Peripherally inserted central catheters have become a priority in infants who require long-term intravenous therapy, but their use involves certain risks.
ObjectiveThe aim of the study was to describe the occurrence of adverse events in newborns with peripherally inserted central catheters and to determine the risk factors associated with them.
MethodsA descriptive cross-sectional study was designed and performed. All neonates with peripherally inserted central catheters from October 1st, 2014 to September 30th, 2015 were included. The adverse events and sociodemographic and clinical variables related to neonates and analysed catheters were recorded.
ResultsA total of 140 catheters were placed in 116 infants. All of them were analysed. Adverse events occurred in 16.4%: catheter-associated bacteraemia (5.7%), obstruction (5.7%), extravasation (2.1%) and phlebitis (2.1%), <27 weeks of pregnancy (OR=1.2, p=.02), birth weight <1000g (OR=6.7, p=.02), with catheters in situ for longer than one week (OR=9.8, p=.02) and with perfusion of antibiotics per catheter (OR=1.3, p<.01). Phlebitis is associated with the insertion of the catheter in LL and head (OR=1.1, p=.03). Factors associated with bacteraemia risk with adjusted prevalence odds ratio are extremely low birth weight neonates (OR=6.38; p=.03) and with a catheter in situ for longer than one week (OR=9.41; p=.04).
ConclusionsThe periodic evaluation of catheter-related adverse events is very useful to prepare improvement plans. This will maximise safety for the most vulnerable newborns, especially those of extremely low birth weight that require very long treatments.
Los catéteres centrales de inserción periférica se han convertido en una prioridad en los neonatos que necesitan terapia intravenosa de larga duración, pero su uso no está exento de riesgos.
ObjetivoDescribir la aparición de eventos adversos en neonatos portadores de catéteres venosos centrales de inserción periférica y determinar factores de riesgo asociados a ellos.
MétodosSe llevó a cabo un estudio descriptivo transversal en el que se incluyeron la totalidad de neonatos portadores de catéteres venosos centrales de inserción periférica desde el 1 de octubre de 2014 hasta el 30 de septiembre de 2015. Se registraron los eventos adversos y variables sociodemográficas y clínicas relacionadas con los neonatos y los catéteres analizados.
ResultadosSe incluyeron en el estudio un total de 116 neonatos y 140 catéteres. Se registraron eventos adversos en el 16,4% de ellos: bacteriemia asociada al catéter (5,7%), obstrucción (5,7%), extravasación (2,1%) y flebitis (2,1%). Los factores asociados a bacteriemia con el odds de prevalencia corresponde a neonatos: <27semanas de gestación (OR=1,2; p=0,02), con peso <1.000g (OR=6,7; p=0,02), portadores de catéter >1semana (OR=9,8; p=0,02) y con perfusión de antibióticos por catéter (OR=1,3; p<0,01). La flebitis se asocia con la inserción del catéter en miembros inferiores y cabeza (OR=1,1; p=0,03). Los factores asociados a bacteriemia con odds de prevalencia ajustada corresponde a neonatos de peso extremo (OR=6,38; p=0,03) y portadores del catéter >7días (OR=9,41; p=0,04).
ConclusionesLa evaluación periódica de eventos adversos relacionados con catéteres es de gran utilidad para elaborar planes de mejora. Esto permitirá extremar la seguridad en los RN más vulnerables, en especial los neonatos con extremo bajo peso que precisan tratamientos muy prolongados.
The peripherally inserted central catheter (PICC) is the vascular access of choice in the newborn (NB) who requires long-term intravenous treatment. This is a safe and effective technique for the prolonged administration of intravenous solutions in higher calibre vessels and causes less irritative inflammatory phenomena in the vascular endothelium. Even so, this technique is not risk free and the main problems are infection or bacteraemia associated with them, together with other mechanical type complications, such as obstruction, extravasation, catheter rupture and non elective extraction.
The nurse plays an essential role as he or she is responsible for insertion, maintenance and prevention of adverse events associated with these catheters. The safety of hospitalised newborns is a challenge for professionals who look after them. The culture of safety entails the periodic assessment of the status and evolution of adverse events in conjunction with clinical practice and professional perceptions.
What does this study contribute?The results of this study, which was conducted in the neonate intensive care unit of a tertiary level general hospital, provide data on the prevention of adverse events relating to PICC in neonates with extremely low weight and who have had a catheter for over a week.
Implications for nursing practiceData obtained provide relevant information for all nurses who are carrying out clinical practice in neonatal intensive care units. Furthermore, these findings are comparable and coincide with general data from other studies. The findings highlight the vulnerability of adverse events associated with catheters, and specifically with bacteraemia in neonates with a low weight for their gestational age and with catheters for over a week. This information is essential for nurses so they may establish and apply extreme aseptic measures in the medical care management these babies require. The use of specific protocols such as “Zero Bacteraemia” increases clinical safety and reduces morbidity of the neonates.
