Concerns exist around the generalizability of randomised controlled trials (RCTs) for adolescents with major depressive disorder (MDD). This review assesses whether adolescents with MDD treated in RCTs are representative of clinical samples.
MethodsA systematic narrative review of selection criteria used in RCTs for adolescent MDD (PROSPERO CRD42018096298). Included were studies assessing psychological, pharmacological or combination treatments.
Results52 studies were included. Overall, the reporting of selection criteria (defined as both inclusion and exclusion criteria), in the 23 psychotherapy trials was low (52% did not report on comorbid emotional disorders and 48% did not report on suicidal ideation). In contrast, the majority of selection criteria were reported in the 22 medication trials and the 7 combination trials. Where selection criteria were reported, most adolescents with comorbidities were excluded from psychotherapy and medication trials. The 7 combination trials included more adolescents with comorbidities. Of note, only 10 of the 52 studies reported on self-harm as a selection criteria.
ConclusionReporting of the characteristics of depressed adolescents was poor in psychotherapy trials. Both psychotherapy and medication trials excluded many adolescents with co-morbid conditions, however combination trials tended to be more inclusive. There is concern that many RCTs for adolescent MDD may not be generalizable to clinical populations, particularly with regards to comorbidity, self-harm and suicidal ideation. The findings suggest that clinicians need to view the evidence base and guidelines developed from RCTs with discernment. Pragmatic trials are needed with representative clinical populations and comprehensive reporting of the selection criteria.
Adolescent depression is a serious public health issue worldwide.1 In particular, depressive disorders are the leading cause of years lost to disability for adolescent girls and young women aged 15-24 years.1 Adolescent depression is associated with an increased risk of suicidality,2 comorbid psychiatric conditions,3 a high rate of relapse4–6 and poorer health in adulthood.5 For clinicians the challenge is to interpret the results of many different treatment trials whilst offering personalised care. Clinicians and policy makers also rely on the findings of systematic reviews; however, the conclusions of these reviews are necessarily limited if the trials are not generalizable to the relevant clinical population.
The treatment of adolescent depression remains a challenge at many levels. For example, the FDA ‘black box’ warning around suicidality and the use of antidepressants in adolescents7 led to changes in clinical practice that may have been helpful in some circumstances, such as overprescribing in mild to moderate depression, but may also have caused potential harm. Antidepressants may be useful for some adolescents with moderate to severe depression who are at increased risk of suicide, and there is evidence for under treatment in young people who have tragically taken their own lives.8,9 There is thus a potential danger of putting too much emphasis on a narrative that antidepressants are harmful in all circumstances, or do not work, when there is evidence for a role within the spectrum of treatments for moderate to severe depression.10,11
In recent years, a number of analyses have assessed the efficacy of treatments for adolescent depression. In a network meta-analysis, Cipriani et al.,12 cautioned that antidepressants do not seem to offer a clear advantage for children and adolescents, and gave a limited endorsement for fluoxetine despite a standardised mean difference of 0.51. It has been argued that these conclusions may have undervalued the potential benefits of antidepressants particularly in more complex clinical populations.13 However, as the authors noted, the poor methodology and lack of reliable data on suicidality in many of these trials makes interpretation of findings difficult. Although the available data on comorbidity was examined in a sub-analysis, and did not affect the principle findings, it was unclear how the samples recruited to these trials compared to clinical populations.
Generally, there has been less scrutiny of psychological treatment trials when compared to antidepressant trials, although concerns also exist regarding the quality of available data, as well as adverse effects.14–16 In a network meta-analysis, Zhou et al.17 reported that interpersonal therapy for adolescents (IPT-A) and cognitive-behavioural therapy (CBT) were significantly more effective than most control conditions. However, studies on treatment-resistant and psychotic depression were excluded, and effects were less pronounced when comorbid psychiatric disorders were present. The authors noted that this may have led to an overestimation of the effect size, because the most difficult cases were not considered, and waitlist controls may have inflated the effect of psychotherapies. In addition, data on suicidal behaviour was not examined, because this data was lacking in almost all studies. The authors stated that these variables are important for clinicians and patients to make decisions on selecting appropriate treatment and that the findings on comorbidity need replication as these subgroups were small. These findings again raise the question of generalisability to children and young people seen in clinical practice.
