The clinical guideline for the management of sepsis, recommends using arterial blood samples for glycaemic control. A multicentre study in 86 Spanish intensive care units (ICU) revealed that 85.4% of ICUs used capillary puncture.
ObjectiveTo analyse the reliability of glycaemia by comparing different blood samples (arterial, venous, capillary) and instruments (glucometers, gasometers, central laboratory). Secondarily, to estimate the effect of confounding variables and the performance of measuring instruments as determined by different quality standards.
MethodologySystematic review and meta-analysis with search in PubMed, CINAHL and Embase databases in September-2021 and September-2022, with no time or language limits. Grey literature sources: DART-Europe, OpenGrey and Google Scholar. Results summarised by qualitative (description of results, study characteristics) and quantitative (meta-analysis to assess standardised mean difference) synthesis. Methodological quality of articles assessed with Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2). Protocol: https://osf.io/ DOI 10.17605/OSF.IO/T8KYP.
ResultsA total of 32 articles and 5451 patients were included. No discrepancies were obtained between arterial glucometer vs laboratory samples [bias (95%CI): 0.01 (−0.12 to 0.14) mg/dL]. In contrast, arterial samples with a gasometer did significantly overestimate [bias (95%CI): 0.12 (0.01 to 0.24) mg/dL]. The same trend is seen in capillaries with a glucometer, although not significantly [bias (95%CI): 0.07 (-−0.02 to 0.15) mg/dL]. There is discrepancy between studies on the effect of haematocrit and acid-base balance. The greatest consensus is on the poor agreement of glucometer with capillary vs laboratory samples in the presence of shock and vasopressor support, renal failure or during vitamin C treatment.
ConclusionsThe evidence to date recommends the use of arterial blood with a blood glucose meter for better reliability of glycaemic analysis and less effect of possible confounding variables, frequently present in the critically ill adult patient.
La guía clínica para el manejo de la sepsis, recomienda usar muestras de sangre arterial para el control glucémico. Un estudio multicéntrico en 86 unidades de cuidados intensivos (UCI) españolas reveló que el 85,4% de las UCI utilizaban punción capilar.
ObjetivoAnalizar la fiabilidad de la glucemia comparando diferentes muestras sanguíneas (arterial, venosa, capilar) e instrumentos (glucómetros, gasómetros, laboratorio central). Secundariamente, estimar el efecto de variables confusoras y el rendimiento de los instrumentos de medición determinados por las diferentes normas de calidad.
MetodologíaRevisión sistemática y metanálisis con búsqueda en bases de datos PubMed, CINAHL y Embase en septiembre-2021 y septiembre-2022, sin límites temporales ni idiomáticos. Fuentes de literatura gris: DART-Europe, OpenGrey y Google Académico. Resultados resumidos mediante síntesis cualitativa (descripción de resultados, características de los estudios) y cuantitativa (metanálisis para evaluar la diferencia de medias estandarizadas). Calidad metodológica de artículos evaluada con Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2). Protocolo: https://osf.io/ DOI 10.17605/OSF.IO/T8KYP.
ResultadosSe incluyeron un total de 32 artículos y 5451 pacientes. No se obtuvieron discrepancias entre muestras arteriales con glucómetro vs laboratorio [sesgo (IC95%):0,01 (−0,12 a 0,14) mg/dL]. En cambio, arteriales con gasómetro sí sobrestimaron de forma significativa [sesgo (IC95%):0,12 (0,01 a 0,24) mg/dL]. La misma tendencia presentan capilares con glucómetro, aunque no de forma significativa [sesgo (IC95%):0,07 (−0,02 a 0,15) mg/dL]. Hay discrepancia entre los estudios sobre el efecto del hematocrito y el equilibrio ácido-base. El mayor consenso se da en la poca concordancia del glucómetro con muestras capilares vs laboratorio en presencia de shock y soporte vasopresor, situación de fallo renal o durante el tratamiento con vitamina C.
ConclusionesLa evidencia hasta el momento recomienda el uso de sangre arterial con gasómetro para una mejor fiabilidad del análisis glucémico y menor efecto de posibles variables confusoras, frecuentemente presentes en el enfermo crítico adulto.
According to the clinical guide for the management of sepsis, Surviving Sepsis Campaign,1 intravenous insulin should be administered when glycaemia values are >180 mg/dL, keeping it within a range from 140 to 180 mg/dL (strong recommendation). Although the latest edition of the clinical guide for sepsis treatment1 does not offer recommendations about how to monitor glycaemia, the 2016 guide2 states that it should be measured every 1−2 hrs. until glycaemia levels and insulin infusion rates stabilize. It is recommended that arterial blood samples should be used to monitor glycaemia instead of capillary blood (weak recommendation, evidence of low quality) due to a possible lack of precision, especially in the ranges of hypoglycaemia and hyperglycaemia and in patients in shock with vasopressor treatment.2
Bedside glucometers should only be used when the patient has no catheters to obtain samples of venous or arterial blood, when the only alternative is therefore capillary puncture.3 The latest generation of glucometers are more promising in terms of reliability, although special care has to be taken when certain factors may make them less accurate, such as haematocrit, partial oxygen pressure and vasoactive drugs.4,5
The most recent systematic review found in the literature,6 from 2013, concluded that the most reliable method for analysis of glycaemia uses arterial blood with a gas analyser, followed by arterial blood with a glucometer. Nevertheless, within the hypoglycaemia range (< 81 mg/dL), the incidence of error in devices using arterial samples was greater than it was in normal ranges of glycaemia (odds ratio of gas analyser vs. glucometer error: 1.86 vs. 2.33).
Within the context of the MOviPre multicentre national study to analyse early mobilisation in Spanish intensive care units (ICU), and given that hyperglycaemia is a risk factor for muscular weakness, the collaborating researchers were asked about the type of blood sample and analyser they used for the analysis of glycaemia.7 85.4% of the ICU used capillary puncture to analyse glycaemia, and 94.4% used glucometers. Of the glucometers used, only 36.2% were AccuChek®. This glucometer is able to reduce the deviation generated by haematocrit when measuring glycaemia, and only one ICU (1.2%) used Stata-Strip®, a latest generation glucometer. These results were ratified by García del Moral-Martín et al.8 in Andalusia, in a broad homogeneous sample, as representative at the level of an autonomous community. Nevertheless, although the evidence about how glycaemia monitoring should take place was published more than five years ago, it has yet to be applied in practice.
Given that the latest guide1 on sepsis management does not examine how to monitor glycaemia and recommends broadening research to control it more safely, we consider it to be timely to undertake a review that is able to offer new evidence about glucose monitoring in critical adult patients. We will analyse the reliability of this control, comparing different blood samples and instruments, and secondarily estimate the effect of the confusion variables and the performance of measuring instruments as determined by different quality standards.
The question for this review was drawn up using the acronym PICOS (patient/population, intervention, comparison, outcome-results, study type), where each item corresponds to: (P) critical adults patients treated in an ICU, and who may correspond to different types: medical, surgical, polyvalent, cardiological or neurological, as well as emergency department critical units; (I) studies that evaluate the precision of glycaemia analysis by glucometers or gas analysers; (C) with or without comparison with glycaemia values analysed in a central laboratory; (O) the magnitude of the difference in comparison with glycaemia measurements in different samples, according to the instruments and analytical methods used (the main result) and the performance of glucometers and/or gas analysers according to the different quality criteria or interchangeability (secondary result), and analysis of the influence of possible confusion variables on the inaccuracy of the measurement (secondary result), and (S) observational, experimental and quasi-experimental studies.
The research question was: what type of blood (arterial, venous, capillary) and analytical instrument (glucometer, gas analyser) should I use to measure glycaemia at the bedside of a critical adult patient?
MethodologyA systematic review and meta-analysis according to Joanna Briggs Institute (JBI) methodology for reviews of exactitude of diagnostic tests, and reported according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.9,10 The study protocol has been published in OSF (https://osf.io/ DOI 10.17605/OSF.IO/T8KYP).
Inclusion criteriaTo respond to the research question a bibliographical search was undertaken in any published language and without exclusion due to publication date. The following inclusion criteria were applied: (1) study type: randomized and non-randomized clinical trials, before and after studies, observational, prospective and retrospective studies, cases-control and cross-sectional studies, as well as qualitative studies; (2) studies whose abstract reports comparisons that were implemented using different analytical instruments (intraclass correlation coefficient, Bland and Altman); (3) studies in which critical adults patients participated; (4) studies which analysed the precision of glycaemia analysis using bedside instruments (point of care [POC]) using glucometers or gas analysers, and which express their results according to the concordance between glycaemia values measured by the different instruments and samples (arterial, venous or capillary).
Studies which evaluated glycaemia using laboratory-manipulated human blood samples were excluded, as were those which analysed continuous interstitial glucose monitors. Studies undertaken in major burns units, congress summaries, books and editorials were all excluded, following the recommendations for a systematic review.
Sources of information and the search strategyIn September 2021 we carried out a preliminary search to locate systemic reviews that had been published or were underway, as well as to identify potentially relevant papers and identify terms and descriptors that would be relevant for the definitive search. The databases consulted were PROSPERO (https://www.crd.york.ac.uk/prospero/), Cochrane Database of Systematic Reviews (Wiley, 1995-), PubMed (1945-), Embase (Elsevier, 1947-), JBI EBP Database (Ovid) and ClinicalTrials.gov.
The definitive bibliographical search to identify potentially relevant documents was carried out in September 2021 and updated in September 2022. It included the databases PubMed (1946-), Embase (Elsevier, 1947-) and CINAHL (EBSCO, 1937-).
