Streptococcus pneumoniae causes serious diseases in the susceptible population. The 13-valent pneumococci conjugate vaccine (PCV13) was included in the children’s calendar in 2011. The objective of the study was to analyze the evolution of pneumococcal serotypes and their resistance after PCV13.
MethodsThis study included the pneumococci serotyped in Galicia in 2011–2021. Antibiotic susceptibility was analyzed following EUCAST criteria. The data was analyzed in 3 sub-periods: initial (2011–2013), middle (2014–2017) and final (2018–2021). The prevalence of serotypes and their percentage of resistance to the most representative antibiotics were calculated.
ResultsA total of 2.869 isolates were included. Initially, 42.7% isolates presented capsular types included in PCV13, compared to 15.4% at the end. Those included in PCV20 and not in PCV13 and PCV15 were 12.5% at baseline and 41.3% at the end; 26.4% of the isolates throughout the study had serotypes not included in any vaccine. The prevalence of serotype 8 multiplied almost by 8 and that of 12F tripled. The 19A serotype was initially the most resistant, while the resistance of serotypes 11A and 15A increased throughout the study.
ConclusionsThe introduction of PCV13 in the pediatric population determined a change in pneumococcal serotypes towards those included in PCV20 and those not included in any vaccine. Serotype 19A was initially the most resistant and the 15A, not included in any vaccine, deserves special follow-up. Serotype 8, which increased the most, did not show remarkable resistance.
Streptococcus pneumoniae causa enfermedades graves en la población susceptible. La vacuna neumocócica conjugada (PCV) 13-valente (PCV13) se incluyó en el calendario infantil en 2011. Este estudio analiza la evolución de los serotipos de neumococo y de sus resistencias tras la PCV13.
MétodosSe incluyeron los neumococos serotipados en Galicia en 2011-2021. Se estudió la sensibilidad antibiótica siguiendo criterios EUCAST. Se analizaron los datos en 3 subperíodos: inicial (2011-2013), medio (2014-2017) y final (2018-2021). Se calcularon las prevalencias de los serotipos y el porcentaje de resistencia a los antibióticos más representativos.
ResultadosSe incluyeron 2.869 aislados. Inicialmente el 42,7% presentaba tipos capsulares incluidos en la PCV13, frente al 15,4% al final. Los incluidos en la PCV20 y no en la PCV13 y PCV15 fueron el 12,5% inicialmente y el 41,3% al final. El 26,4% de los serotipos a lo largo del estudio no estaban incluidos en ninguna vacuna. La prevalencia del serotipo 8 se multiplicó casi por 8 y la del 12F se triplicó. El serotipo 19A fue el más resistente inicialmente. La resistencia de los serotipos 11A y 15A aumentó a lo largo del estudio.
ConclusionesLa introducción de la PCV13 en la población infantil determinó un cambio en los serotipos de neumococo hacia los incluidos en la PCV20 y los no incluidos en ninguna vacuna. El serotipo 19A inicialmente fue el más resistente, y el 15A, no incluido en ninguna vacuna, merece un especial seguimiento. El serotipo 8, que fue el que más se incrementó, no mostró resistencia destacable.
Streptococcus pneumoniae (pneumococcus) is a microorganism classified into different serotypes according to the antigenic properties of the polysaccharides of its external capsule.1,2 It causes mild infections (otitis, sinusitis or bronchitis) and severe invasive pneumococcal disease (IPD), such as bacteraemia, meningitis or pneumonia. In fact, pneumococcal pneumonia is considered one of the most prevalent serious diseases, both in developed and developing countries.3 This disease mainly affects children under two years of age, adults over 65 years of age and immunosuppressed people.
To prevent pneumococcal disease (PD), different vaccines have been developed, which have as many valences as there are serotypes against which they are directed: the 7-valent pneumococcal conjugate vaccine (PCV7), the 13-valent pneumococcal conjugate vaccine (PCV13), the 15-valent (PCV15) and 20-valent (PCV20) pneumococcal conjugate vaccines and the 23-valent pneumococcal polysaccharide vaccine (PPV23).
The technical agreement to start a pilot study evaluating the impact of the PCV13 vaccine on IPD led to the free introduction of this vaccine in the Galician childhood immunisation schedule in January 2011.4 The PCV13 vaccine was offered to all children born on or after 1 November 2010, and to all children born on or after 1 January 2010 who had started vaccination with PCV7. The regimen was three doses (at two, four and 12 months) and among those born between 2010 and 2015, the coverage achieved for the three doses was 93%. The study ended in 2015 and, after evaluating the impact of the vaccine on the incidence of IPD,5 it was decided to permanently include the PCV13 vaccine in the childhood immunisation schedule (February 2015) and also introduce it free of charge in the adult schedule (July 2017).6
IPD surveillance remains essential because the introduction of the vaccine induces changes in circulating serotypes, based on the indirect effect of vaccination.7,8 Recently, Houseman et al.9 published an article showing an increase in IPD associated with PPV23-only serotypes and non-vaccine serotypes, and highlighted the need for new research to verify whether this phenomenon was a specific or generalised result due to changes in the vaccination programme.
Furthermore, after vaccination with the PCV7, the prevalence of serotype 19A isolates increased, many of them multiresistant, and although serotype 19A is covered by the PCV13, a niche may be created for other serotypes. Several authors have identified an increase in the prevalence of serotypes 15A and 35B resistant to multiple antibiotics.10
The main objective of this study was to analyse the evolution of IPD due to PCV13 serotypes and non-PCV13 serotypes in Galicia, as well as to study the evolution of resistance to antibiotics in the strains of the serotypes identified.