In a more recent meta-analysis spanning five decades of psychological research, Weisz et al.18 showed that the overall effect size in psychotherapy trials for adolescent depression was 0.36 post-treatment and 0.21 at follow-up. This is a modest effect size and has remained unchanged over the past thirteen years.19 Of particular concern, was that across the four specific targeted problems, treatment of depression showed the most disappointing effects; in fact, by teacher report, treatments for depression were worse than control conditions. As the authors stated, this finding is noteworthy in light of professional guidelines that recommend psychological therapy as the first-line treatment for youth depression, together with the concerns regarding representativeness of clinical populations.
The use of selection criteria (inclusion and exclusion criteria) in RCTs is a necessary part of trial design but leads to concerns that RCTs do not mimic clinical practice, and that potential participants with more severe symptoms, comorbidity or suicidality are often excluded.20 Blanco et al.,21 used exclusion criteria from two of the meta-analyses described above and applied them to data from the National Comorbidity Survey: Adolescent Supplement (NCS-A). The NCS-A is a nationally representative, face-to-face survey aiming to provide estimates of prevalence and patterns of service use for DSM-V mental disorders among US adolescents.22 Blanco et al.,21 showed that at least 6 out of 10 adolescents with MDD in the US population would have been excluded from the medication trials assessed by the Cipriani meta-analysis,12 and at least 4 out of 10 adolescents would have been excluded from the psychotherapy trials assessed by the Zhou meta-analysis.17
Similar results have been found in the adult population group. In 2008, Blanco et al.,23 applied a standard set of exclusion criteria for clinical trials to patients included in the National Epidemiologic Survey for Alcohol and Related Conditions (NESARC), which is the largest psychiatric epidemiologic study in the United States to date (n = 43,093). They found that over two thirds of people who had already been treated for MDD in the NESARC would have been excluded from traditional clinical trials. This lack of generalisability is also the case for specific treatments for MDD. Zimmerman et al.,24,25 report that over the past 25 years only a small minority of patients being prescribed antidepressant medication would have been included in most clinical trials, and this has worsened over time, so that clinical trials are becoming less representative of clinical settings. Similar concerns were reported by Wisniewski et al.26 who applied common clinical trial exclusion criteria to participants in the STAR*D project, which had broad inclusion criteria. Morrison et al.,27 performed similar research on psychotherapeutic treatments of MDD and found that nearly all the patients who were receiving psychotherapy in their study would have been excluded from most clinical trials.
The aim of this study was to analyse the use of selection criteria in RCTs of adolescents with Major Depressive Disorder (MDD) and compare the study populations to the information available on the characteristics of adolescents with depression.
Primary aimThe primary aim was to describe the reporting of inclusion and exclusion criteria used by RCTs for MDD in adolescents
Secondary aim- 1.
To analyse the inclusion of adolescents with MDD plus the following characteristics in RCTs for MDD in adolescents
- a.
Comorbid mental health problems
- b.
Comorbid physical health problems
- c.
Previous treatment for depression
- d.
Risk to self
- e.
Age of onset and duration of MDD
- a.
- 2.
To analyse the absence of reporting of the above characteristics.
This review compliments but differs from the existing literature by including additional trials which assessed the combination of treatment with medication and psychological therapy, as well as examining the absence of reporting of selection criteria. To our knowledge, this absence of reporting has not been systematically documented before and is important in understanding the potential limitations of clinical trials.
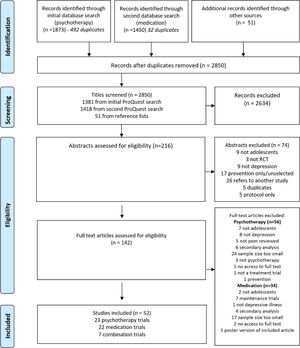
MethodsTwo authors (AM & LK) reviewed all titles independently, shortlisted potentially relevant studies, read the full text articles to confirm suitability for inclusion in this review and extracted the required data (Fig. 1). Relevant reviews and meta-analyses were hand-searched for any additional studies (a list of reviews which were hand searched is available on request). Any discrepancies were decided by consensus of all three authors. Inclusion criteria were (1) peer-reviewed RCTs (with or without a placebo arm) assessing psychological, pharmacological or combination treatments, (2) minimum of 20 participants in each arm, (3) adolescents (mean age 11-19 at the time of enrolment), (4) diagnosis of MDD.