From a healthcare viewpoint, the term “paediatric” applies to children aged under 18 years. According to the World health Organisation (WHO) this extensive period is divided into: newborns (NB) or neonates (from 0 to 28 days), infants and young children (from 1 month to 2 years), pre-school aged children (from 2 to 5 years), primary school children (from 6 to 11 years) and adolescents (form 12 to 18 years).1 The Spanish neonatology Society (SEN for its initials in Spanish) classifies NB children according to their gestational age when they were born. According to this classification: NB is the term used when they are born at 37 weeks or more and preterm NB when the child is born before 37 completed weeks of gestation, with gestation being a physiological variable fixed at 280±15 days. Preterm NB are divided into moderate to late preterm (they are born at 32–36 weeks), highly preterm (between 28–31 weeks) and extremely preterm (born at 27 or fewer gestation weeks).2,3
The most prevalent pathologies of premature babies derive from their immaturity, mainly from their gestational age and hypoxia (ineffective postnatal respiratory adaptation after trans-placental oxygenation). As a result, most morbidity and mortality affects highly and extremely premature NB, and it is necessary for them to be admitted to neonatal units for clinical care management.3
In recent years, survival of the most premature children has increased thanks to specialised care, new technologies and more prolonged treatments. These treatments require increased techniques or invasive procedures (surgical interventions, insertion of catheters, etc.), increasing hospital stays, and specifically in the neonatal intensive care units (NICU).2
During hospitalisation, these children usually require the administration of intravenous liquids and drugs for long periods of time. In these cases, the central venous accesses have become the pathway of choice.5 There are different forms of central venous access: through the umbilical cord; through direct access in the femoral, jugular or subclavian veins, and access to the vena cava through the peripheral veins using peripherally inserted central catheters (PICC) for this.4,5 The choice of the type of access depends on the immaturity and pathology of the preterm child, always choosing the pathway of lowest risk and best performance, maintaining the best possible vascular state of the child.4,5 The main pathway of choice is umbilical, the principal complications of which are severe sepsis and thrombosis. It is therefore recommended that the catheter be removed as soon as possible (between day 5 and 7). Femoral, jugular and subclavian venous access are not exempt from complications either. Apart from leading to the same complications as the umbilical pathway there may be other more specific ones, depending on the venous access, such as pneumothorax or haemorrages.4,5 As a result PICC has become the vascular access of choice in NB who need long-term intravenous therapy. This is a safe and effective venous access technique for intermediate (more than 7 days and less than 3 months) or prolonged (over 3 months) administration of intravenous solutions (fluids, parenteral nutrition, vasoactive drugs and antibiotics).3–6 However, this route is not exempt from risks. The main problem derived from its use are infections, or bacteraemias, as well as other mechanical type complications, such as obstruction, extravasation, catheter rupture and non elective extraction.2,4–10 Its use for blood perfusion or certain drugs such as prostaglandins is advisable (in neonates prostaglandin E1 or alprostadil are used for the opening and/or maintenance of patent ductus arteriosus in certain cardiopathies). In these cases peripheral venous catheters are used although use is reserved due to its risks (mainly extravasation and phlebitis). These risks determine the low duration of these catheters, between 2 or 3 days, and the necessity to frequently replace them.4,5
The nurse is responsible for the insertion, maintenance and prevention of adverse events (AE) associated with PICC.9,11 The simplest and most satisfactory cannulation of this route occurs when the patient's vascular accesses are intact, although this requires the presence of trained professionals with extensive experience in insertion and managment.12 During recent years several studies have demonstrated that the use of ultrasound facilitates the PICC insertion procedure, increasing safety and success with this technique of both insertion and correct location.12,13
The safety of hospitalised patients is a challenge for all healthcare professionals. Safety culture through periodic assessments leads to knowledge on the state and development of AE related to clinical practice and the perceptions of professionals in this regard.14 This challenge is all the more extreme in the NICU where the preterm NB are highly vulnerable, and where there is a high risk of morbimortality associated with their anatomical-physiological and clinical traits.
In our centre, from 1st June 2104 the “Zero Bacteraemia” protocol was applied. This protocol, driven by the WHO and endorsed by the Ministry of Health, Social Policy and Equality (MSSSI for its initials in Spanish),2 contains a series of measures to be implemented by nurses to reduce AE related to vascular catheters. These measures consist of hand hygiene and disinfection, sterile management of catheters in both insertion and maintenance, skin disinfection with aqueous-based 2% chlorhexidine and the use of alcohol-based .5% chlorhexidine for the three-way port and connectors.2,15 This aim of this study, based on this new working methodology on the culture of safety was to describe the risk factors associated with AE in NB who had PICC in a NICU following the application of the “Zero Bacteraemia” protocol.
MethodDesign, population and sampleA descriptive, cross-sectional study was conducted. The population were all NB admitted to the NICU of a tertiary level general hospital, and who had a PICC. The sample comprised all children with this type of catheters during the study period, form 1st October 2014 until 30th September 2015. Sample size was 140 PICC, analysed in 116 children. Estimation of the proportion of risk of AE associated with catheters was 5% precision, with a 95% confidence interval and a 10% proportion.16
VariablesThe variables included were as follows:
The presentation of an AE (yes or no) as the dependent and principal study variable.
Independent variables:
- -
Those relating to the NB: gestational age, weight and sex. Gestational age and weight categories were determined according to those established by the Spanish Paediatric Society.3 Therefore newborns ≤27 weeks were considered extremely preterm, NB between 28 and 31 weeks as highly preterm, NB between 32 and 36 weeks as late to moderate preterm and NB born at ≥37 weeks as full term. With regards to weight, the NB were divided into extremely low weight at ≤999g, very low weight at between 1000 and 1499g, low weight at between 1500 and 2499g and normal weight at ≥2500g.
- -
Those relating to the catheter: calibre measured using the French scale (Fr) (1 or 2Fr), anatomical catheter insertion area: upper limbs (UL), lower limbs (LL) or head (with correct location of the catheter tip according to radiological confirmation in superior or inferior vena cava); number of days which the catheter was inserted, type of catheter perfusions (differentiating perfusions with and without antibiotics) and number of catheters which were inserted into the same patient (one, two or more than two). The type of AE was specified when it presented.
For data collection an ad hoc document was used. Data were extracted from computerised clinical records of each of the newborns included in the sample. Recording was carried out during January 2016, when all necessary data required for investigation was made available (specifically relating to AE associated with catheters through the catheter tip analytical and culture study register). Compliance with study variables was met during the morning shift in the NICU by three nurses from the unit, so that analysis and data collection was always completed in pairs to eliminate registration and codification bias.
