaco y régimen de dosificación. La terapia empírica frente a P. aeruginosa variaría considerablemente si se consideraran ambas herramientas de vigilancia microbiológica. En este estudio, el análisis PK/PD ha permitido preservar el valor terapéutico de antimicrobianos con bajos valores de sensibilidad, como los carbapenems, y la selección de los antimicrobianos de mayor eficacia, entre aquellos que presentaban altos valores de sensibilidad.
According to the National Healthcare Safety Network, Pseudomonas aeruginosa is the fifth most common cause of hospital-acquired infections,1 it is a ubiquitous Gram-negative microorganism associated with a high mortality. In fact, P. aeruginosa is included in the group ESKAPE, acronym introduced by Rice2 in 2008 to designate a group of bacteria who escape the lethal action of antibiotics: Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, P. aeruginosa and Enterobacter species. These bacteria are increasingly prevalent in our hospitals and increasingly resistant to many of the antimicrobial agents,3 which is a serious health problem that needs to be addressed urgently.
Alert to antimicrobial resistance crisis, the May 2015 World Health Assembly adopted a global action plan on this issue.4 The main goal of this plan is to ensure, for as long as possible, continuity of successful treatment and prevention of infectious diseases with effective and safe medicines that are quality-assured, used in a responsible way, and accessible to all who need them.
The therapeutic approaches against P. aeruginosa infections are particularly challenging due to its intrinsically resistance to the majority of antimicrobial agents, and its ability to become resistant in the course of the antibiotic treatment, which greatly complicates the selection of appropriate treatment, and subsequently, increases the morbidity and mortality.5 In this sense, the inadequate empirical therapy, suboptimal dosing and delays in the initiation of appropriate treatment are associated with high mortality rates and contribute to an increased length of hospital stay.6
Surveillance has been recognized as a fundamental component in the control of organisms with resistance to antimicrobial agents. Surveillance of antimicrobial susceptibility enables the assessment of the burden of disease, determination of risk factors, and identification of temporal trends in occurrence and resistance patterns of infectious diseases. Such information may be used to establish empirical antimicrobial therapy recommendations,7 although it would imply that clinicians should be familiarized with the local epidemiologic surveillance programs to choose wisely the most appropriate empirical therapy against pseudomonal infections.6
Another useful tool to guide antipseudomonal therapy is the pharmacokinetic/pharmacodynamic (PK/PD) analysis with Monte Carlo simulation, which provides a reasonable prediction of the probability of success for a treatment, incorporating the variability of the pharmacokinetic parameters and the bacterial population MIC distribution (local MIC distributions).6,8 In a recent surveillance study carried out with P. aeruginosa isolates from an intensive care unit (ICU), we concluded that both, susceptibility rates and the success probability associated to the activity of the antimicrobial agent are complementary tools, and they have to be considered together to optimize the antimicrobial dose regimen for clinical making-decisions.9
Therefore, considering the alarming increase of P. aeruginosa resistance against several antimicrobials and the consequent loss of treatment options that this fact entails, the aim of the present study was to evaluate the adequacy of the antimicrobial therapy against P. aeruginosa, in admitted patients in a tertiary Spanish Hospital, excluding those at Intensive Care Units (ICU). With this goal in mind, firstly, the changes in the susceptibility of P. aeruginosa strains to the antimicrobials in an 18 years period (2000–2017) were analyzed. Moreover, the therapy success probability over the 18 years was also evaluated by applying a PK/PD modeling approach as a microbiological surveillance tool, by using PK/PD indexes as surrogate markers of efficacy.
MethodsMicrobiological data acquisition and calculation of susceptibilityThe susceptibility of P. aeruginosa against amikacin, cefepime, ceftazidime, ciprofloxacin, gentamicin, levofloxacin, imipenem, meropenem, piperacillin/tazobactam and tobramycin was studied. MIC distributions corresponding to clinical isolates collected from the admitted patients at the University Hospital of Araba (HUA), for every antimicrobial agent were extracted from the hospital database. Data were collected from 2000 to 2017, excluding those from ICUs.