The search strategies were prepared by an experienced librarian (CCA) and revised by another information specialist (JMM) prior to their execution using the Peer Review of Electronic Search Strategies11 checklist. The search strategy for each database is described in the supplementary material (Appendix A Table S1). The search was designed with the aid of the following tools: Yale MeSH Analyzer,12 PubReMiner13 and Polyglot Search Translator.14
The search in electronic databases was supplemented by a search in DART-Europe for access to theses, OpenGrey and Google Académico, and experts were contacted too. A supplementary search was also undertaken in the references lists of the studies selected for inclusion in this review, to identify additional relevant studies. A systematic search of citations was then carried out, compiling all of the studies that cited the selected papers for inclusion by using CitationChaser (https://estech.shinyapps.io/citationchaser/).15
The authors of key papers and summaries were also contacted to request more information about their studies.
The final results of the search were exported to Zotero and duplicates were eliminated.
The selection processPrior to selecting studies we carried out a pilot test with 15 papers in each one of both screening process phases, considering a coincidence in more than 75% as the consensus criterion; both reviewers obtained an agreement of 96%. All of the references were independently screened using their titles and abstracts according to the inclusion criteria described above. After the selection of titles and abstracts the complete texts of the remaining papers were recovered. The complete texts were then reviewed independently according to the inclusion criteria. Discrepancies were resolved by agreement. The resulting references were then loaded in the Rayyan programme (https://www.rayyan.ai/).16
Data extractionA standard formulation was used that had initially been piloted in 5 of the included studies. The reviewers extracted data from the selected studies: the first author, year and country of publication, methodology, number of patients, number of paired samples, sample type used, instruments used, the analytical method used by the instruments, results in terms or reliability, confusion variables analysed, analysis based on quality criteria and the authors’ conclusions.
Critical evaluation of the studiesThe methodological quality of the selected papers was independently evaluated by 2 reviewers using the Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2) tool.17 The reviewers then went on to agree a shared result, resolving any discrepancies by consensus.
Data analysisThe results were summarised by qualitative and quantitative synthesis. In the qualitative synthesis a table was prepared containing the description of the results and the characteristics of the studies. A meta-analysis was performed in the quantitative synthesis to evaluate the magnitude of the difference in the results. All of the meta-analytical methods used in this study are based on the guide by Harrer et al.18
Version 4.0.3 of R software was used for all of the analyses and graphs. All of the studies with sufficient data to calculate the difference of standardized averages were included in the meta-analyses. Averages were calculated based on the median and range in the studies that did not present their results in this form.19 When standard deviations were not available they were calculated using the P value, the tabulated t value, the difference between averages and the standard error.20
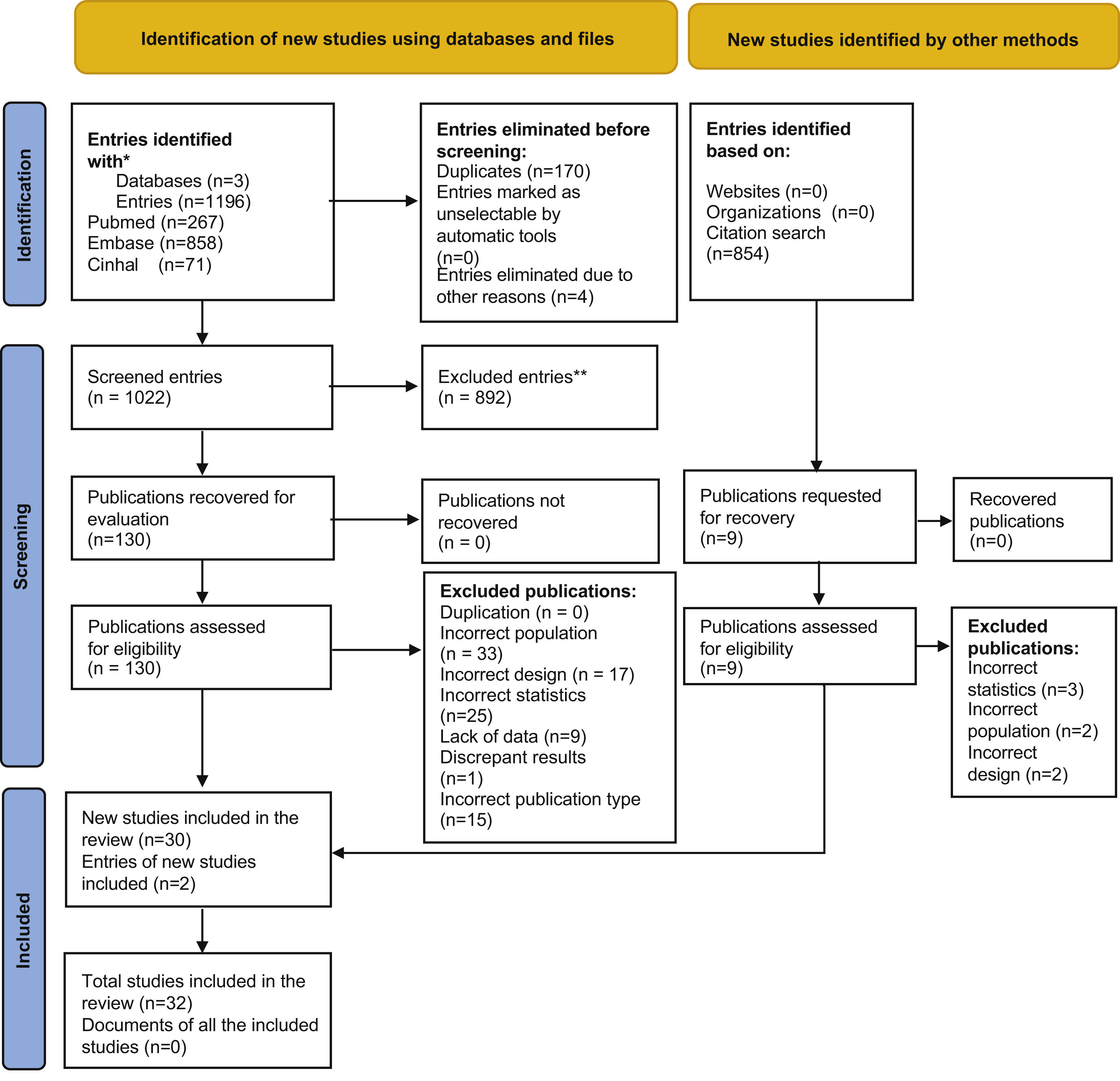
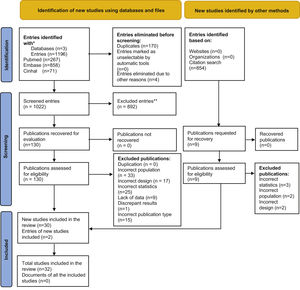
Results1,196 abstracts from the database search were analysed, of which the complete text was evaluated in 130 cases. 30 of these papers plus 2 others located in a secondary review of a citation search were included. The selection process was recorded in sufficient detail to complete a PRISMA flow diagram (Fig. 1) and a list of excluded studies with the reasons for exclusion.
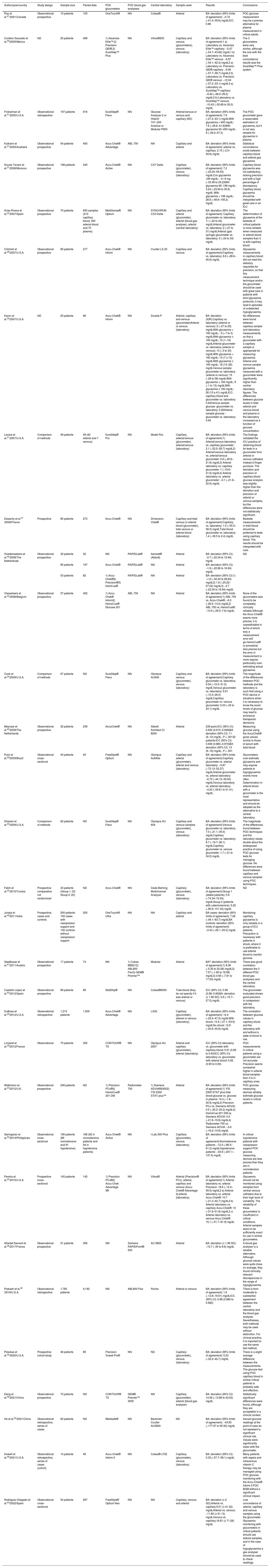
Of the 32 studies included, 26 are prospective observational studies, 5 are retrospective and one consists of cases and controls. 10 studies compare different instruments, 2 compare different samples and 20 studies compare different samples and instruments. A total number of 5.451 patients were analysed in all 32 studies (Table 1).
Characteristics and results of the studies included in the systematic review (n = 32).