PRISMA flow diagram39.
RCTs which recruited adolescents with depressive symptoms were included if the mean level of symptoms was indicative of likely MDD. These studies were discussed individually and consensus was reached between the authors.
Trials of treatment resistant depression were excluded because the management of the depression was likely to be different. Secondary analyses of included RCTs were excluded because the participants were the same as the original RCT participants (or a subset of the original study population) and the aim of this review was to assess the reporting in RCTs. RCTs with less than 20 participants were excluded to ensure a focus on larger trials, which were most likely to influence treatment guidelines and clinical practice. Trials of complementary and alternative treatments were not included as these are not a mainstay of treatments for adolescents with MDD in clinical services.
Two searches were completed using ProQuest (which searches six databases - British Nursing Index, Hospital Premium Collection, MEDLINE®, PsycARTICLES, PsycINFO, PTSDpubs) from inception to the end of January 2020. The searches were kept deliberately broad to ensure that no relevant studies were missed. However, results were limited to English language articles. The first search was tailored towards finding psychotherapy trials and used the terms “noft(depress*) AND noft(adolesc*) AND noft(randomised control trial) AND noft(child)”. The second search was tailored towards finding pharmacology trials and used the terms “noft(depress*) AND noft(adolesc*) AND noft(trial) AND noft(placebo) AND noft(controlled) AND noft(randomized)”. A search of the Cochrane database was also completed and the reference lists of any relevant Cochrane reviews were searched. The protocol was registered on PROSPERO prior to formal screening of results (reference number CRD42018096298) and can be found at https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42018096298. PRISMA guidelines were followed although not all aspects of the PRISMA checklist were relevant to this narrative review because the review analyses the inclusion and exclusion criteria of RCTs rather than the results of the RCTs.28
The analysis was complex because RCTs have used various ways to report inclusion and exclusion criteria. Characteristics of young people were sometimes described in the methodology and the results sections, for example. The term ‘selection criteria’ was used to gather all exclusion criteria and inclusion criteria from the whole paper, even where there was a lack of explicit reporting of inclusion and exclusion criteria. The criteria that were reported in more than one study are shown in Table 1. These characteristics have been shown to influence treatment outcomes e.g.29–38
Basic demographics, sample size, the studied interventions, location of the study, measures used at baseline, age of onset, duration of MDD, severity of depression in recruited participants, comorbidities of the participants and reported suicidality were also extracted from the included RCTs (available on request).
Reporting of inclusion and exclusion criteria.
Key: ADHD =Attention Deficit Hyperactivity Disorder, Behavioural disorder includes Conduct Disorder and Oppositional Defiant Disorder, BPAD = Bipolar Affective Disorder, Neurodevelopmental disorder includes Learning Disability and Autism
The term ‘not reported’ in Table 1 indicates that trial authors did not report, either in their methods or in their results, on whether adolescents with that particular selection criteria were included or excluded. When selection criteria were explicitly reported, details were extracted, and a summary given in the second and third column of Table 1.
ResultsFifty-two studies (total number of adolescents studied=8261) were included in the review, consisting of 23 psychotherapy trials (n = 2508), 22 medication trials (n = 4627) and 7 combination trials (n = 1126).
