Despite the benefits of mobilisation in the critical patient, the evidence in patients with Levitronix® CentriMag as a bridge to heart transplantation (HT) is scarce. The objective of this study is to analyze the impact of mobility on these patients.
MethodsRetrospective observational study of patients who received a HT with Levitronix® CentriMag admitted between 2010 and 2019 to a tertiary hospital. Degree of mobility and nutritional status were assessed at the time of HT. Outcomes including infections, length of hospital admission and mortality were evaluated.
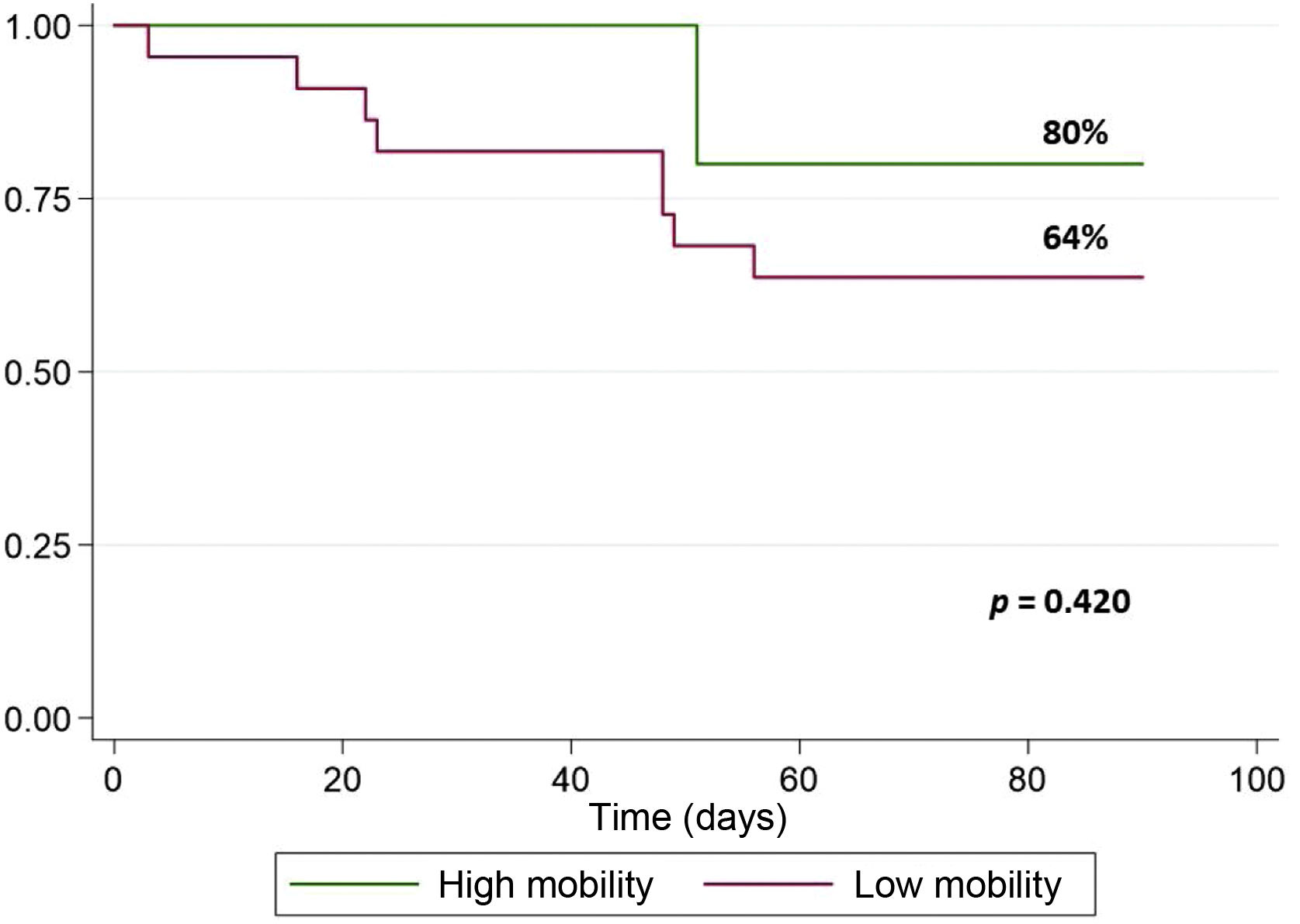
Results27 patients were included and divided in two groups according to degree of mobility (22 with low mobility and 5 with high mobility). 90-day survival after HT was 63.6% in patients with low mobility and 80% in high mobility group; no statistically significant differences were observed. No differences were observed regarding ICU discharge after HT at 30 days. Nevertheless, lower albumin levels were observed in low mobility group (24,5 g/L (IQR: 23–30) vs 33 g/L (IQR: 26–36); p = .029). Invasive mechanical ventilation (IMV) post HT was longer in patients with low mobility (p = .014). There were no significant differences in appearance of pressure ulcers, or post HT infections among mobility groups.