Statistical analysisAll collected data was introduced into a data base and analysed with the statistical package SPSS® (IBM SPSS Statistics) version 20.0. In the descriptive analysis of general characteristics of the sample the numerical variables were written as an arithmetic mean and standard deviation and the results of the categorical variables in frequencies and percentages. The hypothesis of normality of distribution in the continuous variables was assessed using the Kolmogorov–Smirnov test. Any AE which presented and were related to PICC insertion in all of the newborns studied were identified, with presentation of frequencies and percentages. A bivariate analysis was performed to identify which factors had an impact on AE presentation. Here the dependent variable (presentation or non presentation of AE) was related to the other independent variables studied (gestational age, sex, weight, catheter calibre, time of catheter use in days, catheter tip, medication with/without antibiotics and catheter number). The Chi-square test or Fisher exact test was calculated for small samples, considering a significance level of p<.05 in these tests.
In addition to prevalence measures the reason for prevalence was indicated, determining the non adjusted odds ratio (OR) and the confidence interval (CI) of 95%. Finally, the model was adjusted for the variables which obtained significance in bivariate analysis, resulting in the forward method final logistic regression model with an entry criteria of .05 and exit criteria of .10.
Ethical and legal aspectsPrior to data collection a formal request for permission from the staff in charge of the NICU (supervisor and head of the medical service unit) was made. All the parents of the NB in the sample had given their consent for data to be used for medical and research purposes. Authorisation of this consent is contained in the documents provided in the unit. During data collection each child was assigned a code so as to maintain confidentiality. The data obtained were treated in accordance with the general data protection regulation of the European Union 2016/679 from the European Parliament, application in Spain on 25th May 2018, and law 41/2002, of 14th November governing the autonomy of the patient and the rights and obligations pertaining to clinical information and documentation.
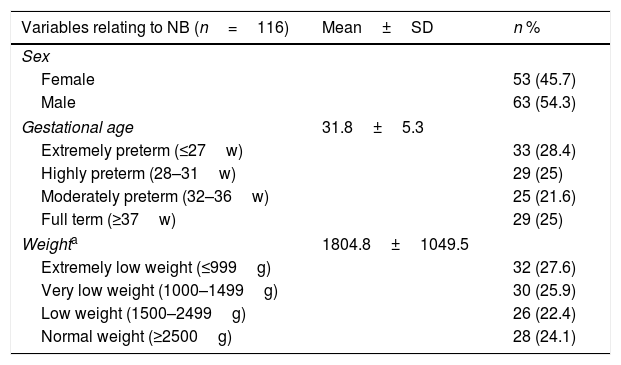
ResultSociodemographic and clinical characteristics of the sampleA total of 116 NB admitted to the NICU were analysed during the study period. Of these, 54.3% were boys and 45.7% were girls, with a gestational mean age of 31.8±5.3 weeks. Median weight was 1415g (980–2450) (Table 1).
Description of the sociodemographic and clinical sample variables.
| Variables relating to NB (n=116) | Mean±SD | n % |
|---|---|---|
| Sex | ||
| Female | 53 (45.7) | |
| Male | 63 (54.3) | |
| Gestational age | 31.8±5.3 | |
| Extremely preterm (≤27w) | 33 (28.4) | |
| Highly preterm (28–31w) | 29 (25) | |
| Moderately preterm (32–36w) | 25 (21.6) | |
| Full term (≥37w) | 29 (25) | |
| Weighta | 1804.8±1049.5 | |
| Extremely low weight (≤999g) | 32 (27.6) | |
| Very low weight (1000–1499g) | 30 (25.9) | |
| Low weight (1500–2499g) | 26 (22.4) | |
| Normal weight (≥2500g) | 28 (24.1) | |
| Variables relating to PICC (n=140) | Mean±SD | n % |
|---|---|---|
| Catheter puncture site | ||
| Upper limb | 94 (67.1) | |
| Lower limb | 28 (20) | |
| Head | 18 (12.9) | |
| Anatomical location of catheter | ||
| Vena cava | 95 (67.9) | |
| Subclavian vein | 35 (25) | |
| Others | 10 (7.1) | |
| Catheter calibre | ||
| 1 French | 89 (63.6) | |
| 2 French | 51 (36.4) | |
| Catheter permanence (1–49 days)b | 9.5±8.1 | |
| 1–7 days | 78 (55.8) | |
| 8–14 days | 38 (27.1) | |
| ≥15 days | 24 (17.1) | |
| Catheter perfusion therapy | ||
| Without antibiotics(parenteral nutrition, lipids, others) | 71 (50.7) | |
| With antibiotics | 69 (49.3) | |
| Number of catheters inserted in the same child (1–4 catheters) | ||
| First | 1.2±0.5 | 116 (82.9) |
| Second or more | 24 (17.1) | |
SD: standard deviation.
In the 116 newborns studies, a total of 140 PICC were analysed, in most cases (63.6%) of 1Fr. Twenty four of these required more than two catheters (from 2 to 4) during their stay for their treatment. Most catheters were inserted in extremely preterm babies (35.7%), highly preterm (22.9%) babies and 57.9%, in babies with weight under 1500g. 67.1% of catheters were cannulated in MMSS. The catheters were inserted for an average of 7 days (5–10.75). Almost half of the catheter perfusion therapy were antibiotics (49.3%) (Table 1).
Presentation of adverse eventsMost of the catheters studied did not present with AE (83.6%). Ranked in order of decreasing frequency, the adverse events were: catheter-associated bacteraemia (5.7%), obstruction (5.7%), extravasation (2.1%) and phlebitis (2.1%). Only in one case was the removal of the catheter required due to its poor function (.8%).