RCTs which may at first have appeared to recruit participants with milder symptoms of depression but reported a mean level of symptoms indicative of likely MDD were included. Examples of this are Wright et al.,40 which reported an inclusion criteria of ≥20 on the Mood and Feelings Questionnaire (MFQ) but their sample had mean baseline MFQ scores of 37 in the intervention group and 34.8 in the controls, which would be indicative of MDD.41 Another example is Merry et al.,42 which had broad inclusion criteria in respect to severity of depressive symptoms but both their control and intervention groups had mean baseline MFQ ≥27, which would also be indicative of MDD.41 Kerfoot et al.43 had an inclusion criteria of MFQ ≥23 but mean baseline MFQ scores were 33.7 in the intervention group and 34.2 in the controls. Kowalenko et al.,44 had an inclusion criteria of ≥18 on the Children's Depression Index (CDI) and reported a mean baseline CDI 20.9 in the intervention group and 22.3 in the controls, which is above the recognised cut off of 19.45
Table 1 shows the two stages of the analysis described in the method section.
The data supplement (available on request) gives an expansion of table one, showing details from each of the 52 named studies and references to the studies included in the analysis.
Absence of reportingComorbidityThere was a marked absence of reporting of comorbidity in the psychotherapy trials. Six out of the 9 comorbidities were not reported in 50% or more of these trials. More than half of the psychotherapy trials did not report on whether participants with comorbid emotional disorders were included (n = 12, 52%). Most psychotherapy trials did not report on comorbid ADHD (n = 19, 83%), personality disorder (n = 19, 83%), eating disorder (n = 17, 74%), schizophrenia (n = 14, 61%) and BPAD (n = 12, 52%). A significant minority did not report neurodevelopmental disorder (n = 11, 48%), psychotic disorder (n =9, 39%) and behavioural disorder (n = 9, 39%).
There was also an absence of reporting of comorbidity in the combination trials with more than half of the trials not reporting on comorbid ADHD (n = 5, 71%), comorbid eating disorder (n = 6, 86%) or personality disorder (n = 6, 86%). Nearly half did not report on comorbid emotional disorder (n = 3, 43%) and nearly a third did not report on comorbid behavioural disorder (n = 2, 29%), BPAD (n = 2, 29%), psychotic disorder (n = 2, 29%), or schizophrenia (n = 2, 29%). Reporting was better for comorbid neurodevelopmental disorder with only one trial not reporting (14%).
The majority of comorbidities were generally reported in the medication trials with the exception of personality disorder (omitted by n = 15, 68%). The remaining comorbidities were reported in the majority of trials with minimal omissions (ADHD (n = 4, 18%), behavioural disorder (n = 3, 14%), BPAD (n = 3, 14%), eating disorder (n = 1, 5%), emotional disorder (n = 3, 14%), psychotic disorder (n = 2, 9%), and schizophrenia (n = 3, 14%), however, a substantial minority did not report on comorbid neurodevelopmental disorder (n = 5, 23%).
Suicidality and self-harmThe reporting of self-harm was even less comprehensive with the majority of trials failing to report on this: 91% (n = 21) of psychotherapy trials, 72% (n = 16) of medication trials and 71% (n = 5) of combination trials did not report on whether adolescents with a history of self-harm were included in the trial.
Although the majority of trials reported on suicidal ideation, a significant minority did not: 48% (n = 11) of psychotherapy trials, 32% (n = 7) of medication trials and 29% (n = 2) of combination trials did not provide this information. Around a third of psychotherapy trials (n = 9, 39%) and medication trials (n = 7, 32%) did not report on immediate suicide risk. However, all combination trials reported on immediate suicide risk.
Previous response to treatmentOf the psychotherapy trials, most (n = 18, 78%) did not report on any previous non-response to treatment and almost half did not report on whether the participants were having concurrent psychological therapy (n = 11, 48%). 35% (n = 8) did not report on concurrent treatment with medication. Of the combination trials more than half (n = 4, 57%) did not report on previous non-response to treatment or concurrent treatment with psychological therapy (n = 4, 57%). Most reported on concurrent treatment with medication (omitted by 29% (n = 2)). Of the medication trials, more than half did not report on previous non-response to treatment (n = 13, 59%) or concurrent treatment with psychological therapy (n = 12, 55%). Most medication trials reported on concurrent treatment with medication (omitted by 5% (n = 1)).
Physical health and pregnancyOverall rates of reporting in psychotherapy trials were low with most (n = 21, 91%) not reporting on whether they included young people who were pregnant or not using contraception and two thirds (n = 14, 61%) not reporting on whether they included young people with physical health problems. All medication trials reported on physical health problems (n = 22) but 32% (n = 7) did not report on pregnancy/contraception, and 29% (n = 2) of combination trials did not report on physical health and pregnancy/contraception.