| Author/year/country | Study design | Sample size | Paired data | POC glucometers | POC blood gas analysers | Central laboratory | Sample used | Results | Conclusions | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ray et al.41/2001/Canada | Observational prospective | 10 patients | 105 | OneTouch® | NN | Cobas® | Arterial | BA: deviation (95% limits of agreement): −0.72 (−41.4–39.6) mg/dLICC: 0.86 | POC glucose measurement may be a precise alternative for plasma measurement in critical adults | |||
| Cordero Saucedo et al.39/2005/México | ND | 25 patients | 489 | 1) Ascensia Elite™,2) Precision QID®,3) SureStep™ Plus | NN | Vitros®250 | Capillary and venous (glucometers), venous (laboratory) | BA: deviation (95% limits of agreement):1 a) Laboratory vs. Ascensia Elite™ capillary: −5,47 (−54.7–43.82) mg/dL1 b) Laboratory vs. Ascensia Elite™ venous: −5.87 (−54.1–42.4) mg/dL2 a) Laboratory vs. Precision QID® capillary: −9.46 (−57.7–38.7) mg/dL2 b) Laboratory vs. Precision QID® venous: −12.04 (−57.2–33.1) mg/dL3 a) Laboratory vs. SureStep™ capillary: −9.40 (−48.0–29.2) mg/dL3 b) Laboratory vs. SureStep™ venous: −10.43 (−50.89 to 30.0) mg/dL | The 3 glucometers were very similar, although the one with the best concordance results was the SureStep™ Plus system | |||
| Finkielman et al.51/2005/U.S.A. | Observational retrospective | 197 patients | 816 | SureStep® Flexx | NN | Glucose Analyzer 2 or Hitachi 747−200 Analizer or Modular P800 | Arterial/venous or venous and capillary (ND) | BA: deviation (95% limits of agreement): 7.9 (−27.2–43.1) mg/dLWith glycaemia > 400 mg/dL: 7.6 (−26.6–41.8)With glycaemia 50−400 mg/dL: 9 (−29.2–47.3) | The POC glucometer gave a reasonable estimation of glycaemia, but it is not very reliable for glycaemia in plasma | |||
| Kulkarni et al.37/2005/Australia | Observational prospective | 54 patients | 493 | Accu-Chek® Advantage | ABL 700 | NN | Capillary and arterial | BA: deviation (95% limits of agreement): arterial vs. capillary: 2.15 (−2.5–29.8) mg/dL | Statistical concordance exists between capillary blood and arterial gas glycaemia | |||
| Soussi Tanani et al.31/2006/Morocco | Observational prospective | 198 patients | 245 | Accu-Chek® Active | NN | CX7 Delta | Capillary (glucometer), venous (laboratory) | BA: deviation (95% limits of agreement): 7.2 (−43.24–59.45) mg/dLCon glycaemia <99 mg/dL: −4.14 mg (−33.48 to 25.2)With glycaemia 99−198 mg/dL: 3.24 (−23.94 to 30.6) mg/dL With glycaemia > 198 mg/dL: 28.8 (−48.6–106.2) mg/dL | Capillary blood glycaemia was not satisfactory, lacking precision and with a high percentage of discrepancy. Capillary blood glycaemia should be interpreted with great care in an ICU | |||
| Arias-Rivera et al.22/2007/Spain | Observational prospective | 70 patients | 630 samples (210 capillary blood, 350 arterial blood and 70 plasma) | MediSense® Optium | NN | SYNCHRON CX3 Delta | Capillary and arterial (glucometer), arterial (blood gas analyser), arterial (central laboratory) | BA: deviation (95% limits of agreement): Capillary glucometer vs. laboratory: 11 (−22 to 44) mg/dLArterial glucometer vs. laboratory: 2 (−27 to 31) mg/dLArterial (gas syringe) glucometer vs. laboratory: 5 (−24 to 34) mg/dL | The determination of glycaemia at the bedside of critical patients is more reliable when measured in arterial blood samples than it is with capillary blood | |||
| Critchell et al.28/2007/U.S.A. | Observational prospective | 80 patients | 277 | Accu-Chek® Inform | NN | Coulter LX-20 | Capillary and venous | BA: deviation (95% limits of agreement):Capillary vs. laboratory: 8.6 (−28.6–45.8) mg/dL | Glycaemia measurements in capillary blood did not meet the statutory requisites for precision, so that this measurement technique and/or the glucometer should be used with great care in patients with strict glycaemia protocols; it may lead to episodes of undetected hypoglycaemia | |||
| Karon et al.35/2007/U.S.A. | ND | 20 patients | 96 | Accu-Chek® Inform | NN | Double P | Arterial, capillary and venous (glucometer)Arterial or venous (laboratory) | BA: deviation (IQR):Capillary vs. laboratory (arterial or venous): 2 (−27 to 29) mg/dLWith glycaemia < 160 mg/dL: −3 (−7 to 3) mg/dLWith glycaemia ≥ 160 mg/dL: 10 (1–16) mg/dLArterial glucometer vs. laboratory (arterial or venous): 15 (−3 to 33) mg/dLWith glycaemia < 160 mg/dL: 10 (7 a 13) mg/dLWith glycaemia ≥ 160 mg/dL: 18 (13–26) mg/dLVenous sample glucometer vs. laboratory (arterial or venous): 15 (−28 to 58) mg/dLWith glycaemia < 160 mg/dL: 9 (−1 to 12) mg/dLWith glycaemia ≥ 160 mg/dL: 26 (15 a 41) mg/dLICC: capillary blood and glucometer vs. laboratory: 0.94Venous sample glucose -glucometer vs. laboratory: 0.92Arterial sample glucose -glucometer vs. laboratory: 0.94 | No differences were found between capillary sample and laboratory measurements, so that a glucometer with a capillary sample is appropriate for measuring glycaemia. Arterial and venous sample glycaemia measured with a glucometer were significantly higher than central laboratory figures. The differences between glucose levels in total arterial and venous blood and plasma in the laboratory increased as a function of glucose concentration | |||
| Lacara et al.21/2007/U.S.A. | Comparison of methods | 49 patients | 49 (42 arterial and 7 venous) | SureStep® Pro | NN | Model RxL | Capillary, arterial/venous (glucometer), arterial/venous (laboratory) | BA: deviation (95% limits of agreement):1) Arterial/venous laboratory vs. capillary glucometer: 2.1 (−22.5–26.7) mg/dL2) Arterial/venous laboratory vs. arterial/venous glucometer: 0.6 (−20.6–21.8) mg/dL3) Arterial laboratory vs. capillary glucometer: 1 (−19.6–21.6) mg/dL4) Arterial laboratory vs. arterial glucometer: −0.1 (−21.6–22.6) mg/dL | The findings validated the ICU practice of obtaining blood for tests in a glucometer from arterial or venous catheters instead of finger puncture. The deviation and precision of capillary blood glucose analysis was slightly higher than the deviation and precision of arterial or venous samples, but the differences were not statistically significant. | |||
| Desachy et al.27 /2008/France | Prospective | 85 patients | 273 | Accu-Chek® | NN | Dimension Vista® | Capillary and total venous or arterial blood (glucometer), total venous or arterial blood (laboratory) | BA: deviation (95% limits of agreement):Capillary vs. laboratory: 1.5 (−55.3–58.3) mg/dLTotal blood glucometer vs. laboratory: 1.4 (−39.5 to 2.4) mg/dL | Bedside measurements in total blood should be preferred to tests using capillary blood. The results should be interpreted with care. | |||
| Hoedemaekers et al.45/2008/The Netherlands | Observational prospective | 32 patients | 32 | NN | RAPIDLab® | Aeroset® (Abbott) | Arterial | BA: deviation (95% CI): −2.7 (−22.34 to 16.94) mg/dL | ND | |||
| 85 patients | 197 | Accu-Chek® | RAPIDLab® | NN | Arterial | BA: deviation (95% CI): −1.8 (−22.66 to 16.94) mg/dL | ||||||
| 53 patients | 82 | 1) Accu-Chek®2) Precision®3) HemCue® | RAPIDLab® | NN | Arterial | BA: deviation (95% CI):1) −1.8 (−32.43 to 28.83) mg/dL2) 1.8 (−25.22–27.02) mg/dL3) −2.7 (−22.34 to 16.94) mg/dL | ||||||
| Vlasselaers et al.44/2008/Belgium | Observational prospective | 37 patients | 452 | 1) Accu-Chek® Inform2) HemoCue® Glucose 201 | ABL 700 | NN | Arterial | BA: deviation (95% limits of agreement):1) ABL 700 vs. Accu-Chek®: −6.3 (−26.5–14.0) mg/dL2) ABL 700 vs. HemoCue®: −10.9 (−29.5–7.6) mg/dL | None of the glucometers was found to be completely clinically reliable.Although the Accu-Chek® seems more precise, it is unpredictable in terms of which way a measurement error will go.HemoCue® is somewhat less precise but the error in measurement is more regular, particularly over-estimating actual glycaemia. | |||
| Cook et al.24/2009/U.S.A. | Comparison of methods | 67 patients | ND | SureStep® Flexx | NN | Olympus AU640 | Capillary and venous (glucometer), venous (laboratory) | BA: deviation (95% limits of agreement):Capillary glucometer vs. laboratory: 9.54 (−12.5–31.5) mg/dLVenous glucometer vs. laboratory: 9.51 (−10.3–26.5) mg/dLCapillary glucometer vs. venous glucometer: 0.03 (−24 to 24.1) mg/dL | The magnitude of the differences between POC methods and the laboratory is such that using a POC device in situations when it is necessary to know the exact levels of glucose may lead to erroneous therapeutic decisions. | |||
| Meynaar et al.42/2009/The Netherlands | Observational prospective | 32 patients | 239 | AccuChek® | NN | Abbott Architect CI 8200 | Arterial | 239 pairs:ICC (95% CI): 0.934 (0.915−0.948)BA deviation (95% CI): 11 (9−13) mg/dL, P < .00132 patients:ICC (95% CI): 0.939 (0.880−0.970)BA deviation (95% CI): 13 (9−16) mg/dL, P < .001 | Measuring glucose using the AccuChek® gives values similar to those of serum with total blood | |||
| Pulzi et al.29/2009/Brazil | Observational cross-sectional | 40 patients | 40 | FreeStyle® Optium | NN | Olympus Au640e | Capillary and arterial (glucometer), arterial and venous (laboratory) | BA: deviation (95% limits of agreement):Capillary glucometer vs. arterial laboratory: −9.87 (−72.12–52.37) mg/dLArterial glucometer vs. arterial laboratory: −6.75 (−44.13–30.63) mg/dLVenous laboratory vs. arterial laboratory: −4.20 (−59.81 to 51.41) mg/dL | Glucometers over-estimate glycaemia and may expose patients to hypoglycaemic events more often. Determination in arterial blood with a glucometer is the most representative and should be adopted as the alternative to a central laboratory. | |||