MethodsThis study was conducted in Galicia, a region located in the north-west of Spain that in 2021 had 2,695,645 inhabitants.
All isolates of pneumococcus in invasive samples sent for serotyping to the reference laboratory of the autonomous community of Galicia at the Microbiology Department of the Hospital Universitario de Ferrol [Ferrol University Hospital] from 2011 to 2021 were included in the study. Serotyping was performed by latex agglutination and/or Quellung reaction11 (Statens Serum Institut, Copenhagen, Denmark). In addition, antibiotic susceptibility to penicillin, cefotaxime, vancomycin, erythromycin, levofloxacin, chloramphenicol, tetracycline, co-trimoxazole and clindamycin was studied. For the interpretation of the results, the 2022 EUCAST criteria were followed12 and the data were analysed when the antibiogram result was interpreted as resistant.
Serotyping was carried out with the support of Pfizer laboratories, and during the first few months, in order to validate the technique, the samples were sent to the National Centre for Microbiology.
The different serotypes were stratified for analysis into six groups based on their inclusion in the different vaccines: serotypes included in the PCV7 (4, 6B, 9V, 14, 18C, 19F and 23F), those included in the PCV13 that are not in the PCV7 (1, 3, 5, 6A, 7F and 19A), those included in the PCV15 that are not in the PCV13 (22F and 33F), those included in the PCV20 that are not in the PCV15 (8, 10A, 11A, 12F and 15B), those included in the PPV23 that are not in the PCV20 (2, 9N, 17F and 20) as well as serotypes that are not in any vaccine.
The age groups defined for the analyses were: patients younger than five years, age five to 44, 45–64, and 65 years and older. The study period was divided into three sub-periods: initial, between 2011 and 2013; middle, between 2014 and 2017 and final, between 2018 and 2021.
In each period, the prevalence of the serotypes and the percentage of resistance of the serotyped pneumococci to the different antibiotics were calculated.
The Stata v16.1 program was used to manage and analyse the data.
ResultsIn the period 2011–2021, 2869 strains of pneumococci isolated in invasive samples were sent to be serotyped from the different hospitals in Galicia (89.5% of these in blood samples, 5.4% in CSF, 2.3% in pleural fluid and 2.8% in other types of sample).
The annual number of isolates varied between 211 in 2014 and 293 in 2017 in the first two periods, but in 2018 and 2019 there were more than 350 isolates and in 2020 and 2021 they dropped to 172 and 107, respectively.
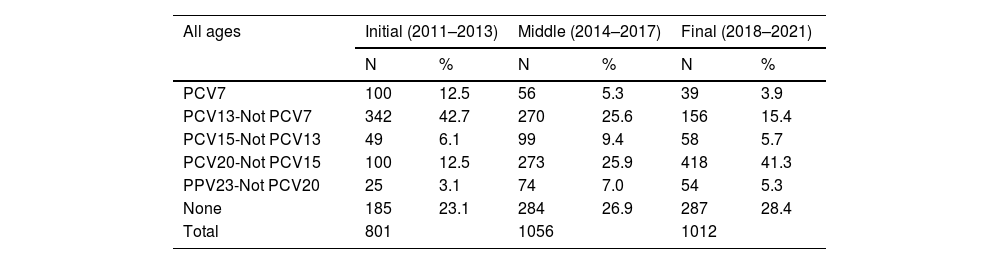
Table 1 shows the prevalence of the serotypes in Galicia between 2011 and 2021, by age group, for each of the three periods analysed. The serotypes included in the PCV13 went from 55.2% in the initial stage to 19.3% in the final stage, and the decrease was evident in all age groups. The serotypes included in the PCV20 vaccine and not included in the PCV13 or the PCV15 went from the initial 12.5%–41.3% in the final period, and the increase was also evident in all age groups. A total of 756 isolates (26.4%) were serotypes not included in any vaccine, and this percentage increased from 23.1% (n = 185) in the initial period to 28.4% (n = 287) in the final period.
Prevalence of typified pneumococcal serotypes in Galicia from 2011 to 2021, by period and age group.