ConclusionsPatients with high mobility had a shorter time of IMV and a better nutritional status. No complications were observed associated to mobility. No differences were observed between the degree of mobility and 90-day mortality, ICU stay or post HT adverse events.
Pese a los beneficios de la movilización en el paciente crítico, la evidencia de su aplicación en pacientes portadores de Levitronix® CentriMag como puente a trasplante cardíaco (TC) es escasa. El objetivo del estudio fue analizar el impacto de la movilidad en estos pacientes.
MétodosEstudio observacional retrospectivo. Se incluyeron los pacientes sometidos a un TC previamente portadores de Levitronix® CentriMag ingresados entre el 2010 y el 2019 en un hospital de tercer nivel. Se relacionaron las variables grado de movilidad y estado nutricional con la evolución clínica posterior al TC (infecciones, tiempo de estancia en UCI y mortalidad).
ResultadosLos 27 pacientes seleccionados se dividieron en dos grupos según el grado de movilidad (22 baja y 5 alta). Se observó una supervivencia a 90 días post TC del 63,6% en el grupo de pacientes con movilidad baja, mientras que en el grupo con movilidad alta fue del 80%; no se observaron diferencias estadísticamente significativas. Tampoco las hubo en la distribución de las altas de UCI desde el TC a 30 días. Por otro lado, se observaron unos menores niveles de albúmina en el grupo de movilidad baja, con una diferencia estadísticamente significativa (24,5 g/L (RIC: 23-30) vs 33 g/L (RIC: 26-36); p = 0,029). También se observaron diferencias en la mediana de días de ventilación mecánica invasiva (VMI) post TC (p = 0,014), siendo mayor en el grupo de movilidad baja. No se observaron diferencias en la aparición de infecciones ni UPP.
ConclusionesLos pacientes con un grado de movilidad alto presentaron un menor tiempo de VMI y un mejor estado nutricional. No se observaron complicaciones asociadas a la movilidad. No se encontraron diferencias significativas entre el grado de movilidad y la mortalidad a 90 días, el tiempo de ingreso en UCI, la aparición de infecciones o UPP post TC.
What is known
Despite the great benefits of early progressive mobilisation in the critically ill patient, there is sparse evidence for its application in patients with Levitronix® CentriMag ventricular assist devices. The evidence is even more sparse in patients who eventually undergo cardiac transplantation.
What it contributes
To our knowledge, this is the first study to address this subject. This project will be a starting point for future prospective research to lay the foundations for the design of a mobilisation protocol for patients with a Levitronix® CentriMag assist device. These types of devices are increasingly used in our setting.
Implications of the study
The results suggest that patients with a Levitronix® Centrimag ventricular assist device could benefit from early mobility programmes due to shorter invasive mechanical ventilation time and improved nutritional status. However, no complications associated with mobility were observed, nor were there any significant differences between the degree of mobility prior to transplantation and 90-day mortality, time spent in the ICU, onset of infections, or PUs after transplantation.