| Shearer et al.25/2009/U.S.A. | Comparison of methods | 62 patients | ND | SureStep® Flexx | NN | Olympus AU 604 | Capillary and venous samples (glucometer), venous (laboratory) | BA: deviation (95% limits of agreement):Venous glucometer vs. laboratory: 7.0 (−21.1–35.0) mg/dLCapillary glucometer vs. laboratory: 8.7 (−18.7–36.1) mg/dLCapillary glucometer vs. venous glucometer: 1.7 (−31 to 34.5) mg/dL | The magnitude of the differences found between POC techniques and the laboratory raises doubts about the widespread practice of using POC glucose tests for managing glucose. No differences were found between capillary and venous samples using POC techniques. | |||
| Fekih et al.33/2010/Tunisia | Prospective comparative not randomized | 43 patients (Group 1: 23; Group 2: 20) | ND | Accu-Chek® | NN | Dade-Behring Multichannel Analyzer | Capillary (glucometer), venous (laboratory) | BA: deviation (95% limits of agreement):Group 1 (stable patients): 0.9 (−74.34–72.54) mg/dLGroup 2 (patients with catecholamine): 5.22 (−90.9–101.34) mg/dL | ND | |||
| Juneja et al.46/2011/India | Prospective, cases and controls | 200 patients: 100 cases with vasopressor support and 100 controls without vasopressor support | 200 | OneTouch® Ultra | NN | NN | Capillary and arterial | BA cases: deviation (95% limits of agreement): 7.28 (−49.1–63.7) mg/dLBA controls: deviation (95% limits of agreement): −0.43 (−26.1–25.2) mg/dL | Monitoring capillary glycaemia is only reliable in a group of ICU patients. Precaution is necessary with patients in shock, where it is preferable to use arterial blood to monitor glucose. | |||
| Stadlbauer et al.47/2011/Austria | Observational prospective | 17 patients | 74 | NN | 1) Cobas B®2212) ABL800 Flex3) GEM® Premier™ | Modular | Arterial | BAa: deviation (95% limits of agreement):1) 8.36 (−5.35 to 22.08) mg/dL2) 7.57 (−1.85 to 16.99) mg/dL3) 4.56 (−7.91 to 17.03) mg/dL | There was good correlation between the 3 different POC blood gas analysers and the central laboratory. | |||
| Castaño López et al.50/2012/Spain | Observational prospective | 89 patients | 89 | StatStrip® | NN | Cobas®6000 | Total blood (they do not specify if it was arterial or venous) | ICC (95% CI): 0.99 (0.98−0.99)BA: deviation (± 1.96 SD): 5.9 (−15.7–27.5) mg/dL | The glucometer evaluated shows good precision in comparison with the laboratory. | |||
| DuBose et al.26/2012/U.S.A. | Observational retrospective | 1,215 patients | 1,935 | Accu-Chek® Advantage | NN | LX20 | Capillary (glucometer), arterial or venous (laboratory) | BA: deviation (95% limits of agreement): 12.4 (−22.4–47.2) mg/dLWith shock: 13.4 (−27.1–53.9) mg/dLNo shock: 12.6 (−20.6–45.8) mg/dL | The correlation between glucose values in capillary blood and the laboratory with and without a state of shock is low. | |||
| Lonjaret et al.30/2012/France | Observational | 75 patients | 302 | CONTOUR® TS | NN | Olympus AU 2007 | Arterial and capillary (glucometer), arterial (laboratory) | ICC (95% CI) laboratory vs. glucometer with capillary blood: 0.91 (0.89 to 0.93)ICC (95% CI) laboratory vs. glucometer with arterial blood: 0.92 (0.90 to 0.93) | POC measurements in critical patients using a glucometer are not accurate. Precision seems somewhat higher in arterial blood samples than it is in capillary ones. | |||
| Watkinson et al.43/2012/U.K. | Observational prospective | 206 patients | ND | 1) Precision PCx®2) HemoCue® 201 DM | Radiometer 700 | 1) Siemens ADVIA®24002) YSI 2300 STAT plus™ | Arterial | BA: deviation (95% limits of agreement):1) YSI 2300 STAT plus total blood glucose vs. glucose in plasma: 14.4 (−1.8–30.6) mg/dL2) Precision PCx vs. Siemens ADVIA: 0.0 (−25.2–25.2) mg/dL3) HemoCue 201 DM vs. Siemens ADVIA: 0.0 (−21.6–19.8) mg/dL4) Radiometer 700 vs. Siemens ADVIA: −3.6 (−16.2–10.8) mg/dL | POC glucose measuring devices reliably estimate glucose levels in critical patients. | |||
| Garingarao et al.32/2014/Philippines | Observational cross-sectional | 180 patients (89 normotensive and 91 hypotensive) | 186 (92 in normotensive patients and 94 in hypotensive patients) | Accu-Chek® Active | NN | I-Lab 300 Plus | Capillary (glucometer), venous (laboratory) | BA: deviation (95% limits of agreement):Normotensive patients: −12.4 (−86.0–61.2) mg/dLHypotensive patients: −34.9 (−207.1–137.4) mg/dL | In critical hypotensive patients with vasopressor support POC glucose measuring devices are less precise than they are in normotensive patients. | |||
| Pereira et al.23/2015/U.S.A. | Prospective cross-sectional | 145 patients | 145 | 1) Precision PCx®2) Accu-Chek Advantage II® | NN | Vitros® | Arterial (Precision® PCx), arterial, capillary and venous (Accu-Chek® Advantage II),arterial (laboratory) | BA: deviation (95% limits of agreement):1) Arterial laboratory vs. arterial Precision: 18.6 (−12.4–49.5) mg/dL2 a) Arterial laboratory vs. arterial Accu-Chek®: 10.7 (−21.3–42.7) mg/dL2 b) Arterial laboratory vs. capillary Accu-Chek®: 10 (−31.8–51.8) mg/dL2 c) Arterial laboratory vs. venous Accu-Chek®: 15,1 (−51.7–81.9) mg/dL | Glycaemia should not be monitored using samples from central venous catheters due to their high level of variability. The reliability of these glucometers is insufficient in critical conditions. Arterial samples seem to be sufficiently exact for use in similar glucometers. | |||
| Allardet Servent et al.48/2017/France | Observational prospective | 51 patients | 306 | NN | Siemens RAPIDPoint® 500 | AU 5800 | Arterial | BA: deviation (± 1.96 SD): −10.7 (−30 to 8.6) mg/dL | A blood gas analyser is a reliable alternative. Although glucose values were quite close on average, they found clinically relevant discrepancies in the range of hypoglycaemia. | |||
| Prakash et al.49 /2018/U.S.A. | Observational retrospective | 1,765 patients | 9,192 | NN | ABL800 Flex | Roche | Arterial or venous | BA: deviation (95% limits of agreement): 1.8 (−12.8–16.61) mg/dLICC (95% CI): 0.98 (0.980 to 0.982) | There is from moderate to substantial agreement between the central laboratory and the blood gas analyser. Nevertheless, both methods may be used without distinction. For clinical practice, it is important to use the same test method. | |||
| Pilackas et al.34/2020/U.S.A. | Prospective cohort study | 46 patients | 85 | Precision Xceed Pro® | NN | ND | Capillary (glucometer), venous (laboratory) | BA: deviation (95% limits of agreement): 5.23 (−32.2–42.7) mg/dL | There is a slight average difference between the measurements. The glucose test using POC capillary blood in similar critical patients is probably safe and effective. | |||
| Deng et al.38/2021/China | Observational prospective | 73 patients | ND | CONTOUR® TS | GEM® Premier™ 3000 | NN | Capillary (glucometer), arterial (blood gas analyser) | BA: deviation (95% CI): 14.58 (−13.86 to 43.02) mg/dL | Statistically significant differences were found, although they are acceptable in a clinical context. | |||
| He et al.52/2021/China | Observational retrospective, series of cases | 82 patients | ND | Medisafe® | NN | Beckman Coulter AU5800 | ND | BA: deviation (95% limits of agreement): −43.83 (−177.97 to 90.32) mg/dL | Inexact glucose readings at the point of case do not represent a significant clinical risk. Values were significantly lower with the glucometer. | |||
| Howell et al.36/2021/U.S.A. | Observational retrospective, series of cases (cohort) | 14 patients | 46 | Accu-Chek® Inform II | NN | Cobas® c702 | Capillary (glucometer), venous (laboratory) | BA: deviation (95% CI): 0.33 (−57.7–58.1) mg/dL | Many patients with sepsis and intravenous vitamin C therapy may be managed using POC glucose monitoring with the Accu-Chek® Inform II POC BGM without a significant clinical impact. | |||
| Rodríguez-Delgado et al.40/2022/Spain | Observational cross-sectional | 54 patients | 297 | FreeStyle® Optium Neo | NN | NN | Capillary, venous and arterial | BA: deviation (± 2 SD):Arterial vs. capillary:5.01 (± 41.32) mg/dLArterial vs. venous:−11.80 (± 61.13) mg/dLVenous vs. capillary:16.81 (± 71.26) mg/dL | Low concordance of arterial, capillary and venous samples using the glucometer. Glycaemia monitoring with glucometers in critical patients should use arterial samples, and in the case of hypoglycaemia a gas analyser should be used to check readings. | |||
BA: Bland and Altman; ICC: intraclass correlation coefficient; SD: standard deviation; 95% CI: 95% confidence interval; ND: not described; NN: not necessary; POC: point of care; IQR: interquartile range; ICU: Intensive Care Unit.
A very heterogeneous set of glucometers were analysed in this review, and different methods of analysis were used (Appendix A Supplementary Material Table S2).
Reliability of capillary determinations by glucometer19 studies used capillary samples measured by a glucometer compared with central laboratory values or a POC blood gas analyser. Of the 17 studies that compared glycaemia measured using a glucometer with a laboratory measurement, 11 authors21–31 contraindicate their use and 2 authors32,33 do not recommend them if patients are receiving intravenous treatment with catecholamine. Only 3 studies34–36 consider that the differences found between both types of measurement should not restrict the use of glucometers when monitoring glycaemia, as do both studies37,38 that compared capillary samples measured with a glucometer vs. a POC blood gas analyser. Cordero Saucedo et al.39 consider that more studies are required to evaluate efficacy: their results are not conclusive. Rodríguez-Delgado et al.40 compared capillary, arterial and venous samples using the same glucometer, and they advise against using capillary samples, especially when patients are receiving a perfusion of vasoactive drugs.