| All ages | Initial (2011–2013) | Middle (2014–2017) | Final (2018–2021) | |||
|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |
| PCV7 | 100 | 12.5 | 56 | 5.3 | 39 | 3.9 |
| PCV13-Not PCV7 | 342 | 42.7 | 270 | 25.6 | 156 | 15.4 |
| PCV15-Not PCV13 | 49 | 6.1 | 99 | 9.4 | 58 | 5.7 |
| PCV20-Not PCV15 | 100 | 12.5 | 273 | 25.9 | 418 | 41.3 |
| PPV23-Not PCV20 | 25 | 3.1 | 74 | 7.0 | 54 | 5.3 |
| None | 185 | 23.1 | 284 | 26.9 | 287 | 28.4 |
| Total | 801 | 1056 | 1012 | |||
| 0–4 years old | N | % | N | % | N | % |
|---|---|---|---|---|---|---|
| PCV7 | 2 | 5.7 | 7 | 10.9 | 0 | 0.0 |
| PCV13-Not PCV7 | 16 | 45.7 | 10 | 15.6 | 2 | 5.3 |
| PCV15-Not PCV13 | 4 | 11.4 | 6 | 9.4 | 3 | 7.9 |
| PCV20-Not PCV15 | 2 | 5.7 | 16 | 25.0 | 14 | 36.8 |
| PPV23-Not PCV20 | 0 | 0.0 | 3 | 4.7 | 0 | 0.0 |
| None | 11 | 31.4 | 22 | 34.4 | 19 | 50.0 |
| Total | 35 | 64 | 38 |
| 5–44 years old | N | % | N | % | N | % |
|---|---|---|---|---|---|---|
| PCV7 | 14 | 12.3 | 9 | 5.6 | 5 | 3.7 |
| PCV13-Not PCV7 | 55 | 48.2 | 44 | 27.2 | 15 | 11.2 |
| PCV15-Not PCV13 | 6 | 5.3 | 14 | 8.6 | 2 | 1.5 |
| PCV20-Not PCV15 | 19 | 16.7 | 61 | 37.7 | 85 | 63.4 |
| PPV23-Not PCV20 | 8 | 7.0 | 11 | 6.8 | 3 | 2.2 |
| None | 12 | 10.5 | 23 | 14.2 | 24 | 17.9 |
| Total | 114 | 162 | 134 |
| 45–64 years old | N | % | N | % | N | % |
|---|---|---|---|---|---|---|
| PCV7 | 29 | 13.5 | 15 | 5.5 | 18 | 5.9 |
| PCV13-Not PCV7 | 93 | 43.3 | 70 | 25.5 | 31 | 10.2 |
| PCV15-Not PCV13 | 10 | 4.7 | 19 | 6.9 | 15 | 5.0 |
| PCV20-Not PCV15 | 31 | 14.4 | 84 | 30.5 | 150 | 49.5 |
| PPV23-Not PCV20 | 3 | 1.4 | 21 | 7.6 | 24 | 7.9 |
| None | 49 | 22.8 | 66 | 24.0 | 65 | 21.5 |
| Total | 215 | 275 | 303 |
| ≥65 years old | N | % | N | % | N | % |
|---|---|---|---|---|---|---|
| PCV7 | 55 | 12.6 | 25 | 4.5 | 16 | 3.0 |
| PCV13-Not PCV7 | 177 | 40.6 | 146 | 26.3 | 108 | 20.1 |
| PCV15-Not PCV13 | 29 | 6.7 | 60 | 10.8 | 38 | 7.1 |
| PCV20-Not PCV15 | 48 | 11.0 | 112 | 20.2 | 169 | 31.5 |
| PPV23-Not PCV20 | 14 | 3.2 | 39 | 7.0 | 27 | 5.0 |
| None | 113 | 25.9 | 173 | 31.2 | 178 | 33.2 |
| Total | 436 | 555 | 536 |
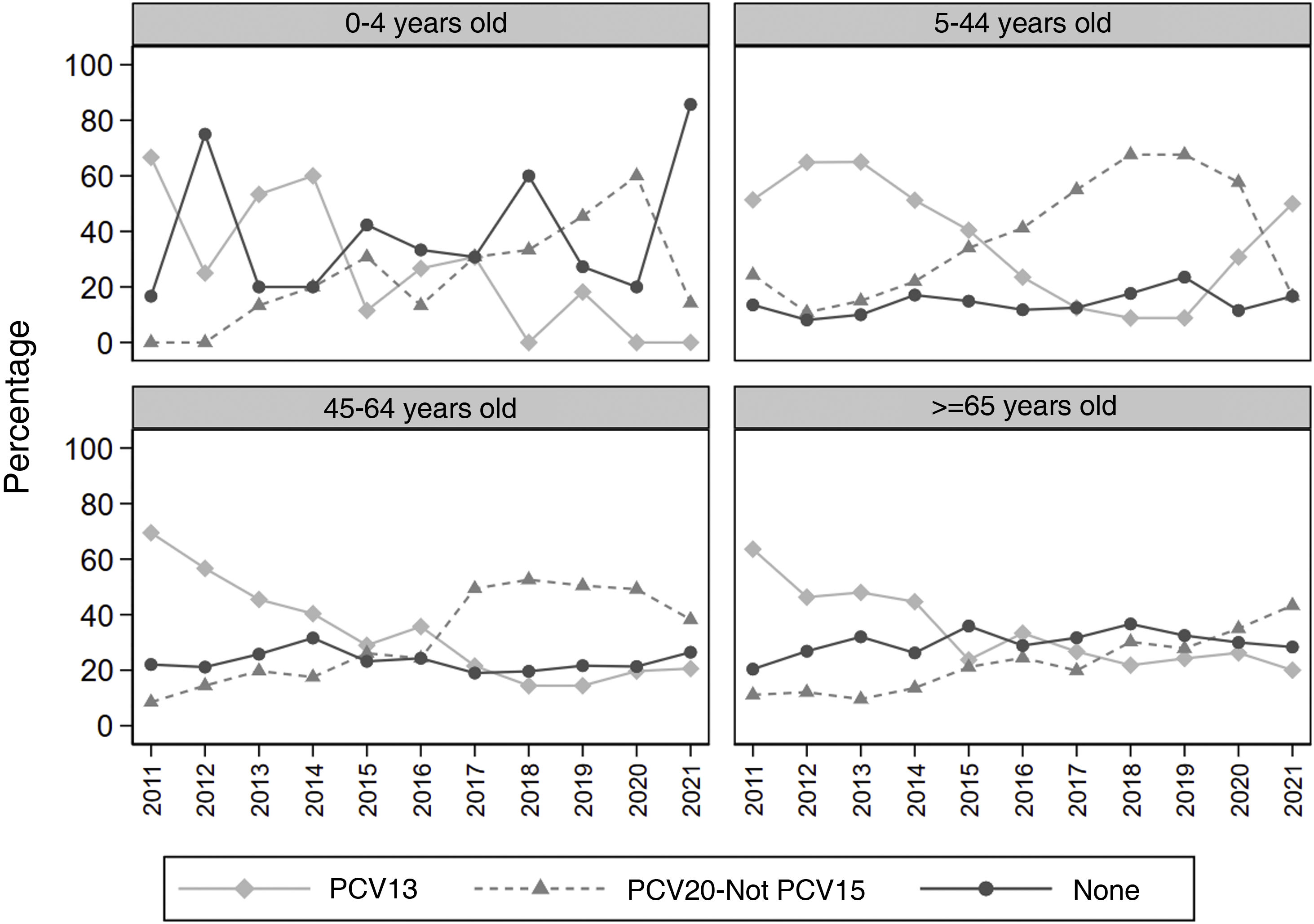
Fig. 1 shows, for each age group, the annual evolution of the percentage of serotypes in the three vaccine groups with the highest prevalence. The prevalence of PCV13 serotypes decreased throughout the study period in all age groups, but in 2019 there was a rebound that was maintained in 2019 and 2020, especially in the five to 44 years age group. The serotypes not included in any vaccine show a stable or slightly increasing trend in those over five years of age, but in the zero to four years age group, a sharp increase is observed in the year 2021.