The era of mechanical circulatory support (MCS) began in the 1950s in parallel with the development of cardiac surgery. In recent years there has been a quantum leap in the field of MCS: the recent development of smaller, more durable, and safer devices. This has enabled new practices and therapies in MCS: bridge to decision, bridge to transplant, bridge to recovery and, increasingly, as destination therapy as an alternative to transplantation.1
Levitronix® CentriMag is a centrally inserted extracorporeal, centrifugal continuous-flow ventricular assist device. It has a maximum recommended duration of 30 days2 and can provide right, left, and biventricular support in patients with cardiogenic shock. Depending on the individual patient’s requirements, an oxygenating membrane can be added to the circuit. Once the assist device has been inserted, the patient must remain hospitalised, usually in an intensive care unit (ICU). An estimated 35 CentriMag devices are implanted every year in Spain according to the ESPAMACS registry3 (created by the Spanish Society of Thoracic and Cardiovascular Surgery [SECTCV] in 2012).4
MCS devices, like the Levitronix® CentriMag, provide good haemodynamic support for the patient and share a similar complication rate: bleeding (28%), kidney failure (28%), infections (24%), thrombosis (7%), neurological complications (7%), haemolysis (3%), and device failure (.08).5
The first patients with MCS devices were put on complete bed rest to prevent complications such as bleeding or accidental decannulation during mobilisation, and to promote rest and healing.6 However, prolonged bed rest is associated with the need for prolonged mechanical ventilation, the risk of associated pneumonia, longer hospital stay, pressure ulcers (PUs), increased risk of falls, increased incidence of delirium,7 and substantial and prolonged neuromuscular complications significantly affecting physical function and quality of life after hospital discharge.8
Thanks to the constant evolution of surgical implantation methods and cannula fixation, the benefits of mobilisation in this type of patient have been observed. Early mobilisation of the critically ill patient improves their physical condition, and reduces the number of days on mechanical ventilation and ICU stay.7,9,10
A systematic review and meta-analysis of studies involving mobilisation interventions in intensive care units (ICU) showed that these interventions are safe, with a very low rate of associated complications (2.6%) and an even lower rate of the consequences of these complications (.6%).7 The goals of early progressive mobility in patients with MCS devices are no different from those of any other patient admitted to an ICU (early extubation, progressive mobility, and nutritional support6), but there are more limitations, barriers, and safety considerations when they are mobilised.6 The placement of the cannulas of MCS devices is a major determinant in the ability to progress with mobility; in the case of CMG-type assist devices, central placement of cannulas allows appropriate exercise to be started gradually, unlike other devices that are usually inserted peripherally (femoral).11
Despite the great benefits of early and progressive mobilisation in the critically ill patient, the evidence of its application in patients with CMG-type ventricular assist devices is scarce6,12 and absent in patients eventually to undergo heart transplantation (HT), where they remain under this type of MCS until the time of surgery (HT). Furthermore, although nutritional status deficit is highly associated with worse clinical outcomes in critically ill patients,13,14 the evidence in this patient profile is practically nil.
The aim of the study was to assess the degree of mobility in these patients prior to HT and its impact on 90-day mortality. We also analysed the relationship between degree of mobility and nutritional status prior to HT with the risk of infections and its impact on progress after HT.
MethodsScope and study populationWe designed a longitudinal retrospective study between January 2010 and December 2019 in the cardiac intensive care unit of the Hospital Universitario de Bellvitge, a referral unit for high complexity conditions for more than two million inhabitants and with a heart transplantation programme.
Consecutive non-probabilistic sampling was used. We included adult patients (>18 years) who had undergone heart transplantation between January 2010 and December 2019 and who had previously had short-duration CMG-type MCS. Patients unable to mobilise (patients with spinal cord injury, polytrauma, etc.) were excluded).
VariablesDemographic variables (age, sex, weight, and height), clinical variables prior to admission (cardiological diagnosis, pathological history, and cardiovascular risk factors) and clinical variables associated with HT and admission to the ICU (INTERMACS score, date of inclusion on the HT list, type of MCS prior to CMG and associated complications, inotropic treatment, requirement for renal replacement therapy or IMV, CMG support time and date of HT) were collected.
Patients who underwent mobilisation during MCS were differentiated. It was also recorded whether they were followed up by the hospital's physiotherapy team. The nutritional status of the patients at the time of HT was analysed, indicating the type of nutrition (absolute diet, parenteral, enteral, oral, or other), and analytical values (albumin [g/L] and prealbumin [mg/L]).
The patients’ progress after HT was recorded, analysing as the main variable mortality 90 days after HT (peri-transplant), as well as onset of PUs and infections (bacterial, fungal, viral, or other), post-HT IMV time, and time to discharge from the ICU.