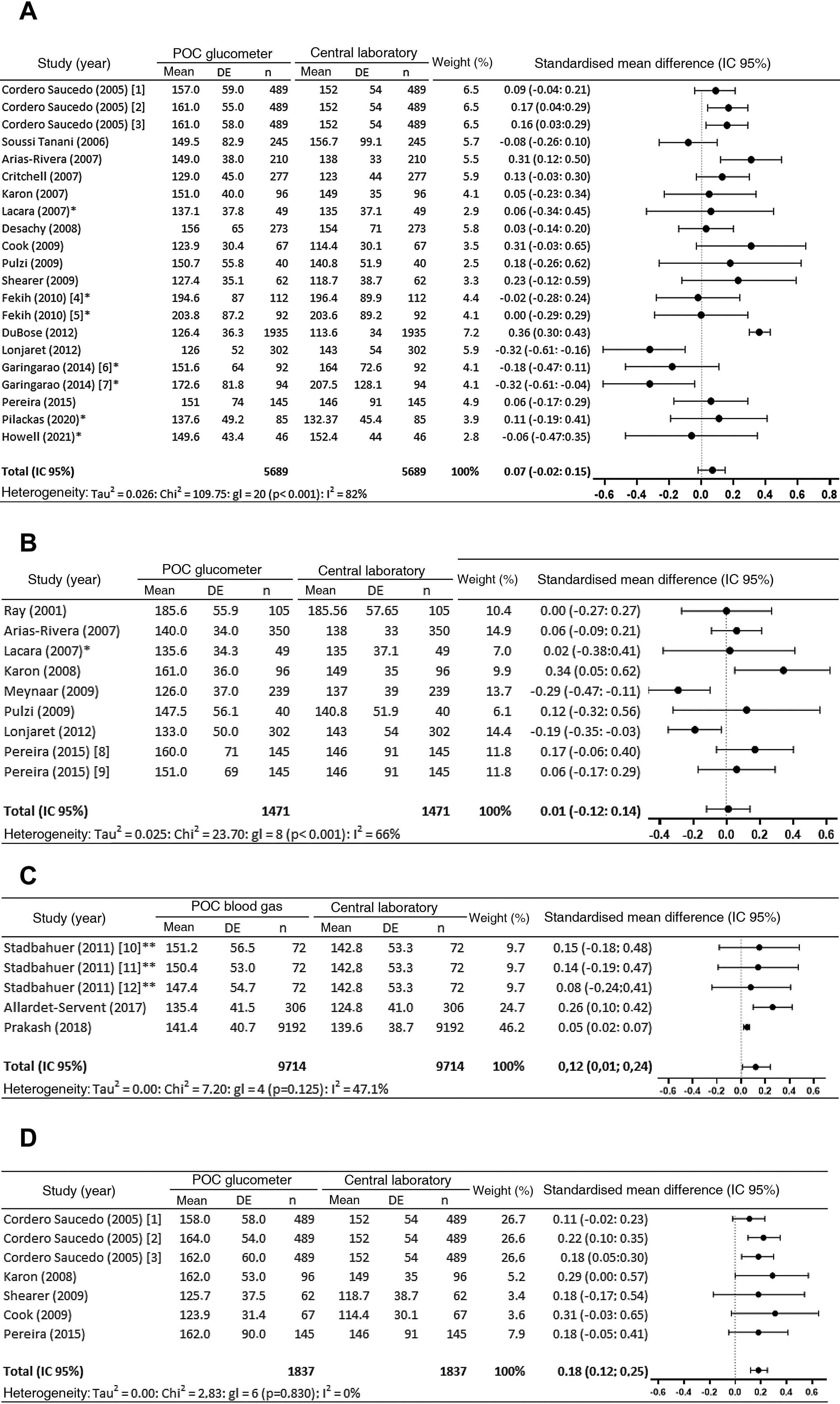
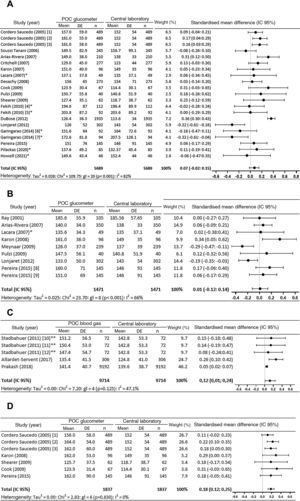
In the meta-analysis of the 17 studies (21 comparisons) that analysed capillary samples using a glucometer vs. laboratory technique, 4 comparisons in 3 studies22,26,39 found that glucometer readings were significant over-estimates, while 2 comparisons30,32 found they were significant under-estimates. Globally the determinations measured using a glucometer were found to be over-estimates, although the differences in the measurements (95% CI) are not significant (0.07 [−0.02 to 0.15] mg/dL) (Fig. 2a).
Meta-analysis. (2a) Meta-analysis of capillary samples with a POC glucometer vs. central laboratory. (2b) Meta-analysis of arterial samples with a POC glucometer vs. central laboratory. (2c) Meta-analysis of arterial samples with a POC blood gas analyser vs. central laboratory. (2d) Meta-analysis of venous samples with a POC glucometer vs. central laboratory.
95% CI: 95% confidence interval; df: degrees of freedom; 1: Ascensia Elite™; 2: Precision Q.I.D®; 3: SureStep™ Plus; 4: stable patients; 5: patients with catecholamine; 6: normotensive patients; 7: hypotensive patients; 8: Precision PCX®; 9: Accu-check Advantage II®; 10: Cobas B 221; 11: ABL800 Flex; 12: GEM Premier; *: estimated data; **: data supplied by the author.
11 authors analysed arterial samples, 9 comparing glucometer determinations with laboratory values21–23,29,30,35,41–43 and 2 comparing a glucometer with a POC blood gas analyser.44,45 OF the 9 studies that compared glycaemia determinations by a glucometer and a laboratory, 7 recommend using them21–23,29,41–43 and 230,35 consider that there is no good concordance between glucometer and laboratory, advising against their use, as did both studies that compared a glucometer with a POC blood gas analyser.44,45 Both of the studies40,46 which compared samples from different sources using the same glucometer are in favour of using one with arterial samples.
Significant differences were observed in the meta-analysis of 3 of the 8 studies (9 comparisons) which evaluated the differences between glycaemia measured using a glucometer vs. a central laboratory: Karon et al.35 found that the glucometer over-estimated glycaemia, while Lonjaret et al.30 and Meynaar et al.42 found that it under-estimated it. However, the overall difference in the average (95% CI) is very small and it is not significant (0.01 [−0.12 to 0.14] mg/dL) (Fig. 2b).
The 4 studies43,47–49 which compare glycaemia measured with POC blood gas analysers and in a laboratory consider blood gas analyser measurements to be reliable. In the meta-analysis (Fig. 2c) the blood gas analyser can be seen to always over-estimate, and the differences between the determination measured using a POC blood gas analyser and in a laboratory are significant in 2 comparisons.48,49 The overall determination of the difference in the averages (95% CI) is also significant (0.12 [0.01 to 0.24] mg/dL).
The reliability of determinations in venous blood using a glucometerThe 5 studies found which compare venous glycaemia measured with a glucometer and in a laboratory23–25,35,39 advise against using a glucometer. The meta-analysis of these 5 studies (7 comparisons) showed that the overall value shows a significant over-estimation in the difference between the averages (95% CI) of measurements using the glucometer vs. the laboratory (0.18 [0.12 to 0.25] mg/dL) (Fig. 2c).
Reliability of determinations in samples without a specified originSome authors analysed the reliability of glycaemia measurements without specifying the origin of the sample. Castaño López et al.50 and Lacara et al.21 recommend using a glucometer if the blood sample is arterial or venous, and they advise against this in the case of capillary samples. Finkielman et al. and He et al.51,52 consider that glucometer measurements are not very reliable.
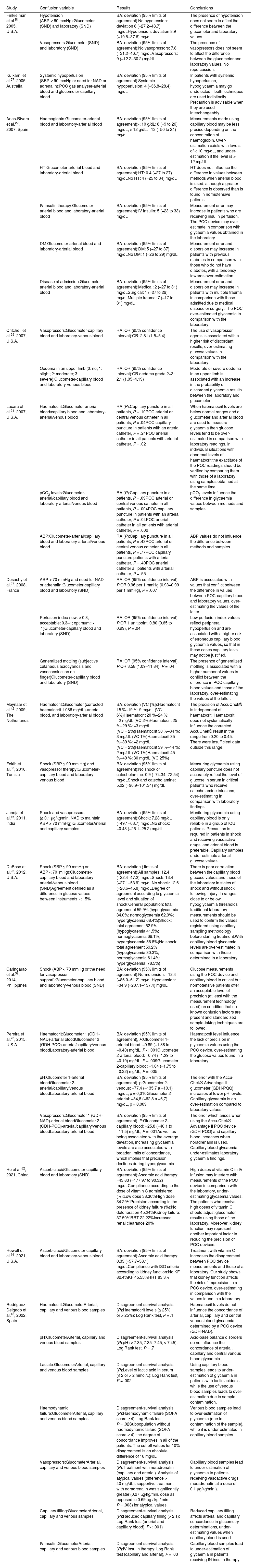
Analysis of the factors which influence the imprecision of glucose measurements by POC devicesOf the 32 papers included in this review, 15 (46.87%) analyse the influence of specific variables on the lack of precision of POC devices in comparison with laboratory measurements. The influence of a total of 18 variables was analysed, as these may explain the lack of agreement between measurements made using different devices (Table 2).