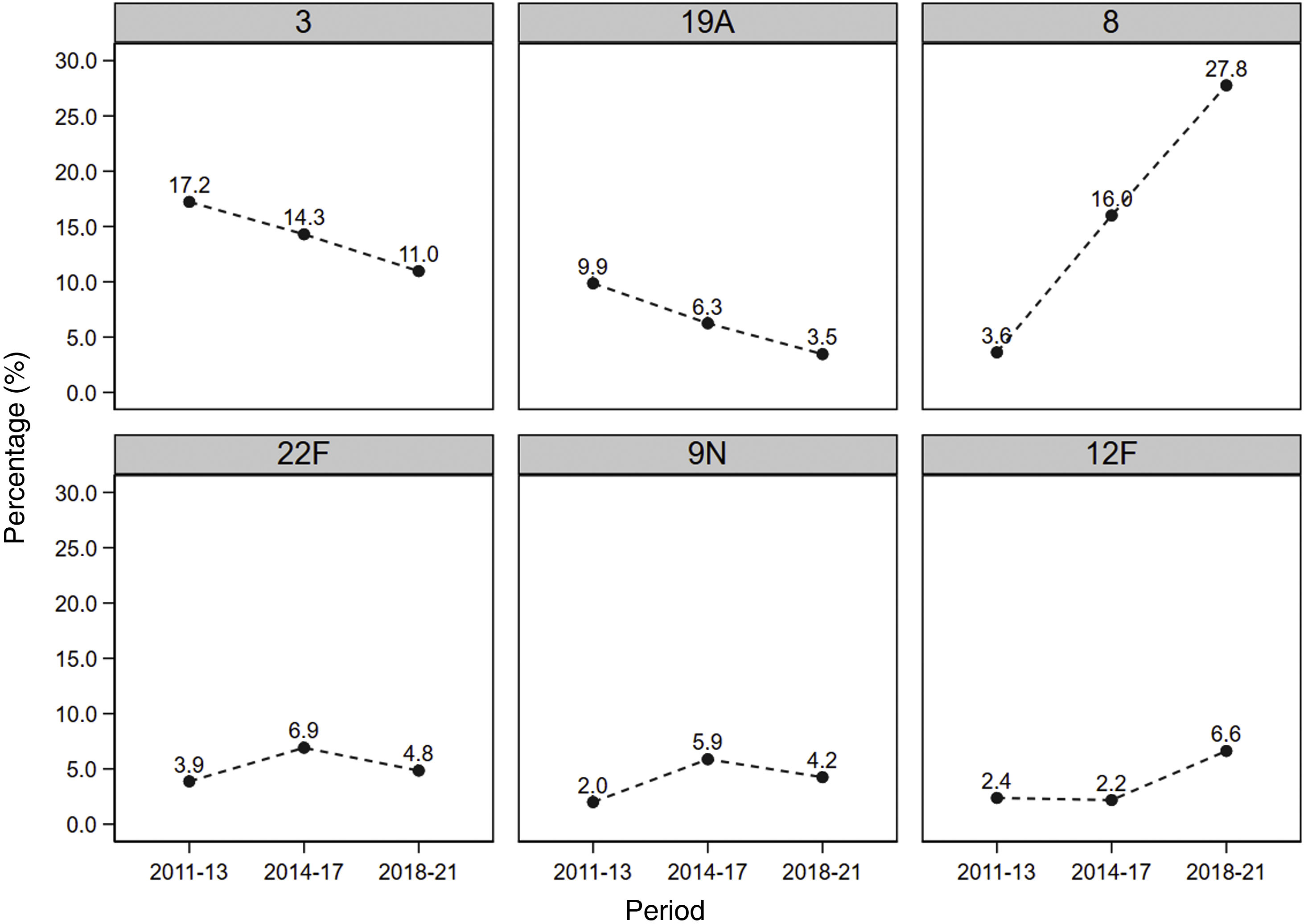
Fig. 2 shows the evolution in the prevalence of the most common serotypes. It can be seen that serotypes 3 and 19A, included in the PCV13, were predominant in the initial period after the introduction of the vaccine, but decreased throughout the study, going from 17.2% initially to 11.0% in the final period, and from 9.9% to 3.5%, respectively. Regarding serotype 8, its prevalence multiplied almost eightfold during the study period, going from an initial 3.6%–27.8% at the end of the study. The prevalence of the 12F serotype almost tripled from the first period to the last, going from 2.4% to 6.6%.