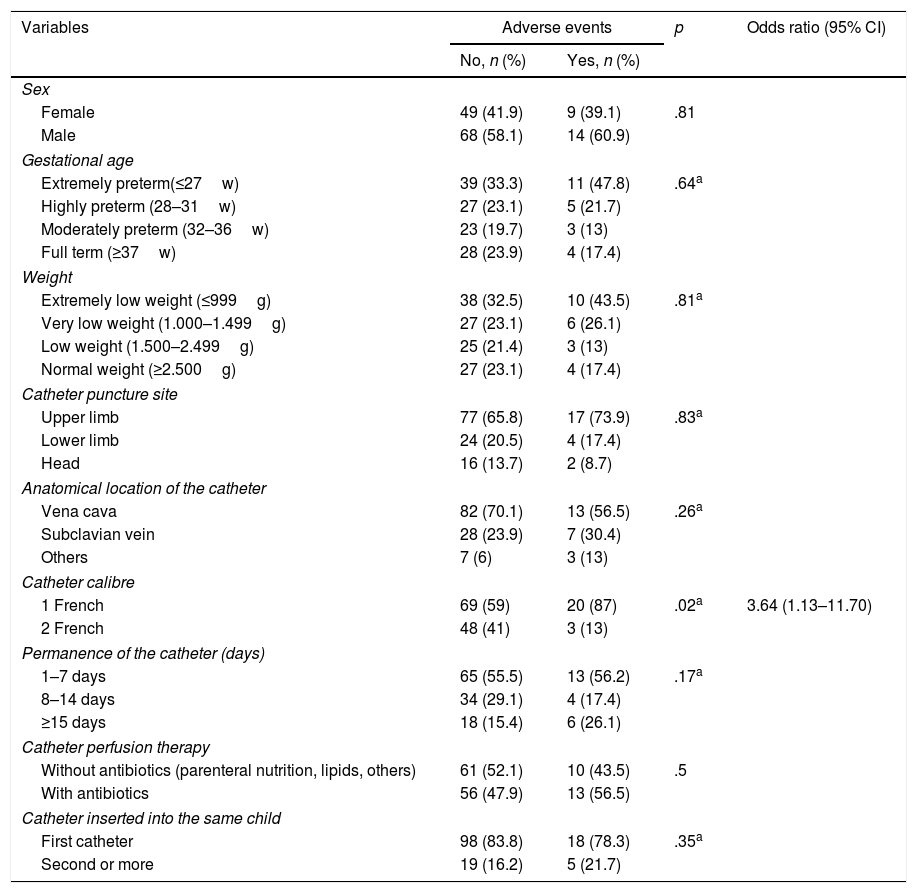
Factors impacting on adverse event presentationCatheter calibre was the only variable associated in any significant way (OR=3.6; p=.02) with the appearance or non appearance of any AE in general. The NB with a small calibre PICC (1Fr) had greater probability of an AE presenting than those who had a superior calibre catheter (2Fr) (Table 2).
Variables associated with adverse event presentation.
| Variables | Adverse events | p | Odds ratio (95% CI) | |
|---|---|---|---|---|
| No, n (%) | Yes, n (%) | |||
| Sex | ||||
| Female | 49 (41.9) | 9 (39.1) | .81 | |
| Male | 68 (58.1) | 14 (60.9) | ||
| Gestational age | ||||
| Extremely preterm(≤27w) | 39 (33.3) | 11 (47.8) | .64a | |
| Highly preterm (28–31w) | 27 (23.1) | 5 (21.7) | ||
| Moderately preterm (32–36w) | 23 (19.7) | 3 (13) | ||
| Full term (≥37w) | 28 (23.9) | 4 (17.4) | ||
| Weight | ||||
| Extremely low weight (≤999g) | 38 (32.5) | 10 (43.5) | .81a | |
| Very low weight (1.000–1.499g) | 27 (23.1) | 6 (26.1) | ||
| Low weight (1.500–2.499g) | 25 (21.4) | 3 (13) | ||
| Normal weight (≥2.500g) | 27 (23.1) | 4 (17.4) | ||
| Catheter puncture site | ||||
| Upper limb | 77 (65.8) | 17 (73.9) | .83a | |
| Lower limb | 24 (20.5) | 4 (17.4) | ||
| Head | 16 (13.7) | 2 (8.7) | ||
| Anatomical location of the catheter | ||||
| Vena cava | 82 (70.1) | 13 (56.5) | .26a | |
| Subclavian vein | 28 (23.9) | 7 (30.4) | ||
| Others | 7 (6) | 3 (13) | ||
| Catheter calibre | ||||
| 1 French | 69 (59) | 20 (87) | .02a | 3.64 (1.13–11.70) |
| 2 French | 48 (41) | 3 (13) | ||
| Permanence of the catheter (days) | ||||
| 1–7 days | 65 (55.5) | 13 (56.2) | .17a | |
| 8–14 days | 34 (29.1) | 4 (17.4) | ||
| ≥15 days | 18 (15.4) | 6 (26.1) | ||
| Catheter perfusion therapy | ||||
| Without antibiotics (parenteral nutrition, lipids, others) | 61 (52.1) | 10 (43.5) | .5 | |
| With antibiotics | 56 (47.9) | 13 (56.5) | ||
| Catheter inserted into the same child | ||||
| First catheter | 98 (83.8) | 18 (78.3) | .35a | |
| Second or more | 19 (16.2) | 5 (21.7) | ||
CI: confidence interval.