Influence of confusion variables on the differences in glycaemia values between methods and/or samples.
| Study | Confusion variable | Results | Conclusions |
|---|---|---|---|
| Finkielman et al.51, 2005, U.S.A. | Hypotension (ABP < 60 mmHg):Glucometer (SND) and laboratory (SND) | BA: deviation (95% limits of agreement):No hypotension: deviation 8 (−27.2–43.7) mg/dLHypotension: deviation 8.9 (−19.8–37.6) mg/dL | The presence of hypotension does not seem to affect the difference between the glucometer and laboratory values. |
| Vasopressors:Glucometer (SND) and laboratory (SND) | BA: deviation (95% limits of agreement):No vasopressors: 7.8 (−31.2–46.7) mg/dLVasopressors: 9 (−12.2–30.2) mg/dL | The presence of vasopressors does not seem to affect the difference between the glucometer and laboratory values. No repercussion. | |
| Kulkarni et al.37, 2005, Australia | Systemic hypoperfusion (SBP < 90 mmHg or need for NAD or adrenalin):POC gas analyser-arterial blood and glucometer-capillary blood | BA: deviation (95% limits of agreement):Systemic hypoperfusion: 4 (−36.8–28.4) mg/dL | In patients with systemic hypoperfusion, hypoglycaemia may go undetected if both techniques are used indistinctly. Precaution is advisable when they are used interchangeably. |
| Arias-Rivera et al.22, 2007, Spain | Haemoglobin:Glucometer-arterial blood and laboratory-arterial blood | BA: deviation (95% limits of agreement):< 10 g/dL: 8 (−9 to 26) mg/dL; > 12 g/dL: −13 (−50 to 24) mg/dL | Measurements made using capillary blood may be less precise depending on the concentration of haemoglobin. Over-estimation exists with levels of < 10 mg/dL, and under-estimation if the level is > 12 mg/dL |
| HT:Glucometer-arterial blood and laboratory-arterial blood | BA: deviation (95% limits of agreement):HT: 0.4 (−27 to 27) mg/dLNo HT: 4 (−25 to 34) mg/dL | HT does not influence the difference in values between methods when arterial blood is used, although a greater difference is observed than is found in normotensive patients. | |
| IV insulin therapy:Glucometer-arterial blood and laboratory-arterial blood | BA: deviation (95% limits of agreement):IV insulin: 5 (−23 to 33) mg/dL | Measurement error may increase in patients who are receiving insulin perfusion. The POC device may over-estimate in comparison with glycaemia values obtained in the laboratory. | |
| DM:Glucometer-arterial blood and laboratory-arterial blood | BA: deviation (95% limits of agreement):DM: 5 (−27 to 37) mg/dLNo DM: 1 (−26 to 29) mg/dL | Measurement error and dispersion may increase in patients with previous diabetes in comparison with those who do not have diabetes, with a tendency towards over-estimation. | |
| Disease at admission:Glucometer-arterial blood and laboratory-arterial blood | BA: deviation (95% limits of agreement):Medical: 2 (−27 to 31) mg/dLSurgical: 1 (−27 to 29) mg/dLMultiple trauma: 7 (−17 to 31) mg/dL | Measurement error and dispersion may increase in patients with multiple trauma in comparison with those admitted due to medical disease or surgery. The POC over-estimated glycaemia in comparison with the laboratory. | |
| Critchell et al.28, 2007, U.S.A. | Vasopressors:Glucometer-capillary blood and laboratory-venous blood | RA: OR (95% confidence interval):OR: 2.81 (1.5−5.4) | The use of vasopressor agents is associated with a higher risk of discordant results, over-estimating glucose values in comparison with the laboratory. |
| Oedema in an upper limb (0: no; 1: slight; 2: moderate; 3: severe):Glucometer-capillary blood and laboratory-venous blood | RA: OR (95% confidence interval):OR oedema grade 2−3: 2.1 (1.05−4.19) | Moderate or severe oedema in an upper limb is associated with an increase in the probability of discordant glycaemia results between the laboratory and glucometer. | |
| Lacara et al.21, 2007, U.S.A. | Haematocrit:Glucometer-arterial blood/capillary blood and laboratory-arterial/venous blood | RA (P):Capillary puncture in all patients, P = .10POC arterial or central venous catheter in all patients, P = .04POC capillary puncture in patients with an arterial catheter, P = .24POC arterial catheter in all patients with arterial catheter, P = .02 | When haematocrit levels are below normal ranges and a glucometer and arterial blood are used to measure glycaemia then glucose levels tend to be over-estimated in comparison with laboratory readings. In individual situations with abnormal levels of haematocrit the exactitude of the POC readings should be verified by comparing them with those of a laboratory using samples obtained at the same time. |
| pCO2 levels:Glucometer-arterial/capillary blood and laboratory-arterial/venous blood | RA (P):Capillary puncture in all patients, P = .09POC arterial or central venous catheter in all patients, P = .004POC capillary puncture in patients with an arterial catheter, P = .04POC arterial catheter in all patients with arterial catheter, P = .002 | pCO2 levels influence the difference in glycaemia values between methods and samples. | |
| ABP:Glucometer-arterial/capillary blood and laboratory-arterial/venous blood | RA (P):Capillary puncture in all patients, P = .43POC arterial or central venous catheter in all patients, P = .77POC capillary puncture patients with arterial catheter, P = .40POC arterial catheter all patients with arterial catheter, P = .55 | ABP values do not influence the difference between methods and samples | |
| Desachy et al.27, 2008, France | ABP < 70 mmHg and need for NAD or adrenalin:Glucometer-capillary blood and laboratory (SND) | RA: OR (95% confidence interval), P:OR 0.96 per 1 mmHg (0.93−0.99 per 1 mmHg), P = .007 | ABP is associated with values that conflict between the difference in values between POC-capillary blood and laboratory values, over-estimating the values of the latter. |
| Perfusion index (low: < 0.3; acceptable: 0.3–1; optimum: > 1)Glucometer-capillary blood and laboratory (SND) | RA: OR (95% confidence interval), P:OR 1 unit point, 0.80 (0.65 to 0.99), P = .04 | Low perfusion index values reflect peripheral hypoperfusion and are associated with a higher risk of erroneous capillary blood glycaemia values, so that in these cases capillary tests may not be justified. | |
| Generalized mottling (subjective cutaneous acrocyanosis and vasoconstriction on finger)Glucometer-capillary blood and laboratory (SND) | RA: OR (95% confidence interval), P:OR 3.58 (1.09–11.84), P = .04 | The presence of generalized mottling is associated with a higher number of values in conflict between the difference in POC capillary blood values and those of the laboratory, over-estimating the values of the latter. | |
| Meynaar et al.42, 2009, The Netherlands | Haematocrit:Glucometer (corrected haematocrit 1.086 mg/dL)-arterial blood, and laboratory-arterial blood | BA: deviation (VC [%]):Haematocrit 15 %–19 %: 9 mg/dL (VC 6%)Haematocrit 20 %–24 %: −2 mg/dL (VC 2%)Haematocrit 25 %–29 %: −3 mg/dL (VC − 2%)Haematocrit 30 %–34 %: 3 mg/dL (VC 1%)Haematocrit 35 %–39 %: −2 mg/dL (VC − 2%)Haematocrit 39 %–44 %: 2 mg/dL (VC 1%)Haematocrit 45 %–49 %: 30 mg/dL (VC 25%) | The precision of AccuChek® is independent of haematocrit.Haematocrit does not systematically influence the corrected AccuChek® result in the range from 0.20 to 0.45. There were insufficient data outside this range. |
| Fekih et al.33, 2010, Tunisia | Shock (SBP ≤ 90 mm Hg) and vasopressor therapy:Glucometer-capillary blood and laboratory-venous blood | BA: deviation (95% limits of agreement):No shock or catecholamine: 0.9 (−74.34–72.54) mg/dLShock and catecholamine: 5.22 (−90.9–101.34) mg/dL | Measuring glycaemia using capillary puncture does not accurately reflect the level of glucose in serum in critical patients who receive catecholamine infusions, over-estimating in comparison with laboratory findings. |
| Juneja et al.46, 2011, India | Shock and vasopressors (≥ 0.1 µg/kg/min. NAD to maintain ABP > 70 mmHg):GlucometerArterial and capillary samples | BA: deviation (95% limits of agreement):Shock: 7.28 mg/dL (−49.1–63.7) mg/dLNo shock: −0.43 (−26.1–25.2) mg/dL | Monitoring glycaemia using capillary blood is only reliable in a group of ICU patients. Precaution is required in patients in shock and receiving vasoactive drugs, and arterial blood is preferable. Capillary samples under-estimate arterial glucose values. |
| DuBose et al.26, 2012, U.S.A. | Shock (SBP ≤ 90 mmHg or ABP < 70 mHg):Glucometer-capillary blood and laboratory-arterial/venous blood (SND)Agreement defined as a difference in glucose values between instruments < 15% | BA: deviation ( limits of agreement):All samples: 12.4 (−22.4–47.2) mg/dLShock: 13.4 (−27.1–53.9) mg/dLNo shock: 12.6 (−20.6–45.8) mg/dLDegree of agreement according to glycaemia level and situation of shock:General population: total agreement 59.9% (hypoglycaemia 34.0%; normoglycaemia 62.9%; hyperglycaemia 68.4%)Shock: total agreement 62.9% (hypoglycaemia 41.5%; normoglycaemia 69.1%; hyperglycaemia 56.8%)No shock: total agreement 59.2% (hypoglycaemia 30.3%; normoglycaemia 61.4%; hyperglycaemia: 78.5%) | There is poor correlation between the capillary blood glucose values and those of the laboratory in states of shock and without shock following injury. In ranges close to or below hypoglycaemia thresholds traditional laboratory measurements should be used to confirm the values registered using capillary sampling methodology before starting treatment.With capillary blood glycaemia levels are over-estimated in comparison with those determined in a laboratory. |