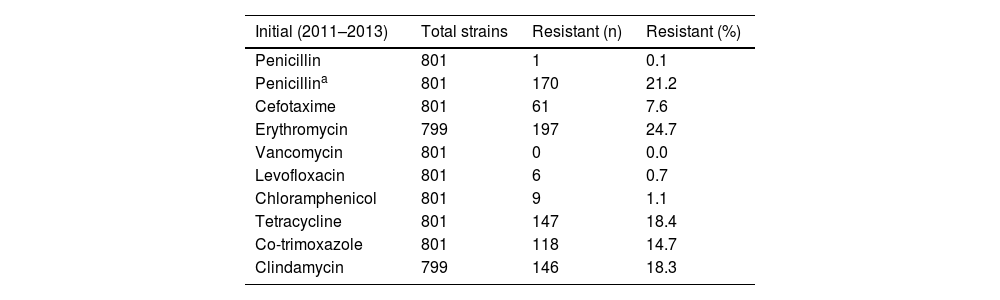
Regarding the susceptibility of the serotyped pneumococci, the percentages of resistance in each of the defined periods are shown in Table 2. All serotyped strains were susceptible to vancomycin, and resistance to levofloxacin (n = 22), chloramphenicol (n = 45) and penicillin with non-meningeal criteria (n = 11) was hardly detected. The percentage of pneumococci resistant to penicillin with meningeal criteria, cefotaxime, erythromycin, tetracyclines and co-trimoxazole decreased between four and six percentage points in the study period, while resistance to clindamycin decreased two percentage points when comparing the initial period and the final period of study. Erythromycin and clindamycin resistance phenotypes were analysed, finding that the M phenotype accounted for 15.4% (n = 96), the MLSBc phenotype 83.7% (n = 522) and the MLSBi phenotype 1% (n = 6).
Percentage of resistant pneumococcal strains during the study period.
| Initial (2011–2013) | Total strains | Resistant (n) | Resistant (%) |
|---|---|---|---|
| Penicillin | 801 | 1 | 0.1 |
| Penicillina | 801 | 170 | 21.2 |
| Cefotaxime | 801 | 61 | 7.6 |
| Erythromycin | 799 | 197 | 24.7 |
| Vancomycin | 801 | 0 | 0.0 |
| Levofloxacin | 801 | 6 | 0.7 |
| Chloramphenicol | 801 | 9 | 1.1 |
| Tetracycline | 801 | 147 | 18.4 |
| Co-trimoxazole | 801 | 118 | 14.7 |
| Clindamycin | 799 | 146 | 18.3 |
| Middle (2014–2017) | Total strains | Resistant (n) | Resistant (%) |
|---|---|---|---|
| Penicillin | 1054 | 5 | 0.5 |
| Penicillina | 1054 | 199 | 18.9 |
| Cefotaxime | 1055 | 38 | 3.6 |
| Erythromycin | 1055 | 249 | 23.6 |
| Vancomycin | 1056 | 0 | 0.0 |
| Levofloxacin | 1056 | 6 | 0.6 |
| Chloramphenicol | 1054 | 25 | 2.4 |
| Tetracycline | 1056 | 221 | 20.9 |
| Co-trimoxazole | 1056 | 117 | 11.1 |
| Clindamycin | 1054 | 213 | 20.2 |
| Final (2018–2021) | Total strains | Resistant (n) | Resistant (%) |
|---|---|---|---|
| Penicillin | 1012 | 5 | 0.5 |
| Penicillina | 1012 | 170 | 16.8 |
| Cefotaxime | 1011 | 25 | 2.5 |
| Erythromycin | 1012 | 188 | 18.6 |
| Vancomycin | 1012 | 0 | 0.0 |
| Levofloxacin | 1012 | 10 | 1.0 |
| Chloramphenicol | 1012 | 11 | 1.1 |
| Tetracycline | 1012 | 145 | 14.3 |