Instrument for collection of variablesThe variables were extracted from the computerised hospital records of the patients. Patient names were anonymised, thus eliminating any means of identification.
A grid was constructed (Table 1) to collect the patients’ degree of mobility at the time of HT, with nine degrees and two differentiated groups: high mobility and low mobility. Patients who prior to transplantation were able to sit up in bed, sit on the edge of the bed, transfer from bed to chair, or ambulate were classified in the high mobility group. The low mobility group included sedated patients and those who were able to perform passive/active-assisted lifting of upper and/or lower limbs.
Degrees of mobility.
| Score by degree of mobility | |
|---|---|
| Low mobility | Sedated patients |
| Passive UL exercises | |
| Passive UL exercises | |
| Active assisted lifting of UL | |
| Active assisted lifting of LL | |
| High mobility | Sitting on bed in "chair mode" |
| Sitting at the edge of the bed | |
| Transfer from bed to chair. Ambulation |
UL: upper limbs; LL: Lower limbs. Scale developed by the authors and in the process of validation.6
A descriptive and inferential analysis of the recruited sample was performed to assess the impact of early mobilisation in patients with CMG-type MCS on clinical progress variables. Quantitative variables are expressed as median and interquartile range (IQR) and qualitative variables as absolute value and proportion.
Non-parametric tests were used for inferential analysis. The Mann-Whitney test was used for quantitative variables and Fisher's exact test for qualitative variables. Between-group mortality was described using Kaplan-Meier curves and between-group differences were analysed using Cox regression. Data analysis was performed with STATA software (Version 15, StataCorp, College Station, Texas, USA.).
Ethical considerationsThe included patients signed their informed consent prior to inclusion. The protocol was designed in compliance with current legal data protection regulations (Organic Law 3/2018 of 5 December on the protection of personal data and guarantee of digital rights and, by extension, the General Data Protection Regulation [EU] 2016/679). The study was approved by the hospital's ethics committee (reference code PR309/20).