| Garingarao et al.32, 2014, Philippines | Shock (ABP < 70 mmHg or the need for vasopressor support):Glucometer-capillary blood and laboratory-venous blood (SND) | BA: deviation (95% limits of agreement):Normotension: −12.4 (−86.0–61.2) mg/dLHypotension: −34.9 (−207.1–137.4) mg/dL | Glucose measurements using the POC device and capillary blood in critical but normotensive patients offer an acceptable level of precision (at least with the measurement technology used) on condition that no known confusion factors are present and standardized sample-taking techniques are followed. |
| Pereira et al.23, 2015, U.S.A. | Haematocrit:Glucometer 1 (GDH-NAD)-arterial bloodGlucometer 2 (GDH-PQQ)-arterial/capillary/venous bloodLaboratory-arterial blood | BA: deviation (95% limits of agreement), P:Glucometer 1-arterial blood: −0.89 (−1.38 to −0.40) mg/dL, P < .001Glucometer 2-arterial blood: −0.74 (−1.29 to −0.19) mg/dL, P = .009Glucometer 2-capillary blood: −1.04 (−1.75 to −0.32) mg/dL, P = .005 | Haematocrit level influence the lack of precision in glycaemia values using the POC device, over-estimating the glucose values found in a laboratory. |
| pH:Glucometer 1-arterial bloodGlucometer 2-arterial/capillary/venous bloodLaboratory-arterial blood | BA: deviation (95% limits of agreement), p:Glucometer 2-venous: −77,4 (−135,7 a −19,1) mg/dL, p = 0,010Glucometer 2-arterial: −34,8 (−62,8 a −6,7) mg/dL, p = 0,009 | The error with the Accu-Chek® Advantage II glucometer (GDH-PQQ) increases at lower pH levels. Capillary glycaemia is an over-estimation compared to laboratory values. | |
| Vasopressors:Glucometer 1 (GDH-NAD)-arterial bloodGlucometer 2 (GDH-PQQ)-arterial/capillary/venous bloodLaboratory-arterial blood | BA: deviation (95% limits of agreement), P:Glucometer 2-capillary blood: −25.8 (−40.1 to −11.5) mg/dL, P = .001As well as being associated with the average deviation, increasing glycaemia levels are also associated with broader limits of concordance, which implies that precision declines during hyperglycaemia. | The error which arises when using the Accu-Chek® Advantage II POC device (GDH-PQQ) and capillary blood increases when noradrenalin is used. Capillary blood glycaemia under-estimates laboratory glycaemia findings. | |
| He et al.52, 2021, China | Ascorbic acidGlucometer-capillary blood and laboratory (SND) | BA: deviation (95% limits of agreement):Ascorbic acid therapy: −43.83 (−177.97 to 90.32) mg/dLCompliance according to the dose of vitamin C administered (%):Low dose 38.30%High dose 34.29%Precision according to the presence of kidney failure (%):No deterioration 45.24%Kidney failure: 37.50%RRT 22.22%Increased renal clearance 20% | High doses of vitamin C in IV infusion may interfere with measurements of the POC device in comparison with the laboratory, under-estimating glycaemia values. The patients who receive high doses of vitamin C should adjust glucometer results using those of the laboratory. Moreover, kidney function may represent another important factor in reducing the precision of POC devices. |
| Howell et al.36, 2021, U.S.A. | Ascorbic acidGlucometer-capillary blood and laboratory-venous blood | BA: deviation (95% limits of agreement):Ascorbic acid therapy: 0.33 (−57.7–58.1) mg/dLCompliance with ISO criteria according to kidney function:No KF 82.4%KF 45.55%RRT 83.3% | Treatment with vitamin C increases the disagreement between POC device measurements and those of a laboratory. Our study shows that kidney function affects the risk of imprecision in a POC device, over-estimating in comparison with the values found in a laboratory. |
| Rodríguez-Delgado et al.40, 2022, Spain | Haematocrit:GlucometerArterial, capillary and venous blood samples | Disagreement-survival analysis (P):Haematocrit levels (≤ 25% or > 25%): Log Rank test, P = .1 | Haematocrit levels do not influence the concordance of arterial, capillary and central venous blood glycaemia determined by a POC device (GDH-NAD). |
| pH:GlucometerArterial, capillary and venous blood samples | Disagreement-survival analysis (P):pH (< 7.35; 7.35−7.45; > 7.45): Log Rank test, P = .7 | Acid-base balance disorders do no influence the concordance of arterial, capillary and central venous blood glycaemia. | |
| Lactate:GlucometerArterial, capillary and venous blood samples | Disagreement-survival analysis (P):Level of lactic acid in serum (≤ 2 or > 2 mmol/L): Log Rank test, P = .002 | Using capillary blood samples leads to under-estimation of glycaemia in patients with lactic acidosis, while the use of venous blood samples leads to over-estimation due to sample contamination. | |
| Haemodynamic failure:GlucometerArterial, capillary and venous blood samples | Disagreement-survival analysis (P):Haemodynamic failure (SOFA score ≥ 4): Log Rank test, P = .02Subpopulation without haemodynamic failure (SOFA score < 4): the degree of concordance improves in all of the patients. The cut-off values for 10% disagreement is an absolute difference of 16 mg/dL | Venous blood samples lead to over-estimation of glycaemia (due to contamination of the sample), while it is under-estimated in capillary blood samples. | |
| Vasopressors:GlucometerArterial, capillary and venous blood samples | Disagreement-survival analysis (P):Treatment with noradrenalin (capillary and arterial). Analysis of atypical values (difference > 40 mg/dL): supportive treatment with noradrenalin was significantly greater (0.27 μg/kg/min. dose as opposed to 0.69 μg / kg / min., P = .003) for atypical values. | Capillary blood samples lead to under-estimation of glycaemia in patients receiving vasoactive drugs (noradrenalin at a dose of 0.1 µg/kg/min.). | |
| Capillary filling:GlucometerArterial, capillary and venous samples | Disagreement-survival analysis (P):Reduced capillary filling (> 2 s): Log Rank test (arterial and capillary blood), P < .001) | Reduced capillary filling affects arterial and capillary concordance in glucometry determinations, under-estimating values when capillary blood is used. | |
| IV insulin:GlucometerArterial, capillary and venous blood samples | Disagreement-survival analysis (P):IV insulin therapy: Log Rank test (capillary and arterial), P = .03 | Capillary blood samples lead to under-estimation of glycaemia in patients receiving IN insulin therapy. |
ABP: Average blood pressure; BA: Bland and Altman; DM: Diabetes mellitus; GDH-NAD: Glucose dehydrogenase with nicotinamide-adenine-nucleotide; GDH-PQQ: Glucose-dehydrogenase-pyrrolquinoline quinone; HT: Hypertension; ICU: Intensive Care Unit; IV: Intravenous; KF: Kidney failure; NAD: Noradrenalin; OR: Odds ratio; POC: Point of care; RA: Regression analysis; RRT: Renal replacement therapy; SBP: Systolic blood pressure; SND: Sample not described; VC: Variation coefficient.
The 4 studies21,23,40,42 which analyse the influence of haematocrit on the lack of precision of POC devices were found to reach different conclusions. Two authors40,42 find that haematocrit does not systematically influence the precision of a POC device, while the other 221,23 conclude that it does affect precision. Pereira et al.23 consider that this imprecision increases when capillary blood samples are used in glucometers which base their analysis on pyrroloquinoline quinone glucose dehydrogenase (GDH-PQQ). Lacara et al.21 observe that when haematocrit levels are below normal ranges, a glucometer with arterial samples tends to over-estimate glucose levels.
HaemoglobinOnly one study22 analysed haemoglobin as a factor which may influence the discrepancy between glycaemia measured in different devices. This study finds that POC measurements of glycaemia in arterial blood are less precise due to the concentration of haemoglobin in a patient; when haemoglobin levels >12 mg/dL, the POC device tends to under-estimate glycaemia, while when haemoglobin levels are <10 mg/dL it overestimates glycaemia.
Acid-base balance: concentration of hydrogen ions and partial pressure of carbon dioxideThree studies analyse the influence of the acid-base balance on the lack of agreement between glycaemia levels measured using different methods. They do so based on the concentration of hydrogen ions (pH)23,40 and partial pressure levels of carbon dioxide (pCO2).21
The results were found to be discrepant. While Rodríguez-Delgado et al.40 determine that acid-base balance disorders do not influence the concordance of arterial and capillary blood glycaemia values in critical patients, Pereira et al.23 determine that glycaemia values are over-estimated when the pH is low, and that this error increases with increasingly low levels of pH values.
Lacara et al.21 conclude that pCO2 should be considered a factor that influences imprecision, although they do not specify how.
Haemodynamic statusNine papers analyse the influence of haemodynamic status on the lack of conformity in the results obtained by instruments and samples using different parameters: lactic acid and the SOFA score,40 state of shock,26,32,33,46 arterial hypotension,51 average arterial pressure21,27 and arterial hypertension.22
In general, 6 of these studies find that haemodynamic status influences the lack of precision of a POC device, 3 find that it under-estimates glycaemia32,40,46 and 3 others find that it leads to over-estimation.26,27,33 Three of these studies21,22,51 found that haemodynamic status does not affect the concordance between POC values and those detected in a laboratory.
Rodríguez-Delgado et al.40 observe that in patients with hyperlactacidaemia (>2 mmol/L), delay in capillary filling (>2 s) and in a situation of haemodynamic failure (SOFA > 4), the concordance between capillary and arterial glycaemia levels is affected. They found that capillary blood samples lead to under-estimation of glycaemia in comparison with levels determined in arterial blood, and that on the contrary, venous samples lead to over-estimation. The only possible explanation is contamination of the sample, so that they advise against using this type of blood sample for this purpose.