| Co-trimoxazole | 1012 | 96 | 9.5 |
| Clindamycin | 1012 | 164 | 16.2 |
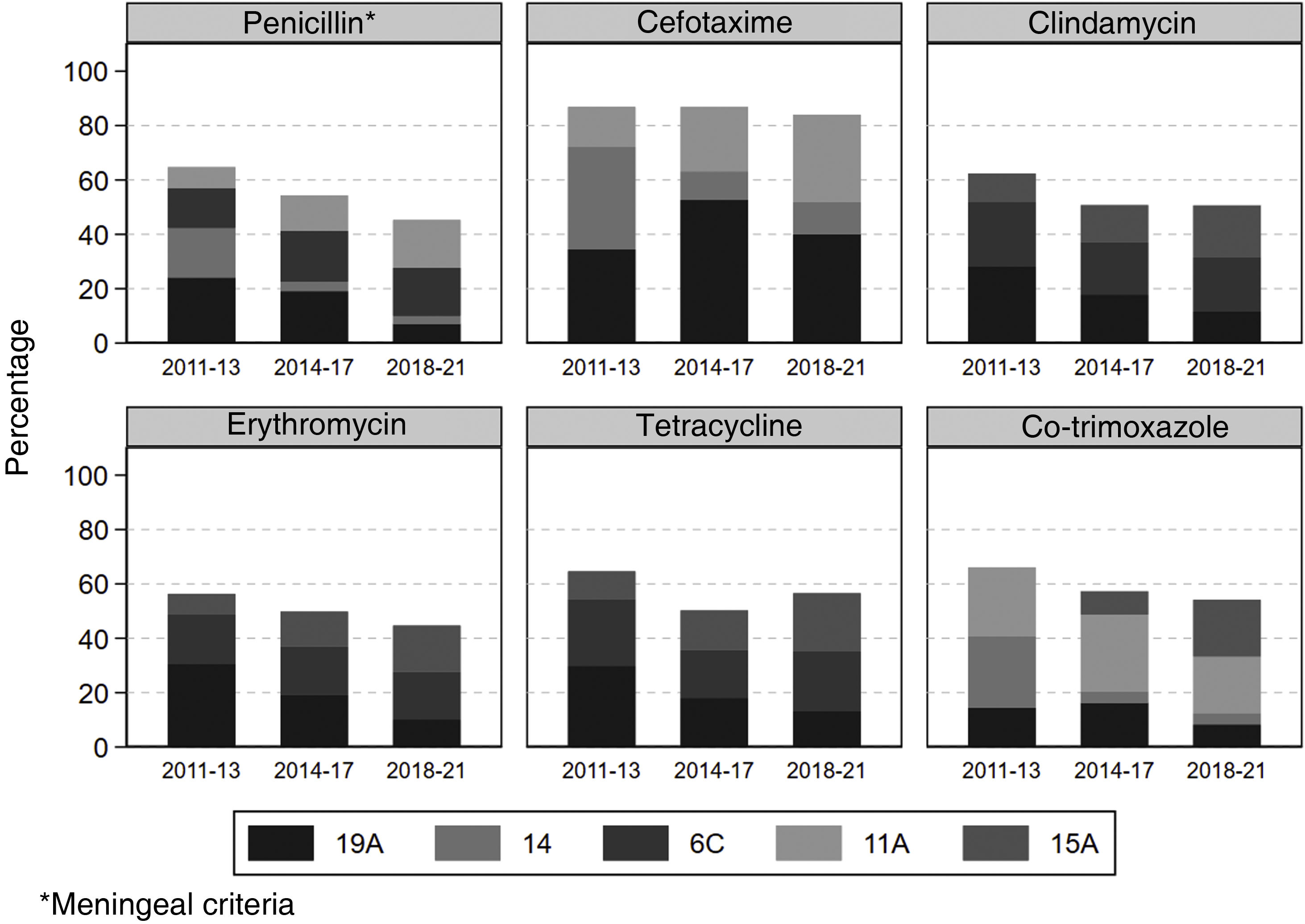
Fig. 3 shows the distribution of pneumococci resistant to each antibiotic based on the most common serotypes. In the initial stage after vaccination, serotype 19A showed the greatest resistance to all the antibiotics analysed, except for cefotaxime and co-trimoxazole, for which serotype 14 showed the greatest resistance with 37.7% and 26.3%, respectively. After the first period, the frequency of serotype 19A among resistant strains decreased in all antibiotics, except for cefotaxime, in which it increased from 34.4% to 40.0%. The increase in the frequency of resistance to penicillin and cefotaxime of serotype 11A (included in the PCV20) in the final period compared to the initial period is noteworthy, more than doubling. Regarding serotype 15A, which is not included in any vaccine, its frequency among strains resistant to cefotaxime, clindamycin, erythromycin, tetracycline and co-trimoxazole increased throughout the study period. Serotype 8, which increased the most during follow-up, did not show remarkable resistance.
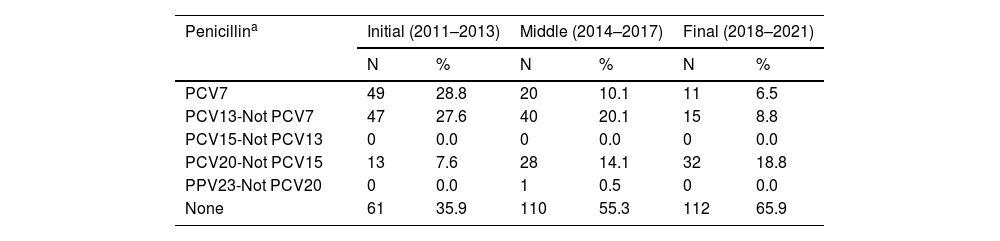
Table 3 shows the distribution of resistant pneumococci by serotype according to their inclusion in the different vaccines, excluding antibiotics with no or a low percentage of resistance. Two groups of antibiotics with similar behaviour are identified. On the one hand, serotypes resistant to penicillin (meningeal criteria), cefotaxime and co-trimoxazole are mostly serotypes included in the PCV13, PCV20-Not PCV15, or not included in any vaccine. On the other hand, serotypes resistant to erythromycin, tetracycline and clindamycin are distributed among all serotype groups, with a residual presence in PCV23-Not PCV20. The serotypes included in PCV13 and those that are not in any vaccine together account for 80% or more of those resistant to these antibiotics in all periods. For all antibiotics, the percentage of resistant serotypes included in the PCV13 decreased by between 27% for cefotaxime and 73% for penicillin, and the percentage of resistant serotypes not included in any vaccine increased by between 46% for cefotaxime and a 290% for co-trimoxazole.