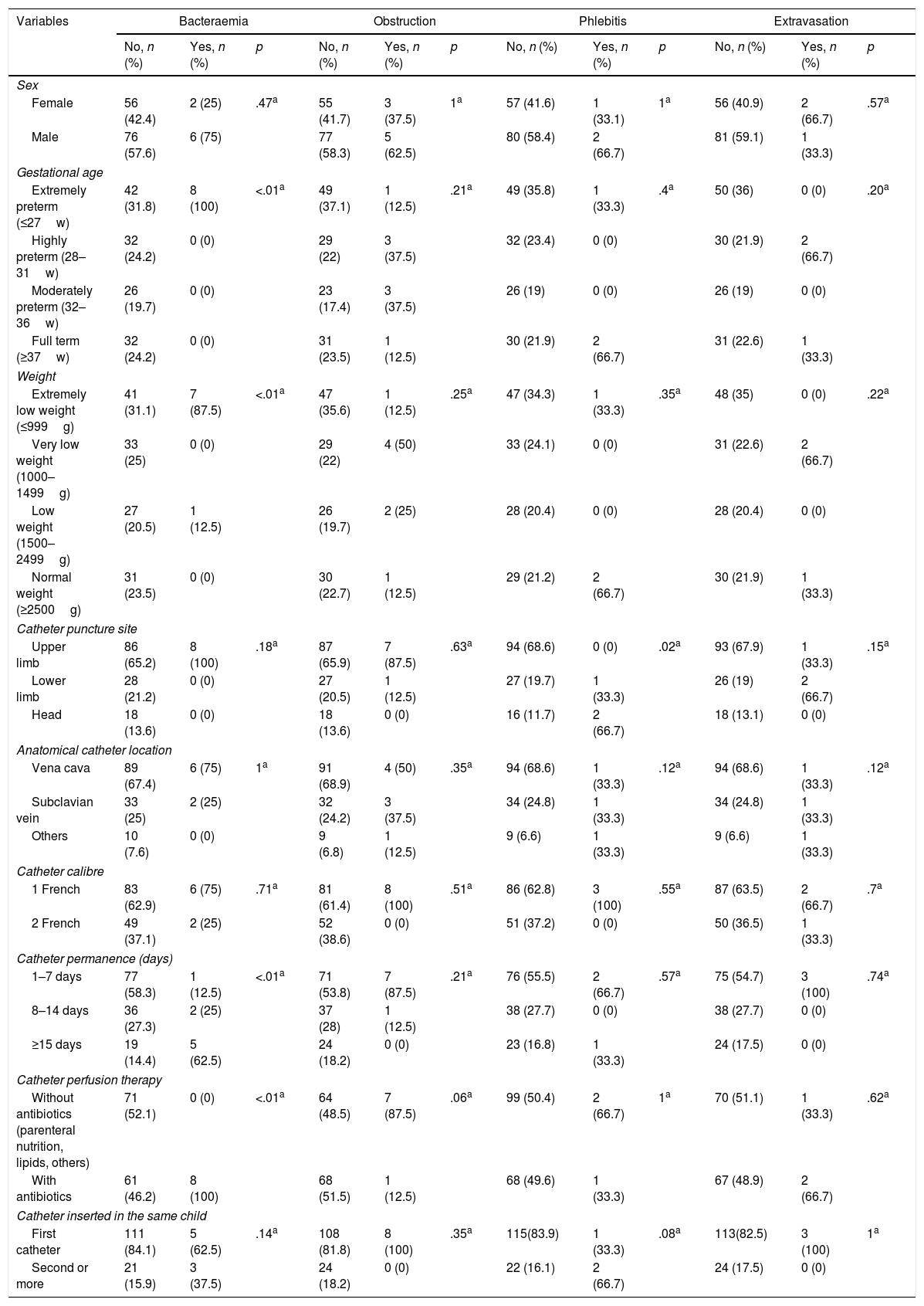
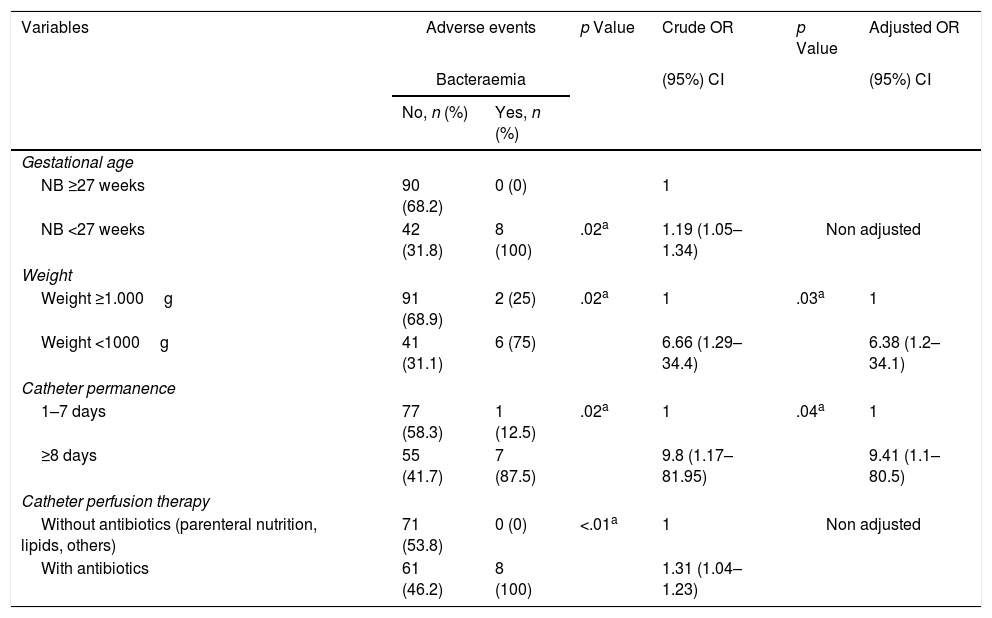
Analysis of the reasons for catheter removal reveal that the bacteraemia was statistically significantly associated with the NB <27weeks (OR=1.2; p=.02), with a weight of <1000g (OR=6,7; p=.02), permanence over 7 days (OR=9.8; p=.02) and with antibiotic therapy infusion (OR=1.3; p<.01). Phlebitis was only associated with the anatomical area where the catheter was located, and in 66.7% of cases it presented in catheters inserted into the head (p=.02). Moreover, the NB with PICC inserted into the LL and the head had more phlebitis compared to those with catheters in UL (OR=1.07; p=.03). No statistical significances were found between the studied variables and risk of obstruction and extravasation (Tables 3 and 4).