On the other hand, 4 of the studies evaluate shock as a confusion variable for lack of POC device precision, although here too a disparity in the results was observed. 2 of these studies26,33 found systematic over-estimation of glycaemia in the POC device in patients who were in shock, as opposed to normotensive patients. DuBose et al.26 also found that this difference in glycaemia values between methods increases within the range of hypoglycaemia. The studies by Juneja et al.46 and Garingarao et al.32 find that shock influences the lack of precision of POC devices, with a clear tendency to over-estimate capillary glycaemia. They therefore advise against using them in haemodynamically unstable patients, and they recommend using arterial blood samples for glucose monitoring. Nevertheless, Finkielman et al.51 conclude that hypotension in a critical patient does not seem to affect the difference in glucose values measured by different devices.
With respect to the possible role of average arterial pressure as a confusion factor, Lacara et al.21 found no relationship between it and differences in glycaemia depending on the test method used, while Desachy et al.27 associated it with a higher number of conflicting values, observing systematic over-estimation of capillary blood glycaemia with a POC device.
For patients with arterial hypertension, the research undertaken by Arias-Rivera et al.22 concludes that it does not affect the precision of a measuring device when arterial blood samples are used.
PerfusionFour studies analyse perfusion as a confusion variable for the precision of POC devices, and the majority conclude that it may be considered to be a factor that influences the precision of these devices.
Critchell et al.28 state that oedema in the fingers in grade II-III affects the precision of a glucometer when it is used for capillary blood samples, with a tendency to over-estimate. On the contrary, Desachy et al.27 evaluate the state of perfusion by using the perfusion index and the presence of cutaneous vascular alterations (generalized mottling), observing that low perfusion index values and the existence of generalized mottling as an indicator of peripheral hypoperfusion are associated with a higher risk of erroneous values in capillary blood glycaemia, which may be over- or under-estimated. Rodríguez-Delgado et al.40 find that reduced capillary filling may affect the concordance between arterial and capillary samples determined by glucometry, although they do not specify how it is affected.
The results obtained by Kulkarni et al.37 are not able to prove that there is an association between a lack of precision when using capillary samples with a glucometer and systemic hypoperfusion.
Vasopressor therapySix studies analyse the influence of vasopressor drugs on the precision of POC devices in critical patients. Five of these studies23,28,33,40,46 show that there is a higher risk of discordant results when glycaemia in capillary blood from critical patients with vasopressor support is measured. It was found to be systematically under-estimated, especially with noradrenalin doses higher than 0.1 µg/kg/min.
On the contrary, Finkielman et al.51 did not find that vasopressor drugs affect glucose determinations measured using a glucometer based on the glucose-oxidase analytical technique, although their study does not specify the type of sample they used.
Other pharmacological therapies: intravenous insulin and vitamin CFour studies analyse the influence of drug therapy on discordancy in glucose values.
The studies by Arias-Rivera et al.22 and Rodríguez-Delgado et al.40 agree that intravenous insulin therapy should be considered a factor that causes imprecision in the determination of glycaemia measured by glucometry, although they disagree about its mode of action: while the first believe that the said values are over-estimated, the latter believe that they are under-estimated.
Two other studies36,52 analyse the influence of treatment with ascorbic acid as a confusion factor in septic patients, and they agree that this therapy increases the risk of POC device imprecision. While Howell et al.36 observe a tendency to over-estimate, He et al.52 determine that this therapy leads to under-estimation in capillary samples, especially in patients with deterioration of kidney function, a need for renal replacement therapy or altered renal clearance.
A history of diabetes mellitus and reasons for admission to intensive careOnly one study was found which analyses the influence of previous metabolic alterations and the type of disease which leads to admission to an ICU. Arias-Rivera et al.22 find that patients with diabetes mellitus are at higher risk of lack of precision in glycaemia determination using arterial blood samples and a POC device, while traumatology is associated with greater discordancy than surgery or medical disease.
Analytical performance of POC devices based on interchangeability criteriaOf the 32 papers selected in the review, 17 (53.12%) analyse the performance of a POC device (glucometer/blood gas analyser) using different interchangeability criteria (Appendix A Supplementary Material. Table S3).
The majority of the studies carried out24,27–30,32,36,42–46,50,52 consider that monitoring glycaemia in critical patients is less precise in terms of concordance than the benchmark method (laboratory analysis). This is chiefly because it is determined using capillary samples, in the presence of shock and vasopressor support, when there is kidney failure or during treatment with vitamin C.
Only 2 authors38,50 conclude that using a glucometer is exact and reliable in terms of quality and device performance when measuring glucose in intensive care settings, above all when arterial samples are used.
On the contrary, 5 studies38,43,45,48,49 favour determining glycaemia in an ICU using a POC blood gas analyser, which they find has a good level of compliance with interchangeability criteria in comparison with the benchmark method (laboratory analysis). No study considers POC blood gas analysers to be a low precision device.
Methodological quality of the included studiesThe majority of the studies are of acceptable quality in spite of their observational design, with a low probability of deviation and low risk in the applicability of their findings. The highest risk of distortion was found in patient selection, where 53% of the studies have an uncertain or high risk (Appendix A Supplementary Material Table S4 and Figure S1).
DiscussionAfter this systematic review of 32 papers, we consider determinations using arterial blood samples and POC glucometers to be reliable for bedside glycaemia monitoring of critical adult patients, although this reliability depends on many factors which in turn could be partially overcome by using latest generation instruments. The determinations made using POC blood gas analysers are also reliable, and their precision is unaffected by confusion factors.
The studies analysed in this review compare the levels of glycaemia measured using arterial blood samples with laboratory determinations. They show a deviation that varies from −0.1 to 18.6 mg/dL when measured using a glucometer and from −3.6 to 10.7 mg/dL when measured with a POC blood gas analyser. These deviations are less than those reported by Inoue et al.6 (from −10 to 23 mg/dL with a glucometer and from −2.7 to 25.2 when a POC blood gas analyser was used). Arterial blood samples are the ones that offer the best concordance in the meta-analysis, and they are recommended by Finfer et al.3
Nevertheless, if no arterial catheter is in place, the recommendation is to extract samples using a venous catheter.3 The meta-analysis of this review shows that the differences in the resulting values in comparison with laboratory determinations are significantly higher, and all of the authors in this review23–25,35,39 advise against using these.
The majority of the studies located analyse glucose measured using capillary blood samples in a glucometer and compared to determination in a laboratory. These studies found deviations from −9.87 to 12,4 mg/dL, and this deviation increases (−34.9–13.4 mg/dL) in unstable patients or those receiving catecholamine. These data are similar to those reported in the review by Inoue et al.,6 with deviations from −16 to 9.9 mg/dL and an increase in inaccuracy in patients treated with vasopressors. On the other hand, as well as finding that glycaemia measured using glucometers is over-estimated, we also found that this deviation increases in the case of higher levels of glycaemia.31,35 The consensus recommendations published by Finfer et al.3 consider that capillary blood samples from ICU patients, and particularly those who are haemodynamically unstable or receiving catecholamine, may lead to major errors in comparison with the benchmark method. Although capillary samples are the most accessible and the least invasive, there is no doubt that they are the least advisable form of sample when monitoring glycaemia in critical patients. Capillary samples would only be recommendable for use in stable patients without an arterial catheter who do not require strict control of glycaemia. Therefore, and as these recommendations state,3 intermittent glycaemia monitoring in a critical and haemodynamically unstable patient should use a POC blood gas analyser instead of a glucometer, as the former comply more closely with the criteria governing the precision and quality of these devices.
Glucose values determined using a POC blood gas analyser differ from 0.5% to 8%43,48,49 in comparison with the benchmark method. This method has a lower percentage of variability (2%)43 and an average difference in glycaemia values of −10.7 mg/dL.48 This appears to be an acceptable deviation in critical patients, and it would be unlikely to lead to a clinically relevant therapeutic error. On the contrary, the habitual practice of using a glucometer should be ruled out, as the performance of these devices is affected by numerous variables. Their lack of exactitude increases under conditions of clinical instability or the need for vasopressor support,29,32,46 kidney failure,36,52 hypoglycaemia,42,44,50 the administration of high doses of ascorbic acid,52 higher scores on severity scales and mortality predictors in critical patients,45 as well as when they are used with capillary blood samples.24,28,30
Although the influence of many variables on the difference in values between POC devices and the benchmark method has been studied, no study has analysed the impact of the deviation caused by the sum of all of the confusion variables on lack of device precision and the resulting clinical risk and morbimortality.
This review has certain limitations. Firstly, the instruments used and the analytical methods are highly heterogeneous, and this hinders comparison of the studies. All of the selected studies are observational and some of them are retrospective, so that the conclusions should be accepted with precaution. No studies report on how the different instruments behave when patients have hypoglycaemia, so that we do not know how reliable they are in these critical situations; we have only found studies that modified the sample in the laboratory, so that they were not included in the review. Some authors do not specify the origin of the blood sample used, so that we cannot compare the studies in question with others to evaluate the reliability of the instruments used.
It should be underlined that the majority of the studies which find that glucometers perform sufficiently well were based on the most permissive international norms governing precision and interchangeability, all of which were designed for non-critical patients. Furthermore, different standards were used to evaluate the instruments, so that this may have distorted the results. Lastly, no single criterion has been agreed to delimit the amount of deviation that is acceptable for an instrument to be considered reliable; each author uses their own criterion, so that we are unable to specify which instruments are reliable, only that some are more reliable than others as they show less deviation.
ConclusionsIn spite of the heterogeneity of the instruments and samples used in the studies that were analysed, we consider that glycaemia monitoring in critical patients who are haemodynamically unstable and require intensive monitoring of glycaemia should be undertaken using arterial blood samples and POC blood gas analysers, as this is more reliable and is not affected by the variability of different confusion factors. Determining glycaemia in capillary blood using glucometry may be suitable in stable patients or when close monitoring of glycaemia is not required.
FinancingThis research received no specific grant from public, commercial or not-for-profit bodies.
Conflict of interestsThe authors have no conflict of interests to declare.
We would like to thank Juan Medino Muñoz, librarian of Fuenlabrada University Hospital, Madrid, for peer reviewing the database search strategy.