Distribution of resistant strains by serotype in each period.
| Penicillina | Initial (2011–2013) | Middle (2014–2017) | Final (2018–2021) | |||
|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |
| PCV7 | 49 | 28.8 | 20 | 10.1 | 11 | 6.5 |
| PCV13-Not PCV7 | 47 | 27.6 | 40 | 20.1 | 15 | 8.8 |
| PCV15-Not PCV13 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| PCV20-Not PCV15 | 13 | 7.6 | 28 | 14.1 | 32 | 18.8 |
| PPV23-Not PCV20 | 0 | 0.0 | 1 | 0.5 | 0 | 0.0 |
| None | 61 | 35.9 | 110 | 55.3 | 112 | 65.9 |
| Cefotaxime | N | % | N | % | N | % |
|---|---|---|---|---|---|---|
| PCV7 | 26 | 42.6 | 7 | 18.4 | 4 | 16.0 |
| PCV13-Not PCV7 | 21 | 34.4 | 20 | 52.6 | 10 | 40.0 |
| PCV15-Not PCV13 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| PCV20-Not PCV15 | 9 | 14.8 | 9 | 23.7 | 8 | 32.0 |
| PPV23-Not PCV20 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| None | 5 | 8.2 | 2 | 5.3 | 3 | 12.0 |
| Erythromycin | N | % | N | % | N | % |
|---|---|---|---|---|---|---|
| PCV7 | 24 | 12.2 | 19 | 7.6 | 14 | 7.4 |
| PCV13-Not PCV7 | 68 | 34.5 | 54 | 21.7 | 23 | 12.2 |
| PCV15-Not PCV13 | 17 | 8.6 | 24 | 9.6 | 14 | 7.4 |
| PCV20-Not PCV15 | 15 | 7.6 | 25 | 10.0 | 15 | 8.0 |
| PPV23-Not PCV20 | 1 | 0.5 | 2 | 0.8 | 4 | 2.1 |
| None | 72 | 36.5 | 125 | 50.2 | 118 | 62.8 |
| Tetracycline | N | % | N | % | N | % |
|---|---|---|---|---|---|---|
| PCV7 | 14 | 9.5 | 16 | 7.2 | 8 | 5.5 |
| PCV13-Not PCV7 | 46 | 31.3 | 51 | 23.1 | 22 | 15.2 |
| PCV15-Not PCV13 | 12 | 8.2 | 16 | 7.2 | 4 | 2.8 |
| PCV20-Not PCV15 | 12 | 8.2 | 24 | 10.9 | 9 | 6.2 |
| PPV23-Not PCV20 | 1 | 0.7 | 3 | 1.4 | 1 | 0.7 |
| None | 62 | 42.2 | 111 | 50.2 | 101 | 69.7 |
| Co-trimoxazole | N | % | N | % | N | % |
|---|---|---|---|---|---|---|
| PCV7 | 48 | 40.7 | 14 | 12.0 | 11 | 11.5 |
| PCV13-Not PCV7 | 18 | 15.3 | 19 | 16.2 | 8 | 8.3 |
| PCV15-Not PCV13 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| PCV20-Not PCV15 | 35 | 29.7 | 38 | 32.5 | 22 | 22.9 |
| PPV23-Not PCV20 | 0 | 0.0 | 2 | 1.7 | 1 | 1.0 |
| None | 17 | 14.4 | 44 | 37.6 | 54 | 56.3 |
| Clindamycin | N | % | N | % | N | % |
|---|---|---|---|---|---|---|
| PCV7 | 17 | 11.6 | 17 | 8.0 | 13 | 7.9 |
| PCV13-Not PCV7 | 44 | 30.1 | 42 | 19.7 | 20 | 12.2 |
| PCV15-Not PCV13 | 17 | 11.6 | 24 | 11.3 | 8 | 4.9 |
| PCV20-Not PCV15 | 5 | 3.4 | 16 | 7.5 | 11 | 6.7 |
| PPV23-Not PCV20 | 0 | 0.0 | 2 | 0.9 | 4 | 2.4 |
| None | 63 | 43.2 | 112 | 52.6 | 108 | 65.9 |
In Galicia, vaccination with the PCV13 has been included in the childhood vaccination schedule since 2011 and in the adult population since 2017.
This study shows how the serotypes included in the PCV13 decreased as a cause of IPD once vaccination was rolled out in the paediatric population, which may demonstrate herd protection in all age groups. The decrease in serotypes included in the PCV13 as a cause of IPD was also reported by other authors in Spain, Europe and the USA.7,8,13–15 The increase in these serotypes in the five to 44-year-old group in 2020 and 2021 is striking, but this increase could be due to the low number of serotyped isolates in those years, so it should be confirmed if the increase is maintained with data after 2021. Of all the serotypes included in the PCV13, the most common were 3 and 19A, which decreased, although not as much as others. Serotype 19A, which was the most common of those resistant to the antibiotics studied, showed a reduction for all the antibiotics evaluated, except for cefotaxime. This decrease in serotype 19A after the PCV13 was also documented by other authors in Japan and Spain.8,16 In contrast, different studies in Spain and Portugal14 found that serotype 3 was maintained despite vaccination. In Portugal,14 the PCV13 was introduced into the childhood vaccination schedule in 2015 and data to 2018 were analysed, observing a modest reduction of the serotypes included in the PCV13 or even stabilisation. However, this effect should arguably be studied in the longer term. In our study, data were analysed 11 years after childhood vaccination with the PCV13, which is more generalisable.