ResultsA total of 27 patients were included with a median age of 56 years (IQR: 48−62.7) and 81.5% were male. The patients spent a median of 22 days (IQR: 13−33) in the ICU post-HT.
As Table 2 shows, 77.8% of the patients had a diagnosis of dilated cardiomyopathy, 25.9% were diabetic and, in addition, 14.8% had chronic obstructive pulmonary disease (COPD). Almost three quarters of the patients received intra-aortic balloon counterpulsation (IABP) prior to HT. A total of 29.6% received extracorporeal membrane oxygenation (ECMO) support prior to HT. A total of 77.8% of the patients had biventricular CMG support at the time of HT, and the remaining 22.2% left CMG support.
Baseline characteristics.
| Variables | Low mobility (n = 22) | High mobility(n = 5) | Total | p |
|---|---|---|---|---|
| Age | 56.7 (49−63.9) | 51.1 (48−56.1) | 56.4 (48−62.7) | .289 |
| Sex (male) | 17 (77.3%) | 5 (100%) | 22 (81.5%) | .547 |
| BMI | 25.7 (23.9−28.7) | 26.6 (25.2−29.5) | 26 (24.2−29.4) | .349 |
| Days in ICU pre HT | 22 (13−29) | 29 (24−41) | 22 (13−33) | .234 |
| Dilated cardiomyopathy | 16 (72.7%) | 5 (100%) | 21 (77.8%) | .555 |
| DM | 4 (18.2%) | 3 (60%) | 7 (25.9%) | .091 |
| HTN | 9 (40.9%) | 2 (40%) | 11 (40.7%) | 1 |
| Hypercholesterolaemia | 9 (40.9%) | 4 (80%) | 13 (48.2%) | .165 |
| COPD | 3 (13.6%) | 1 (20%) | 4 (14.8%) | 1 |
| IAPB pre HT | 17 (77.3%) | 3 (60%) | 20 (74.1%) | .580 |
| Impella pre HT | 3 (13.6%) | 2 (40%) | 5 (18.5%) | .221 |
| ECMO pre HT | 7 (31.8%) | 1 (20%) | 8 (29.6%) | 1 |
| EXCOR pre HT | 1 (4.6%) | 0 | 1 (3.7%) | 1 |
| Left CMG | 5 (22.7%) | 1 (20%) | 6 (22.2%) | 1 |
| Biventricular CMG | 17 (77.3%) | 4 (80%) | 21 (77.8%) | 1 |
| Days of CMG pre HT | 13.5 (4−17) | 13 (12−31) | 13 (8−18) | .49 |
| Inotropes pre HT | 13 (59.1%) | 2 (40%) | 15 (55.6%) | .628 |
| RRT pre HT | 3 (13.6%) | 0 | 3 (11.1%) | 1 |
| IMV pre HT | 11 (50%) | 0 | 11 (40.7%) | .06 |
| IC physiotherapy | 9 (40.9%) | 4 (80%) | 13 (48.2%) | .165 |
| Oral nutrition | 13 (59.1%) | 5 (100%) | 18 (66.7%) | .136 |
| Albumin (g/L) | 24.5 (23−30) | 33 (26−36) | 26 (23−31) | .029 |
| Prealbumin (mg/L) | 160 (113−244) | 222 (140−344) | 166 (114−247) | .239 |