Comparison between reasons for catheter removal and risk factors.
| Variables | Bacteraemia | Obstruction | Phlebitis | Extravasation | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No, n (%) | Yes, n (%) | p | No, n (%) | Yes, n (%) | p | No, n (%) | Yes, n (%) | p | No, n (%) | Yes, n (%) | p | |
| Sex | ||||||||||||
| Female | 56 (42.4) | 2 (25) | .47a | 55 (41.7) | 3 (37.5) | 1a | 57 (41.6) | 1 (33.1) | 1a | 56 (40.9) | 2 (66.7) | .57a |
| Male | 76 (57.6) | 6 (75) | 77 (58.3) | 5 (62.5) | 80 (58.4) | 2 (66.7) | 81 (59.1) | 1 (33.3) | ||||
| Gestational age | ||||||||||||
| Extremely preterm (≤27w) | 42 (31.8) | 8 (100) | <.01a | 49 (37.1) | 1 (12.5) | .21a | 49 (35.8) | 1 (33.3) | .4a | 50 (36) | 0 (0) | .20a |
| Highly preterm (28–31w) | 32 (24.2) | 0 (0) | 29 (22) | 3 (37.5) | 32 (23.4) | 0 (0) | 30 (21.9) | 2 (66.7) | ||||
| Moderately preterm (32–36w) | 26 (19.7) | 0 (0) | 23 (17.4) | 3 (37.5) | 26 (19) | 0 (0) | 26 (19) | 0 (0) | ||||
| Full term (≥37w) | 32 (24.2) | 0 (0) | 31 (23.5) | 1 (12.5) | 30 (21.9) | 2 (66.7) | 31 (22.6) | 1 (33.3) | ||||
| Weight | ||||||||||||
| Extremely low weight (≤999g) | 41 (31.1) | 7 (87.5) | <.01a | 47 (35.6) | 1 (12.5) | .25a | 47 (34.3) | 1 (33.3) | .35a | 48 (35) | 0 (0) | .22a |
| Very low weight (1000–1499g) | 33 (25) | 0 (0) | 29 (22) | 4 (50) | 33 (24.1) | 0 (0) | 31 (22.6) | 2 (66.7) | ||||
| Low weight (1500–2499g) | 27 (20.5) | 1 (12.5) | 26 (19.7) | 2 (25) | 28 (20.4) | 0 (0) | 28 (20.4) | 0 (0) | ||||
| Normal weight (≥2500g) | 31 (23.5) | 0 (0) | 30 (22.7) | 1 (12.5) | 29 (21.2) | 2 (66.7) | 30 (21.9) | 1 (33.3) | ||||
| Catheter puncture site | ||||||||||||
| Upper limb | 86 (65.2) | 8 (100) | .18a | 87 (65.9) | 7 (87.5) | .63a | 94 (68.6) | 0 (0) | .02a | 93 (67.9) | 1 (33.3) | .15a |
| Lower limb | 28 (21.2) | 0 (0) | 27 (20.5) | 1 (12.5) | 27 (19.7) | 1 (33.3) | 26 (19) | 2 (66.7) | ||||
| Head | 18 (13.6) | 0 (0) | 18 (13.6) | 0 (0) | 16 (11.7) | 2 (66.7) | 18 (13.1) | 0 (0) | ||||
| Anatomical catheter location | ||||||||||||
| Vena cava | 89 (67.4) | 6 (75) | 1a | 91 (68.9) | 4 (50) | .35a | 94 (68.6) | 1 (33.3) | .12a | 94 (68.6) | 1 (33.3) | .12a |
| Subclavian vein | 33 (25) | 2 (25) | 32 (24.2) | 3 (37.5) | 34 (24.8) | 1 (33.3) | 34 (24.8) | 1 (33.3) | ||||
| Others | 10 (7.6) | 0 (0) | 9 (6.8) | 1 (12.5) | 9 (6.6) | 1 (33.3) | 9 (6.6) | 1 (33.3) | ||||
| Catheter calibre | ||||||||||||
| 1 French | 83 (62.9) | 6 (75) | .71a | 81 (61.4) | 8 (100) | .51a | 86 (62.8) | 3 (100) | .55a | 87 (63.5) | 2 (66.7) | .7a |
| 2 French | 49 (37.1) | 2 (25) | 52 (38.6) | 0 (0) | 51 (37.2) | 0 (0) | 50 (36.5) | 1 (33.3) | ||||
| Catheter permanence (days) | ||||||||||||
| 1–7 days | 77 (58.3) | 1 (12.5) | <.01a | 71 (53.8) | 7 (87.5) | .21a | 76 (55.5) | 2 (66.7) | .57a | 75 (54.7) | 3 (100) | .74a |
| 8–14 days | 36 (27.3) | 2 (25) | 37 (28) | 1 (12.5) | 38 (27.7) | 0 (0) | 38 (27.7) | 0 (0) | ||||
| ≥15 days | 19 (14.4) | 5 (62.5) | 24 (18.2) | 0 (0) | 23 (16.8) | 1 (33.3) | 24 (17.5) | 0 (0) | ||||
| Catheter perfusion therapy | ||||||||||||
| Without antibiotics (parenteral nutrition, lipids, others) | 71 (52.1) | 0 (0) | <.01a | 64 (48.5) | 7 (87.5) | .06a | 99 (50.4) | 2 (66.7) | 1a | 70 (51.1) | 1 (33.3) | .62a |
| With antibiotics | 61 (46.2) | 8 (100) | 68 (51.5) | 1 (12.5) | 68 (49.6) | 1 (33.3) | 67 (48.9) | 2 (66.7) | ||||
| Catheter inserted in the same child | ||||||||||||
| First catheter | 111 (84.1) | 5 (62.5) | .14a | 108 (81.8) | 8 (100) | .35a | 115(83.9) | 1 (33.3) | .08a | 113(82.5) | 3 (100) | 1a |
| Second or more | 21 (15.9) | 3 (37.5) | 24 (18.2) | 0 (0) | 22 (16.1) | 2 (66.7) | 24 (17.5) | 0 (0) | ||||
Adverse events associated with study NB characteristics.