It was observed throughout the study how, at the same time as the serotypes included in the PCV13 decreased, the serotypes not included increased,15,17,18 especially serotype 8, whose prevalence increased almost eightfold during the study period. An increase in serotype 8 was also observed by other authors.7,13,19–21 Serotype 8 is included in the PCV20, so this vaccine could help mitigate it. This study did not analyse the causes of clinical severity of IPD that could associate this serotype with greater pathogenicity, as was done by Sá-Leao et al.,22 who described it as one associated with invasive disease. However, this serotype did not manifest resistance to antibiotics.
Another notable serotype is 12F, the prevalence of which tripled throughout the study. This serotype is also included in the PCV20 and, like serotype 8, it did not show significant resistance to the antibiotics studied.
The frequency of serotype 11A, also included in the PCV20 and related according to one study23 with the carrier state, more than doubled among serotypes resistant to penicillin and cefotaxime between the initial and final period.
In the group from zero to four years of age, the abrupt increase in the prevalence of IPD due to serotypes not included in any vaccine is striking, which also increased in all age groups, except in the 45–64 years group, where they remained stable. The resistance percentage of these serotypes, analysed together, increased by 46% to cefotaxime and by 290% to co-trimoxazole, with serotype 15A being particularly prevalent. This serotype must be monitored particularly closely and has also been described by Nakano et al.,24 who in Japan associated clone 15A-ST9084 with high resistance urgency, and Shepard et al. in the United Kingdom,10 who warned of the increase in this multiresistant serotype that is not included in any vaccine.
This study highlights the effect of vaccination with the PCV13 in the Galician population, since the sample is representative of the entire community, given that the regional reference laboratory for pneumococcal serotyping receives samples from all the health districts in the community. In addition, the study period (2011–2021) was wide enough to be able to draw conclusions about the evolution of the serotypes and their resistance to antibiotics.
One limitation of the study was that it did not allow for the calculation of incidences, which could indicate a replacement of serotypes, since in the years 2020 and 2021 the number of serotyped pneumococci decreased, and it is unknown if this was due to a decrease in the incidence of IPD, or if it was because the pneumococcal strains were not sent for serotyping due to the COVID-19 pandemic.
In conclusion, throughout the study period it is evident that after the introduction of the PCV13 vaccine in the paediatric population there was a change in the pneumococcal serotypes in Galicia towards the serotypes included in the PCV20 and not in the PCV15, and towards those not included in any vaccine, both in the vaccinated paediatric population and in those aged 65 and over. Although incidence rates were not calculated with the available data and we cannot draw any conclusions regarding serotype replacement, it was observed that the serotypes included in the PCV13 were the predominant serotypes in the initial period analysed, while in the rest of the period it was serotypes 8 and 12F, both included in the PCV20. All pneumococcal strains were susceptible to vancomycin and 99% to levofloxacin. The 19A serotype was the most resistant during the initial stage after vaccination with the PCV13, while the 15A serotype, not included in any vaccine, increased its resistance throughout the study and warrants special monitoring. Serotype 8, which became progressively predominant during the period analysed, did not show remarkable resistance percentages.
Ethical considerationsThis study was conducted according to the guidelines of the Declaration of Helsinki in relation to ethical principles for medical research in human beings. The right to confidentiality was maintained at all times. For this publication, no personal patient data was processed except age group.
FundingThis paper presents the results of a research project led by the Servizo de Microbioloxía [Microbiology Department] of the Complexo Hospitalario Universitario de Ferrol [University of Ferrol Hospital Complex] and with the participation of the Dirección Xeral de Saúde Pública [General Directorate of Public Health] of Galicia.
It was sponsored by the pharmaceutical company Pfizer. File No.: AAB-VAC-2015-01. Financing Entity: Pfizer. S.L U.
Conflicts of interestPedro Miguel Juiz González and Susana Méndez Lage were contracted to take on the project and Andrés Agulla Budiño was the principal investigator of the project. Isabel Losada Castillo and Alberto Malvar Pintos were collaborating researchers on the project. The other authors have no conflicts of interest.
María Rodríguez-Mayo. Complexo Hospitalario Universitario de A Coruña [A Coruña University Hospital Complex].
Gema Barbeito-Castiñeiras. Complejo Hospitalario Universitario de Santiago [Santiago University Hospital Complex].
Francisco José Vasallo-Vidal. Complexo Hospitalario Universitario de Vigo [Vigo University Hospital Complex].
Isabel Paz-Vidal. Complexo Hospitalario Universitario de Ourense [Ourense University Hospital Complex].
Pedro Alonso-Alonso. Hospital Comarcal de Monforte [Monforte County Hospital].
Marta Serrano-López. Hospital da Mariña de Burela [da Mariña de Burela Hospital].
Victoria Pulián-Morais. Complexo Hospitalario Universitario de Pontevedra [Pontevedra University Hospital Complex].
María Domínguez González. Hospital Comarcal de Valdeorras [Valdeorras County Hospital].
Patricia Capón González. Hospital Universitario Lucus Augusti [Lucus Augusti University Hospital].