BMI: body mass index; CMG: Levitronix® Centrimag; COPD: chronic obstructive pulmonary disease; DM: diabetes mellitus; ECMO: extracorporeal membrane oxygenation; EXCOR: Berlin-Heart Excor® circulatory support; HT: heart transplantation; HTN: hypertension; IAPB: intra-aortic balloon counterpulsation; IC: interconsultation; ICU: intensive care unit; IMV: invasive mechanical ventilation; RRT: renal replacement therapy.
Only five patients had a high degree of mobility. Of the 22 with low mobility, six were under sedation, seven were only able to perform passive upper and lower limb exercises, and nine performed active-assisted limb mobility exercises. No events related to dislodgement of cannulas during patient mobilisations were recorded.
A survival rate of 80% was observed in the high mobility group. In the low mobility group a survival rate of 63.6% was observed. The log rank analysis showed no statistically significant differences (Fig. 1).
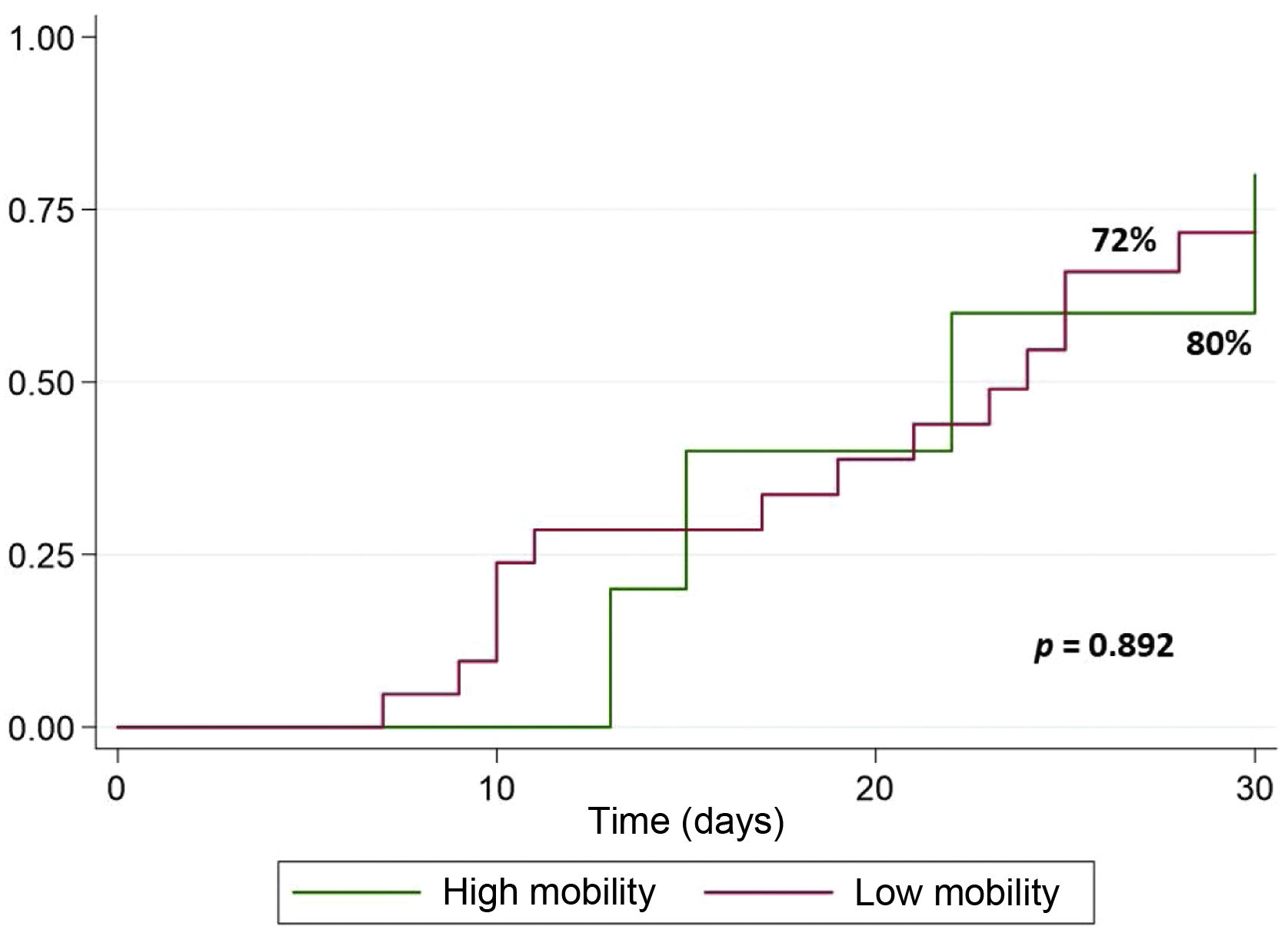
The progress of the patients until discharge from the ICU after HT at 30 days can be seen in Fig. 2. No significant differences were observed in the distribution of discharges.
There was a trend towards a higher proportion of patients arriving for HT with invasive mechanical ventilation (IMV) in the low-mobility group (Table 2).
Lower albumin levels were observed in the low mobility group, with a statistically significant difference (24.5 g/L [IQR: 23−30] vs. 33 g/L [IQR: 26−36]; p = .029). In addition, it was observed that all patients in the high mobility group maintained an oral diet, in contrast to the lower mobility group at only 59% (Table 2). No significant differences were observed with respect to age, sex, pre-HT ICU stay and pre-HT circulatory support (IABP, Impella, ECMO).
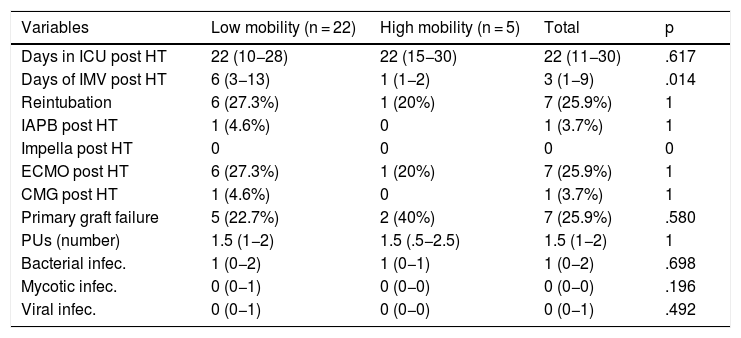
Table 3 shows the main post-HT events in both the high mobility and low mobility groups. We can highlight that a quarter of the patients needed ECMO support after surgery. Statistically significant differences were observed in the median number of days of post-HT IMV, which was greater in the low mobility group (six days [IQR: 3−13] vs. one day [IQR: 1−2]; p = .014) (Table 3). No statistically significant differences were observed in either the number of PUs between groups or the number of infections (bacterial, fungal or viral).