| Variables | Adverse events | p Value | Crude OR | p Value | Adjusted OR | |
|---|---|---|---|---|---|---|
| Bacteraemia | (95%) CI | (95%) CI | ||||
| No, n (%) | Yes, n (%) | |||||
| Gestational age | ||||||
| NB ≥27 weeks | 90 (68.2) | 0 (0) | 1 | |||
| NB <27 weeks | 42 (31.8) | 8 (100) | .02a | 1.19 (1.05–1.34) | Non adjusted | |
| Weight | ||||||
| Weight ≥1.000g | 91 (68.9) | 2 (25) | .02a | 1 | .03a | 1 |
| Weight <1000g | 41 (31.1) | 6 (75) | 6.66 (1.29–34.4) | 6.38 (1.2–34.1) | ||
| Catheter permanence | ||||||
| 1–7 days | 77 (58.3) | 1 (12.5) | .02a | 1 | .04a | 1 |
| ≥8 days | 55 (41.7) | 7 (87.5) | 9.8 (1.17–81.95) | 9.41 (1.1–80.5) | ||
| Catheter perfusion therapy | ||||||
| Without antibiotics (parenteral nutrition, lipids, others) | 71 (53.8) | 0 (0) | <.01a | 1 | Non adjusted | |
| With antibiotics | 61 (46.2) | 8 (100) | 1.31 (1.04–1.23) | |||
| Phlebitis | p Value | Crude OR | p Value | Adjusted OR | ||
|---|---|---|---|---|---|---|
| No, n % | Yes, n % | (95%) CI | (95%) CI | |||
| Catheter puncture site | ||||||
| Upper limb | 94 (68.6) | 0 (0) | .03a | 1 | Non adjusted | |
| Lower limb/head | 43 (31.4) | 3 (100) | 1.07 (.99–1.15) | |||
CI: confidence interval; OR: odds ratio.
Table 4 contains the results obtained after adjusting the logistic regression model in accordance with the variables which appeared to be significant in bivariate analysis (bacteraemia and phlebitis). The results indicated a greater risk of bacteraemia associated with the catheter in NB with extremely low weight (<1000g) (OR=6.8; p=.03) and in those who had had a catheter for over 7 days compared with those who had had it for 7 or fewer days (OR=9.41; p=.04).
DiscussionIn this study the most used PICC calibre in NB analysed was that of 1Fr. This is due to the fact that over half of the sample are extremely preterm and very low weight, with a gestational age of <31 weeks. Newborns with a small catheter (1Fr) were at greater risk of AE than those with higher calibre catheters (2Fr). These results coincide with studies where lower calibre PICC has a shorter duration and higher probability of AE.17,18
In the newborns studied the anatomical area of choice for insertion of the PICC was the upper limbs (67.1%), with the superior vena cava first line choice. This data coincides with that of other studies.11,19,20 It was therefore confirmed that the location of catheters is associated with complications. In a study by Lloreda-Garcia et al.10 the mid-way clavicular PICC presented with more risks of complications than those located in vena cava or brachiocephalic vein. Other authors have described severe complications (pneumothorax, cardiac taponade, thrombosis and even cardiorespiratory arrest) caused by catheter positioning from inappropriate location of its tip.3,8,9 In the sample analysed, no event of these dimensions occurred as a consequence of poor catheter positioning. This may be due to the rapid radiological confirmation of catheter tip location, which has enabled rectification and avoidance of inappropriate positioning. The most common reason for removal of the PICC studied was elective, mainly due to termination of intravenous treatment, which was in line with other published research studies.11,17,19
The percentage of AE in the sample NB with epicutaneous catheters was 16.4%. In the references reviewed figures ranging between 10% and 57.2% were obtained.2,3,9,10,13,16–26 If we compare the percentage obtained in this study which those we reviewed, we observe lower percentages. Although no data prior to this study are available, it is believed that this result may be due to the introduction of the “Zero Bacteraemia” protocol which has had a positive effect despite only being in operation 3 months prior to the beginning of data collection. Notwithstanding, for greater confirmation a more exhaustive longitudinal follow-up is required.
In several studies results were obtained where the non infectious type complications associated with PICC are more common than the infectious type complications.11,18,19 In this study we also found there were more mechanical complications (10.7%) than infectious ones (57%). However, the results obtained were inferior to findings by other authors. Specifically, in the study by Gomes et al.11 there were 21.4% mechanical complications compared with 14.3% of infectious type events. Van den Berg et al.19 also found there were more mechanical type complications (71%) than infectious ones (13%). Equally, in a study conducted in India, Singh et al.18 obtained figures of 12.2% of non infectious complications compared with 10.7% of infectious complications.