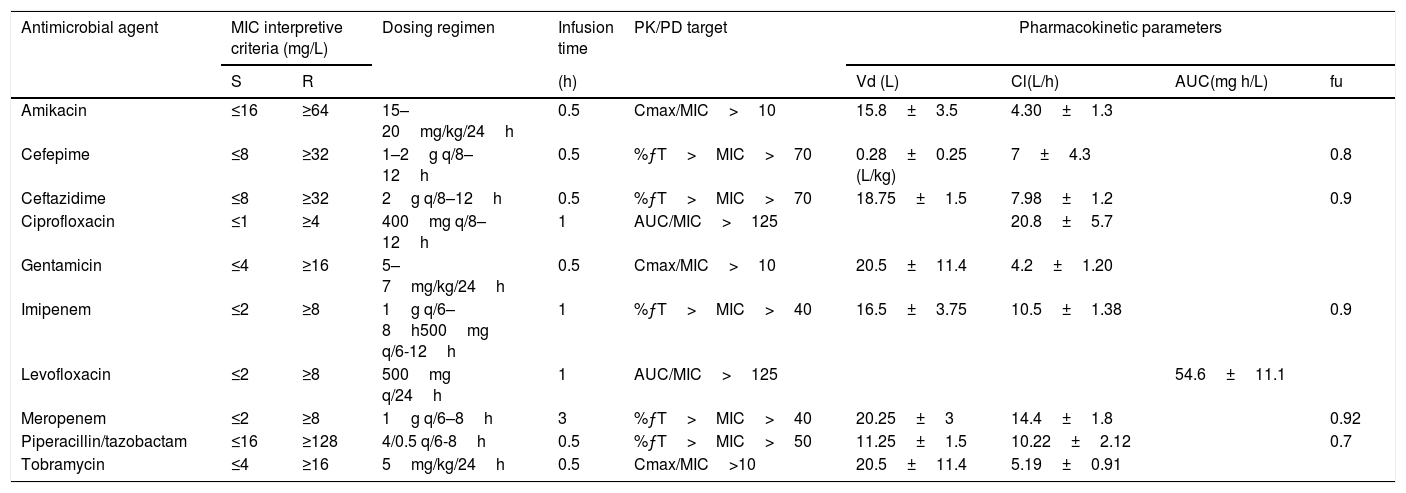
The percentage of susceptible strains was estimated considering the CLSI breakpoints10 (Table 1), and following the methodology recommended by the CLSI guideline,19 that is, considering only the first isolate per patient, per analysis period, irrespective of body site, antimicrobial susceptibility profile, or other phenotypical characteristics and including only species for which there are 30 or more isolates.
| Antimicrobial agent | MIC interpretive criteria (mg/L) | Dosing regimen | Infusion time | PK/PD target | Pharmacokinetic parameters | ||||
|---|---|---|---|---|---|---|---|---|---|
| S | R | (h) | Vd (L) | Cl(L/h) | AUC(mg h/L) | fu | |||
| Amikacin | ≤16 | ≥64 | 15–20mg/kg/24h | 0.5 | Cmax/MIC>10 | 15.8±3.5 | 4.30±1.3 | ||
| Cefepime | ≤8 | ≥32 | 1–2g q/8–12h | 0.5 | %ƒT>MIC>70 | 0.28±0.25 (L/kg) | 7±4.3 | 0.8 | |
| Ceftazidime | ≤8 | ≥32 | 2g q/8–12h | 0.5 | %ƒT>MIC>70 | 18.75±1.5 | 7.98±1.2 | 0.9 | |
| Ciprofloxacin | ≤1 | ≥4 | 400mg q/8–12h | 1 | AUC/MIC>125 | 20.8±5.7 | |||
| Gentamicin | ≤4 | ≥16 | 5–7mg/kg/24h | 0.5 | Cmax/MIC>10 | 20.5±11.4 | 4.2±1.20 | ||
| Imipenem | ≤2 | ≥8 | 1g q/6–8h500mg q/6-12h | 1 | %ƒT>MIC>40 | 16.5±3.75 | 10.5±1.38 | 0.9 | |
| Levofloxacin | ≤2 | ≥8 | 500mg q/24h | 1 | AUC/MIC>125 | 54.6±11.1 | |||
| Meropenem | ≤2 | ≥8 | 1g q/6–8h | 3 | %ƒT>MIC>40 | 20.25±3 | 14.4±1.8 | 0.92 | |
| Piperacillin/tazobactam | ≤16 | ≥128 | 4/0.5 q/6-8h | 0.5 | %ƒT>MIC>50 | 11.25±1.5 | 10.22±2.12 | 0.7 | |
| Tobramycin | ≤4 | ≥16 | 5mg/kg/24h | 0.5 | Cmax/MIC>10 | 20.5±11.4 | 5.19±0.91 | ||
S: Susceptible; R: Resistant; %ƒT>MIC: Percentage of time that the antimicrobial free serum concentration remained above the MIC; AUC: Area under the concentration-time curve; Cmax: Maximum drug plasma concentration; MIC: Minimum Inhibitory Concentration; Vd: Volume of distribution, Cl: clearance; AUC: Area Under the Curve, fu: unbound drug fraction.
The susceptibility data of clinical isolates was analyzed with the WHONET software, version 5.6.
Pharmacokinetic dataPharmacokinetic parameters were obtained from published studies. Prospective studies performed in patients with infections providing the PK parameters and variability were selected. Data from patients in critically ill units were excluded. The PK parameters of all antimicrobials used are shown in Table 1.
PK/PD analysis and Monte Carlo simulationA 10,000 subject Monte Carlo simulation was conducted, with the Oracle® Crystal Ball software, for each antimicrobial and dosing regimen using the PK data from published models (Table 1). The magnitude of value of the PK/PD indexes used as surrogate markers of efficacy for each antimicrobial are also shown in Table 1. The best PK/PD index correlated with the efficacy for all betalactams used is the duration of time that active antimicrobial concentrations exceed the MICs, this time-dependent index is expressed as the percentage of the dosing interval and only the fraction of drug not bound to proteins is considered (%ƒT>MIC). Aminoglycosides and fluoroquinolones present concentration-time dependent bactericide action, and therefore, the PK/PD indexes used have been Cmax/MIC and AUC/MIC, respectively.