Events post HT.
| Variables | Low mobility (n = 22) | High mobility (n = 5) | Total | p |
|---|---|---|---|---|
| Days in ICU post HT | 22 (10−28) | 22 (15−30) | 22 (11−30) | .617 |
| Days of IMV post HT | 6 (3−13) | 1 (1−2) | 3 (1−9) | .014 |
| Reintubation | 6 (27.3%) | 1 (20%) | 7 (25.9%) | 1 |
| IAPB post HT | 1 (4.6%) | 0 | 1 (3.7%) | 1 |
| Impella post HT | 0 | 0 | 0 | 0 |
| ECMO post HT | 6 (27.3%) | 1 (20%) | 7 (25.9%) | 1 |
| CMG post HT | 1 (4.6%) | 0 | 1 (3.7%) | 1 |
| Primary graft failure | 5 (22.7%) | 2 (40%) | 7 (25.9%) | .580 |
| PUs (number) | 1.5 (1−2) | 1.5 (.5−2.5) | 1.5 (1−2) | 1 |
| Bacterial infec. | 1 (0−2) | 1 (0−1) | 1 (0−2) | .698 |
| Mycotic infec. | 0 (0−1) | 0 (0−0) | 0 (0−0) | .196 |
| Viral infec. | 0 (0−1) | 0 (0−0) | 0 (0−1) | .492 |
CMG: Levitronix® Centrimag; ECMO: extracorporeal membrane oxygenation; HT: heart transplantation; IAPB: intra-aortic balloon counterpulsation; ICU: intensive care unit; IMV: invasive mechanical ventilation; Infec.: infections; PUs: pressure ulcers.
The results show that mobilisation in this complex clinical scenario is feasible and safe (0 major cannula dislodgement events). Our study found no significant differences between degree of pre-HT mobility in patients with MCS and 90-day mortality, ICU length of stay, onset of infection or post-HT PUs. Although the median number of days of post-HT ICU stay was the same in both groups, early mobilisation was associated with a significant and clinically relevant difference in the need for post-HT IMV, as it was observed that patients with a high degree of mobility had a shorter post-HT IMV time, and the absence of mobilisation-related complications. The patients with high mobility had a better nutritional status at the time of HT; from these data it can be inferred that high mobility is associated with better nutritional status, avoiding the malnutrition associated with prolonged bed rest.
The use of MCS devices in the critically ill cardiac patient is growing in our country as regional shock code networks are being established.15 Current management of the critically ill patient requires a multidisciplinary approach that overall has an impact on the clinical prognosis of these patients. This includes specific care of nutritional status, physiotherapy and mobilisation, pain control, sedation status, psychological and emotional support, etc. All this care is also particularly relevant in patients with MCS as a bridge to HT, as they have longer hospital stays, undergo multiple invasive procedures and have a level of immunosuppression that aggravates the consequences of potential complications. These patients sometimes have less opportunity to receive this type of care due to the need for sedation and the difficulty in managing the multiple catheters, cannulas, and drains that need to be closely monitored throughout.
Despite the relevance of this care, there is no current evidence on the benefits of mobilising these patients. To our knowledge, this is the first study to address this issue. In our sample, we observed that only five patients had a high degree of mobility, in other words, they were able to at least sit up in bed in chair mode, while the remaining 22 were classified in the low mobility group. These data show that the mobilisation of these patients is still a pending task that needs to be addressed. Nurses play a central and active role in promoting and supervising patient care, as they spend the most time with the patient at the bedside and, therefore, are very often the professionals who best know the needs of these patients.
Although the results do not show a clear benefit in terms of mortality and infections, the patients with high mobility had a shorter IMV time and no complications associated with mobilisation, providing evidence of its safety. These results are in line with other previously published studies in which the safety and potential benefit of physical exercise in patients with long-term ventricular assist devices was also observed.16
The limiting factors for this study are that it is a retrospective study with a small number of patients, and therefore, due to a probable lack of power, no statistically significant differences were found in the relationship between degree of mobility and mortality. All data collection was performed solely by reviewing the patients' medical records. This leads to biases relating to the lack of information in the clinical records, which often did not contain all the information necessary for the collection of data for the study.
ConclusionsPatients undergoing HT with MCS and a high degree of mobility had a shorter IMV time compared to patients with a low level of mobilisation, and better nutritional status. No mobilisation-associated complications were observed. No significant differences were found between pre-HT degree of mobility in patients with MCS and 90-day mortality, ICU length of stay, onset of infections or post-HT PUs. Based on these findings, we believe that more statistically powered, multicentre, prospectively designed studies are needed for a more detailed analysis of the safety, impact of degree of mobility, and nutritional status in patients undergoing HT with MCS.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sector.
Conflict of interestsThe authors have no conflict of interests to declare.