Regarding mechanical complications 5.7% of obstructions were found to be the most common in those analysed. This result is lower than that reflected by other studies, where there was from 13.1% to 30.9% obstruction.9,11,17,21,23,24 Extravasation at 2.1%, was the second most prevalent complication found in our study, a figure which is higher than that obtained by Costa et al.24 (1.2%) and below that from those obtained in other studies (from 5% to 11.9%).9,21,27 However, no significant association was found between the variables studied and these two mechanical complications, and neither from that found in the reviewed references. Catheter rupture occurred in only one case (.8% of all NB studied) with this percentage being higher in the literature consulted (ranging between 8.8% and 16.4%).9,11,21,23,24
Regarding treatment administered through the catheter, 49.3% of the PICC analysed were used to administer therapies with antibiotics. In the bivariate analysis a greater risk of bacteraemia was observed which was associated with the catheter in newborns with antibiotic infusion compared with those who did not have these treatments. No studies were found to match this findings. After logistical regression analysis we confirmed that treatments with antibiotics did not independently impact the presentation of bacteraemia.
Presentation of catheter-associated phlebitis may be from infectious or mechanical causes. In this study we obtained a very low rate of it (2.1%) if we compare it with other research studies. Thus, Donovan et al.21 obtained figures of 3.6% and Ma et al.27 of 11.5%, with this AE being higher in children of lower weight and lower gestational age. Our results show there is an association between the presentation of phlebitis and the anatomical puncture site, so that if the catheter is inserted in the LL and head there is a greater probability that this AE will present than if it is inserted in the UL. These data coincide with several authors who state that there are more complications in PICC inserted in LL than those inserted in UL.27,28 However, there are studies which differ, such as that conducted by Njere et al.,29 which found a lower rate of AE in PICC inserted in LL compared with that in UL and in the study by Callejas et al.,30 which showed a higher rate of infection in catheters inserted in scalp veins and in UL than those inserted in LL.
In our study infectious complications (bacteraemia) were observed in 5.7% of newborns. In the bivariate analysis an association was found between gestational age and extremely low weight of NB with the administration of antibiotics through the catheter and with the days of its permanence. After logistic regression analysis and the calculation of factors which independently impact the presentation of catheter-associated AE, an association was found between bacteraemia and neonates with a weight equal to or lower than 1000g (6.4 times more likely to present with this AE than in neonates who weighed over 1kg) and those with a permanence above 7 days of catheter (with a risk 9.4 times higher compared to those who had the catheter for under a week).
The duration of cannulation essentially depends on the patient characteristics (weight, severity, cannulation complexity, etc.). Permanence of time in catheters is variable according to different reviewed publications and is situated between a mean of 3 and 18 days.7,11,17,18,22–25,29–31 In our study a permanence period with a mean of 7 days was verified (5–10.75), with this data being in line with reviewed studies.
One study conducted in two hospitals in Turkey17 observed a significant increase in the catheter contamination from the third week of permanence. In turn, Njere et al.29 detected that the probability of catheter-associated bacteraemia increased by 3.1 times if it was in situ for 9 days or more, which was a similar result to that obtained in this research, where the probability of catheter-associated bacteraemia increased after 7 days of catheter permanence.
According to a report by the MSSSI,2 the rate of nosocomial sepsis is inversely proportional to weight and gestational age and affects over 20% of NB of very low weight, reaching almost 50% in NB with a weight under 1000g. Several studies describe gestational age and weight as risk factors of bacteraemia related to PICC. Wen et al.,16 Zhao et al.26 and García González et al.32 found there was an association between bacteraemia and NB with a weight of <1500g. Nercelles et al.7 and Van den Berg et al.19 found there was a significant association between the bacteraemia related to the PICC in newborns with a weight under 1000g, coinciding with findings from these two studies with the results of this investigation.
Study limitations and strengthsOne of the limitations of this study was the small NB sample, together with the low rate of AE found. This meant that the independent factors associated with the AE detected, such as obstruction, phlebitis and extravasation could not be studied. Another limitation was not including analysis of umbilical catheters in this study, as has been done in other studies, which impeded us from obtaining information on these catheters and comparing results.
The main strength of this research was that it contained all newborns with PICC over a natural year, with no seasonal bias. The low prevalence of AE found was also key to the study and the unit. This led us to suggest that the “Zero Bacteraemia” protocol had been successful, although further studies are needed to compare results.
ConclusionsCare of the NB in the NICU is associated with a high risk of AE. Their physiological immaturity, the severity of their pathology and the complexity and duration of treatments means that these children are highly vulnerable. Strict asepsis and minimal manipulation of the perfusion systems are effective preventative measures. Any measure adopted to reduce the rate of complications is always justified, since a PICC-associated infection increases morbidity and mortality in the NB and also raises health costs.
This study has examined our clinical practice and obtained further information on the AE related to PICC. The results provide relevant information for all nurses who are undertaking clinical practice in the NICU. These findings will help us to reinforce the safety of the most vulnerable NB, and particularly newborns with extremely low weight that require prolonged treatments. Reinforcing asepsis measures with specific protocols such as the “zero bacteraemia” helps to increase clinical safety and reduce morbimortality in these children. Periodical assessment of catheter-associated AE is highly useful in optimisation plans. Protocols relating to catheter insertion and maintenance will be updated as a result, bearing in mind the risk factors associated with its complications, and improving healthcare quality.
FinancingThis study did not receive any funding or subsidy.
Conflict of interestsThe authors have no conflict of interests to declare in this study.
We would like to thank all the nurses who took part in the study, and the hospital for having granted us permission to conduct it.
Please cite this article as: Padilla-Sánchez C, Montejano-Lozoya R, Benavent-Taengua L, Monedero-Valero A, Borras-Vañó MJ, Ángel-Selfa MJ, et al. Factores de riesgo asociados a eventos adversos en recién nacidos portadores de catéteres centrales de inserción periférica. Enferm Intensiva. 2019;30:170–180.