The probability that a specific value of a PK/PD index associated with the efficacy of the antimicrobial treatment is achieved at a specific population of microorganisms is known as the cumulative fraction of response (CFR).20 It allowed us to calculate the probability of success for a treatment without knowledge of the susceptibility of the specific isolate responsible for the infection, but taking into account the bacterial population MIC distribution. A CFR ≥80% but <90% was associated with moderate probabilities of success, whereas a CFR ≥90% was considered as optimal against that bacterial population.21
Statistical analysisThe percentage of susceptible strains and the probability that PK/PD indices reach the target over time, were calculated over a 18 years period. The annual rates were compared by linear regression for trends. All statistical analyses were performed with IBM® SPSS®, Statistics for Windows, Version 24 (IBM). According to Friedrich et al., 22 an appropriate degree of fit was considered with a coefficient of determination (r2) of at least 0.5 (corresponding to a correlation coefficient of ≥0.7). A p value <0.05 was considered statistically significant.
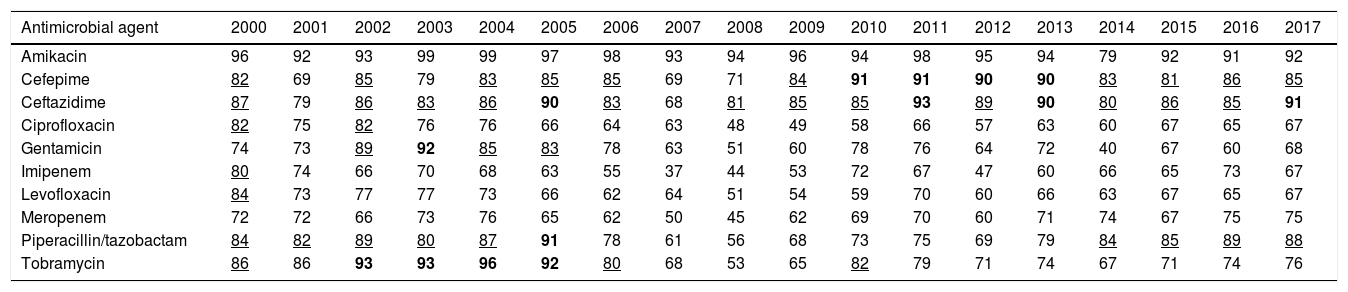
ResultsTable 2 features the antimicrobial year-by-year susceptibility to amikacin, cefepime, ceftazidime, ciprofloxacin, gentamicin, imipenem, levofloxacin, meropenem, piperacillin/tazobactam and tobramycin of P. aeruginosa isolates from the hospitalized patients. As it is shown, the last year evaluated (2017) P. aeruginosa displayed a susceptibility to amikacin, penicillins and cephalosporins equal or higher than 85%; for tobramycin and meropenem, it was 76% and 75%, respectively. Susceptibility to the other antimicrobials was under 70%.
Percentage of P. aeruginosa susceptible strains from 2000 to 2017.
| Antimicrobial agent | 2000 | 2001 | 2002 | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amikacin | 96 | 92 | 93 | 99 | 99 | 97 | 98 | 93 | 94 | 96 | 94 | 98 | 95 | 94 | 79 | 92 | 91 | 92 |
| Cefepime | 82 | 69 | 85 | 79 | 83 | 85 | 85 | 69 | 71 | 84 | 91 | 91 | 90 | 90 | 83 | 81 | 86 | 85 |
| Ceftazidime | 87 | 79 | 86 | 83 | 86 | 90 | 83 | 68 | 81 | 85 | 85 | 93 | 89 | 90 | 80 | 86 | 85 | 91 |
| Ciprofloxacin | 82 | 75 | 82 | 76 | 76 | 66 | 64 | 63 | 48 | 49 | 58 | 66 | 57 | 63 | 60 | 67 | 65 | 67 |
| Gentamicin | 74 | 73 | 89 | 92 | 85 | 83 | 78 | 63 | 51 | 60 | 78 | 76 | 64 | 72 | 40 | 67 | 60 | 68 |
| Imipenem | 80 | 74 | 66 | 70 | 68 | 63 | 55 | 37 | 44 | 53 | 72 | 67 | 47 | 60 | 66 | 65 | 73 | 67 |
| Levofloxacin | 84 | 73 | 77 | 77 | 73 | 66 | 62 | 64 | 51 | 54 | 59 | 70 | 60 | 66 | 63 | 67 | 65 | 67 |
| Meropenem | 72 | 72 | 66 | 73 | 76 | 65 | 62 | 50 | 45 | 62 | 69 | 70 | 60 | 71 | 74 | 67 | 75 | 75 |
| Piperacillin/tazobactam | 84 | 82 | 89 | 80 | 87 | 91 | 78 | 61 | 56 | 68 | 73 | 75 | 69 | 79 | 84 | 85 | 89 | 88 |
| Tobramycin | 86 | 86 | 93 | 93 | 96 | 92 | 80 | 68 | 53 | 65 | 82 | 79 | 71 | 74 | 67 | 71 | 74 | 76 |
In bold: susceptibility ≥90%; Underlined: susceptibility ≥80% and <90%.
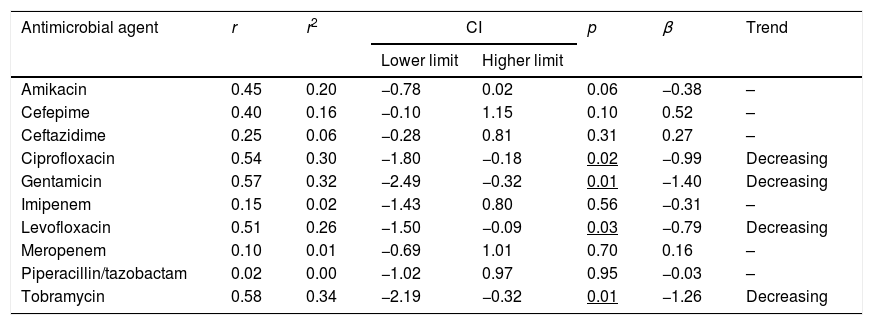
The trend analyses of antimicrobial susceptibility of P. aeruginosa over time are summarized in Table 3. The susceptibility rates to amikacin and betalactams were stable during the evaluated period. In contrast, the susceptibility to quinolones, gentamicin and tobramycin decreased significantly over time.
Trends in susceptibility rates of P. aeruginosa from 2000 to 2017.
| Antimicrobial agent | r | r2 | CI | p | β | Trend | |
|---|---|---|---|---|---|---|---|
| Lower limit | Higher limit | ||||||
| Amikacin | 0.45 | 0.20 | −0.78 | 0.02 | 0.06 | −0.38 | – |
| Cefepime | 0.40 | 0.16 | −0.10 | 1.15 | 0.10 | 0.52 | – |
| Ceftazidime | 0.25 | 0.06 | −0.28 | 0.81 | 0.31 | 0.27 | – |
| Ciprofloxacin | 0.54 | 0.30 | −1.80 | −0.18 | 0.02 | −0.99 | Decreasing |
| Gentamicin | 0.57 | 0.32 | −2.49 | −0.32 | 0.01 | −1.40 | Decreasing |
| Imipenem | 0.15 | 0.02 | −1.43 | 0.80 | 0.56 | −0.31 | – |
| Levofloxacin | 0.51 | 0.26 | −1.50 | −0.09 | 0.03 | −0.79 | Decreasing |
| Meropenem | 0.10 | 0.01 | −0.69 | 1.01 | 0.70 | 0.16 | – |
| Piperacillin/tazobactam | 0.02 | 0.00 | −1.02 | 0.97 | 0.95 | −0.03 | – |
| Tobramycin | 0.58 | 0.34 | −2.19 | −0.32 | 0.01 | −1.26 | Decreasing |
r: correlation coefficient; r2: coefficient of determination; CI: confidence interval; β: slope.
In bold: r2≥0.49; Underlined p<0.05.
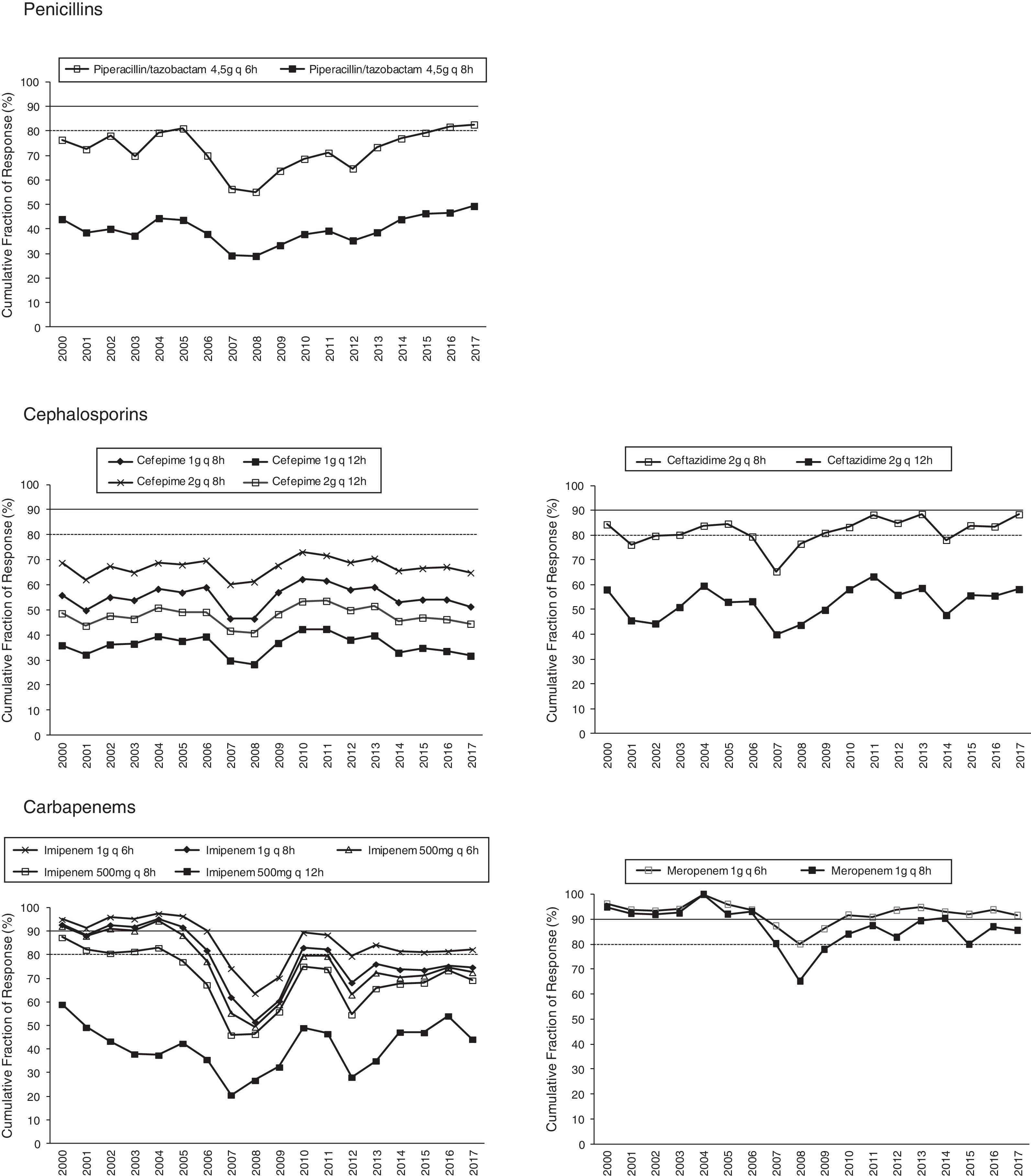
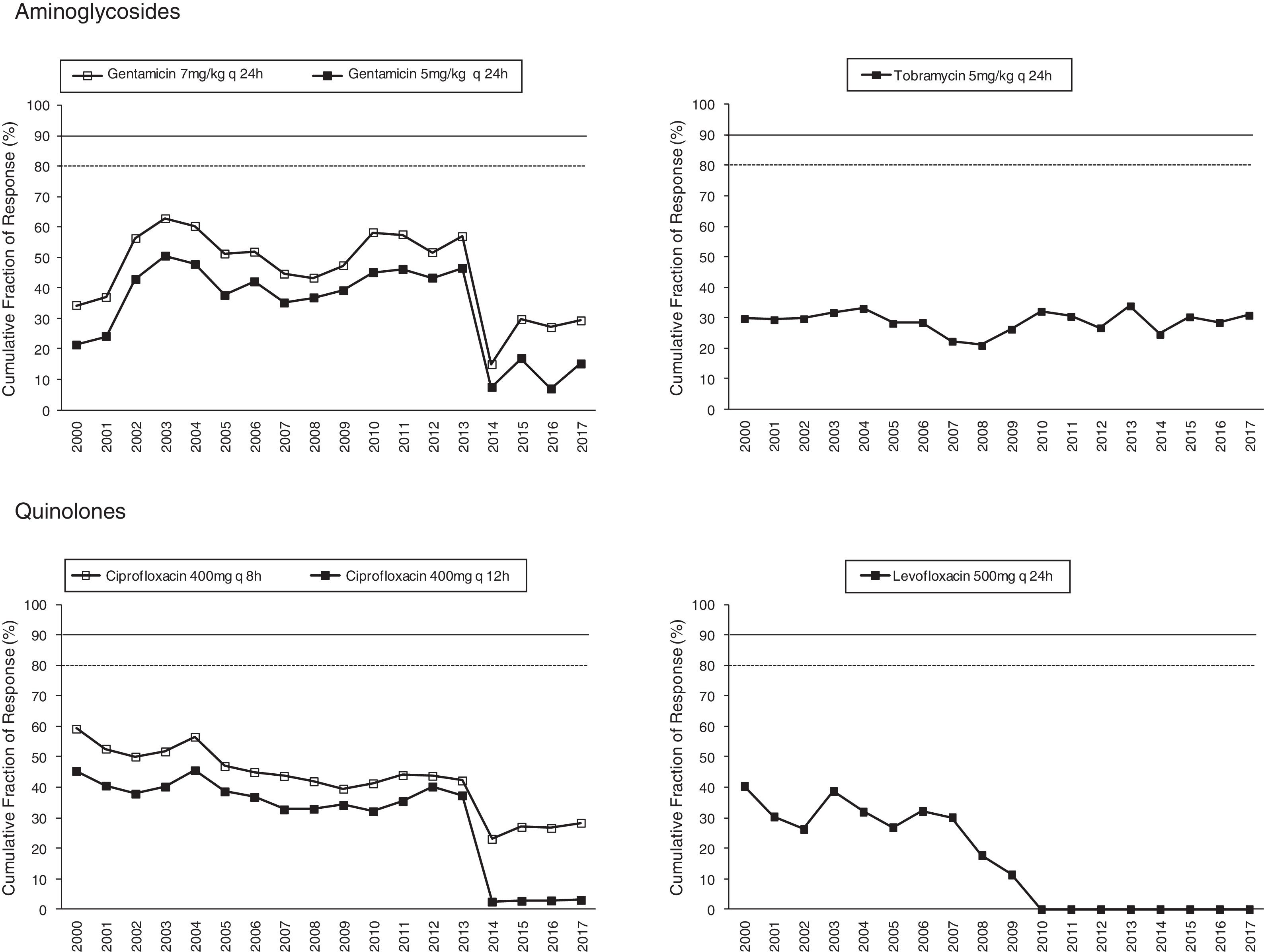
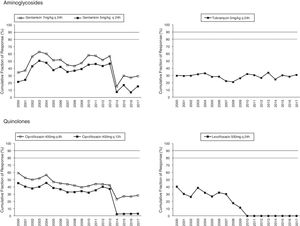
Figs. 1 and 2 provide an overview of the probability of PK/PD target attainment according to MIC distributions (CFR values) for the antimicrobials studied at the selected dosing regimens. In summary, in 2017, the last year evaluated (Fig. 1), only meropenem 1g every 6h (q6h) administered as extended infusion was able to attain CFR >90% (92%). CFRs between 80–90% were attained with ceftazidime 2g q8h (88%), piperacillin/tazobactam 4.5g q6h (83%), meropenem 1g q8h (86%), and imipenem 1g q6h (82%). The CFRs for the other treatments evaluated were always lower than 80%. Fluoroquinolones and aminoglycosides (Fig. 2) achieved the lowest CFR values (<35%). Supplementary Table 1 shows the CFR values obtained annually of each antimicrobial evaluated. CFR values for amikacin have not been included because when antibiograms were carried out, amikacin concentrations tested ranged from 4mg/L to 32mg/L and, therefore, MICs lower than 4mg/L were not available and the CFR could not be adequately calculated.
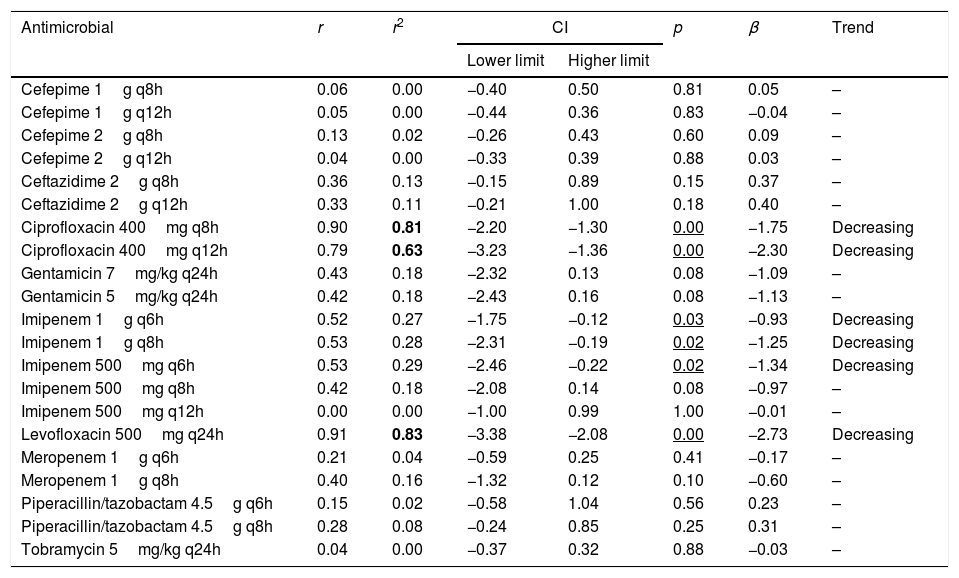
Table 4 presents the linear regression studies for CFR values. A statistically significant trend over time was observed for fluoroquinolones and imipenem. In the case of levofloxacin and ciprofloxacin, a significant decrease was observed in CFR values from 40–59% in 2000 to 0–29% in 2017, depending on the dose but the activity was low from the start of the evaluated period. Imipenem showed a decrease in antimicrobial activity in all dosing regimens except for 500mg q12h, for which CFR values were always under 70%; for 1g q6h antimicrobial activity decreased from 95% to 82%, maintaining moderate activity. For other regimens, CFR values decreased from high probabilities to target attainment (>90%) to low probabilities (CFR<70%).
Linear regression results for CFR.
| Antimicrobial | r | r2 | CI | p | β | Trend | |
|---|---|---|---|---|---|---|---|
| Lower limit | Higher limit | ||||||
| Cefepime 1g q8h | 0.06 | 0.00 | −0.40 | 0.50 | 0.81 | 0.05 | – |
| Cefepime 1g q12h | 0.05 | 0.00 | −0.44 | 0.36 | 0.83 | −0.04 | – |
| Cefepime 2g q8h | 0.13 | 0.02 | −0.26 | 0.43 | 0.60 | 0.09 | – |
| Cefepime 2g q12h | 0.04 | 0.00 | −0.33 | 0.39 | 0.88 | 0.03 | – |
| Ceftazidime 2g q8h | 0.36 | 0.13 | −0.15 | 0.89 | 0.15 | 0.37 | – |
| Ceftazidime 2g q12h | 0.33 | 0.11 | −0.21 | 1.00 | 0.18 | 0.40 | – |
| Ciprofloxacin 400mg q8h | 0.90 | 0.81 | −2.20 | −1.30 | 0.00 | −1.75 | Decreasing |
| Ciprofloxacin 400mg q12h | 0.79 | 0.63 | −3.23 | −1.36 | 0.00 | −2.30 | Decreasing |
| Gentamicin 7mg/kg q24h | 0.43 | 0.18 | −2.32 | 0.13 | 0.08 | −1.09 | – |
| Gentamicin 5mg/kg q24h | 0.42 | 0.18 | −2.43 | 0.16 | 0.08 | −1.13 | – |
| Imipenem 1g q6h | 0.52 | 0.27 | −1.75 | −0.12 | 0.03 | −0.93 | Decreasing |
| Imipenem 1g q8h | 0.53 | 0.28 | −2.31 | −0.19 | 0.02 | −1.25 | Decreasing |
| Imipenem 500mg q6h | 0.53 | 0.29 | −2.46 | −0.22 | 0.02 | −1.34 | Decreasing |
| Imipenem 500mg q8h | 0.42 | 0.18 | −2.08 | 0.14 | 0.08 | −0.97 | – |
| Imipenem 500mg q12h | 0.00 | 0.00 | −1.00 | 0.99 | 1.00 | −0.01 | – |
| Levofloxacin 500mg q24h | 0.91 | 0.83 | −3.38 | −2.08 | 0.00 | −2.73 | Decreasing |
| Meropenem 1g q6h | 0.21 | 0.04 | −0.59 | 0.25 | 0.41 | −0.17 | – |
| Meropenem 1g q8h | 0.40 | 0.16 | −1.32 | 0.12 | 0.10 | −0.60 | – |
| Piperacillin/tazobactam 4.5g q6h | 0.15 | 0.02 | −0.58 | 1.04 | 0.56 | 0.23 | – |
| Piperacillin/tazobactam 4.5g q8h | 0.28 | 0.08 | −0.24 | 0.85 | 0.25 | 0.31 | – |
| Tobramycin 5mg/kg q24h | 0.04 | 0.00 | −0.37 | 0.32 | 0.88 | −0.03 | – |
r: correlation coefficient; r2: coefficient of determination; CI: confidence interval; β: slope.
In bold r2≥0.49; Underlined p<0.05.
Two microbiological surveillance tools have been used to evaluate the antimicrobial therapy against P. aeruginosa: (i) the analysis of susceptibility changes of the antimicrobials over time, and (ii) the analysis of the activity of the empirical antipseudomonal treatments by using a PK/PD modeling approach. After the evaluation over 18 years of the adequacy of the therapy, differences in the expected efficacy of the antimicrobials, in terms of susceptibilities or CFRs, were observed depending on the tool used.
P. aeruginosa is intrinsically resistant to several antimicrobial agents. Recently, Mensa et al.23 have published the estimated prevalence of this bacteria in Spanish hospitals, and overall, resistance rates are over 20–30% for most antipseudomonal antimicrobials, except for amikacin, colistin and the recently introduced ceftolozane-tazobactam with values over 5%. In our study, in 2017 only amikacin and the betalactams ceftazidime, cefepime and piperacillin/tazobactam, showed susceptibilities higher than 85% (Table 2). Over the 18 years evaluated, a decrease in the susceptibility was observed only for gentamicin, tobramycin, and fluoroquinolones (Table 3); but this reduction showed a poor relationship between both variables (r2<0.5) susceptibility and time, despite statistically significant (p<0.05).
Different national action plans on antimicrobial resistance, which have been implemented in the HUA hospital to control the emergence of resistances, have probably influenced substantially the obtained results, that is, the susceptibility rates and their evolution over the time. Bacteremia Zero24 project (2009) and “Zero-VAP” bundle25 (2011), consisting of the implantation of measures to prevent central venous catheter-related bacteremia and a bundle of ventilator-associated pneumonia (VAP) prevention measures, respectively. Although these programs have been implemented in the ICU, they can have an important role in the prevention of the intra-hospital dissemination of resistances. Programs for optimizing the use of antibiotics in hospitals (called PROA), implemented in the HUA hospital since 2015, are promoted by the Ministry of Health, Social Services and Equality of Spain as a Strategic and Action Plan to Reduce the Risk of Selection and Dissemination of Resistance to Antibiotics 2014–2018.26 The main objectives27 of these programs are (i) to improve the clinical results of patients with infections, (ii) to minimize the adverse effects associated with the use of antimicrobials (including the appearance and dissemination of the resistance), and (iii) to ensure the use of cost-effective treatments.
PK/PD analysis is outlined as a needed strategy to wisely optimize dosing regimens of the antimicrobial agents in order to conserve their therapeutic value,8 moreover, its incorporation to clinical routine would contribute to reach the main objectives of the surveillance programs.
In this study, Monte Carlo simulation, pharmacokinetic modeling and institution-specific MIC determination have been used to evaluate antimicrobial dosing regimen for the empirical treatment of P. aeruginosa. It is important to remark that the expected probability of success estimated by applying Monte Carlo simulation for the evaluated antimicrobials do not match their susceptibilities. Meropenem (susceptibility of 75%) administered as 1g q6 or 8h was able to attain high and moderate probabilities of success (92% and 86%, respectively). Ceftazidime and piperacillin/tazobactam (susceptibilities of 91 and 88%, respectively), administered at the highest dose, showed only moderate probabilities to attain the PK/PD target, and cefepime, despite its high susceptibility (85%) provided CFRs under 70% for all dosage regimens evaluated. On the contrary and surprisingly, imipenem at highest dose was able to achieve moderate probability of success (CFR 82%) with only a 67% of susceptible isolates.
In the case of cefepime there is a controversy on its susceptibility breakpoint that could explain the observed differences. According to the CLSI criteria,19 the cefepime MIC breakpoint was ≤8mg/L. However, Bhat et al.28 based on pharmacodynamic and clinical grounds, have suggested to lower the breakpoints for cefepime in countries where the cefepime dosage of 1 to 2g every 12h is the licensed therapy for serious infections, so that organisms with a cefepime MIC of 8μg/ml should be no longer regarded as susceptible to the antibiotic. More recently, Sue et al.29 also demonstrated worse outcomes related to high cefepime MICs for P. aeruginosa, despite being “susceptible” in the currently range, and proposed that the current CLSI criteria for cefepime susceptibility did not predict clinical outcomes appropriately and that the breakpoint of 8mg/L is too high. In our study, if we calculate the susceptibility rate of P. aureginosa against cefepime considering 8mg/L as resistant, the susceptibility rate decreases (for instance, in 2017 from 85% to 53%), and in this case, CFR values would match better their susceptibilities.
The concentration-dependent antimicrobials, fluoroquinolones, gentamicin and tobramycin, showed CFRs under 35%, although susceptibilities ranged from 67 to 76%. Unfortunately, it has not been possible to calculate CFR values for amikacin, because of MIC concentrations tested in the hospital only ranged from 4mg/L to 32mg/L (susceptibility breakpoint MIC ≤16mg/L). This concentration range is adequate to categorize the strains as susceptible or resistant, but it is not useful to estimate the CFR properly, whose value depends on the knowledge of the MIC values corresponding to a wide distribution.
Estimation of CFR is also useful to determine not only which antimicrobial, but also the dose regimen with the best likelihood of success. An increase of the antimicrobial doses not always implies relevant changes in CFR values, for example, increases in ciprofloxacin dose did not significantly improve the probability of target attainment (400mg q12h and 400mg q8h, CFR 3–28% respectively); on the contrary, for piperacillin/tazobactam CFR ranged between 49% (4.5g q6h8) to 83% (4.5g q6h). Moreover, considering the time-dependent activity of all betalactams, the efficacy probably would improve by administering them through extended or continuous infusion, as shown with meropenem, although stability should be considered, as in the case of imipenem, with poor stability at room temperature.
Fluoroquinolones, and also imipenem, showed a statistically significant decreasing trend in the values of CFR over the 18 years evaluated, however, only in the case of ciprofloxacin and levofloxacin the coefficient of determination r2 was higher than 0.5, indicating good correlation.
In order to evaluate properly the results obtained in this theoretical PK/PD analysis, some limitations must be considered. (i) PK information from the patients from whom P. aeruginosa was isolated was not available. Therefore, it was extracted from prospective studies carried out in patients with infections, excluding patients in ICUs and the PK/PD analysis was carried out by using the mean PK parameters and their variability; (ii) PK/PD analysis is conditioned by the place of infection since the CFR would vary according to location, preferably in the case of antibiotics with wide urinary excretion; (iii) colistin has not been evaluated because it is hardly used in the patients admitted at the HUA, with the exception of ICU patients (not included in the study). Additionally, colistin's MIC distribution has not been tested until recent years, and this antimicrobial does not have a widely accepted PK/PD index; (iv) extended infusion of beta-lactams has not been evaluated, except for meropenem; (v) in this study, only the CLSI breakpoints have been considered. EUCAST30 (European Committee on Antimicrobial Susceptibility Testing) and CLSI10 susceptibility breakpoints agree on six of the 10 antimicrobials studied, and the breakpoints of amikacin, imipenem, ciprofloxacin and levofloxacin differ only in one dilution. In 2017, amikacin presented a susceptibility value of 22 percentage points lower by using EUCAST criterion instead CLSI; the difference for the other three affected antimicrobials was less than 10 percentage points.
In brief, empirical antipseudomonal therapy would vary considerably if, in addition to susceptibility data, PK/PD analysis is considered. Based only on susceptibility, amikacin, ceftazidime, piperacillin/tazobactam but also cefepime would be the best therapeutic options. PK/PD analyses was able to identify changes in antimicrobial activity not detected by simply assessing MIC indices, meropenem provided high probabilities to achieve the PK/PD target, followed by ceftazidime, piperacillin/tazobactam and imipenem, with moderate probabilities, all of them administered at the highest doses. In conclusion, PK/PD approach has allowed to preserve the therapeutic value of antimicrobials with low susceptible values, such as carbapenems, and the selection of the most efficacy antimicrobials among those with high rates of sensible isolates. Both microbiological surveillance tools, analysis of susceptibility and PK/PD modeling, should be considering together in the clinical routine to determine the most appropriate antimicrobial drug and its dose regimen, contributing in this way to decrease the risk of treatment failure and resistances development.
Patients’ data protectionNot applicable.
Conflict of interestNothing to declare.
This work was supported by the UPV/EHU (PPG17/65, GIU17/032). A Valero thanks Universia Foundation for her grant.