Age of onset and duration of MDDAge of onset of MDD was not reported by any of the psychotherapy or combination trials. It was reported by ten of the medication trials (mean age of onset ranged from 9.8-14.5 years). Duration of MDD was reported by four psychotherapy trials; one reported a mean duration of 26 weeks,46 one reported 81 weeks,35 one reported that 83% had a duration of “over 1 year”43 and one reported that 76% had a duration “over 6 months”47 (exact durations not reported). Twelve medication trials reported a mean duration of MDD (range 69-185 weeks). Two combination trials3,48 also reported a mean duration of MDD (both reported mean duration of 40 weeks).
Reported comorbidityWhen comorbidity was reported, rates of inclusion were low across all three trial types. In the psychotherapy trials, less than half included participants with comorbid behavioural disorder (n = 10, 43%) or comorbid emotional disorder (n = 9, 39%); few included comorbid ADHD (n = 4, 17%), BPAD (n = 1, 4%), eating disorder (n = 3, 13%) or personality disorder (n = 2, 9%). None of the psychotherapy trials reported inclusion of adolescents with a comorbid neurodevelopmental disorder and only one trial included adolescents with a comorbid psychotic disorder.
Most medication trials explicitly listed comorbid behavioural disorder (n = 13, 59%), BPAD (n = 19, 86%), eating disorder (n = 21, 95%), emotional disorder (n = 16, 73%), neurodevelopmental disorder (n = 17, 77%), psychotic disorder (n = 19, 86%) and schizophrenia (n = 19, 86%) as exclusion criteria. No medication trials reported including participants with comorbid BPAD, eating disorder, neurodevelopmental disorder, personality disorder or schizophrenia. A minority included adolescents with ADHD (n = 8, 36%), behavioural disorder (n = 6, 27%) and a comorbid emotional disorder (n = n = 3, 14%).
Of the combination trials, 57% (n = 4) included adolescents with comorbid emotional disorder and a minority included adolescents with comorbid behavioural disorder (n = 3, 43%), ADHD (n = 2, 29%), eating disorder (n = 1, 14%), personality disorder (n = 1, 14%) or psychotic disorder (n = 1, 14%). Most reported neurodevelopmental disorder (n = 6, 86%) and psychotic disorder (n = 4, 57%) as explicit exclusion criteria.
Reported suicidal ideation and riskOf the studies where selection criteria were reported, the majority of combination trials included young people with suicidal ideation or history of attempted suicide (n = 5, 71%) versus a minority of psychotherapy (n = 8, 35%) and medication trials (n = 3,14%). Suicidal ideation or history of attempted suicide were reported as explicit exclusion criteria in 17% (n = 4) of psychotherapy trials, 55% (n = 12) of medication trials and none of the combination trials. Young people with a history of self-harm were only included in 4% (n = 1) of psychotherapy trials, 23% (n = 5) of medication trials and 29% (n = 2) of combination trials.
Reported Co-existing and previous treatment39% (n = 9) of psychotherapy trials excluded young people who were taking medication and 32% (n = 7) of medication trials excluded young people who were receiving psychological therapy. Only 9% (n = 2) of psychotherapy trials and 5% (n = 1) of medication trials included young people who had previously not responded to an intervention for depression.
Reported physical health and pregnancyComorbid physical health problems were listed as an explicit exclusion criteria in all of the medication trials (n = 22, 100%), more than half of the combination trials (n = 4, 57%) and around one third of the psychotherapy trials (n = 7, 30%). Whilst it would be expected that pregnancy /not using contraception would be an exclusion criteria for all medication and combination trials, it was only reported as such in two thirds of medication trials (n = 15, 68%) and 71% of combination trials (n = 5).
DiscussionThis study found that there is poor reporting of key characteristics of participants in most psychotherapy RCTs, and the majority of trials did not report on comorbid emotional disorders, eating disorders, ADHD, schizophrenia, and previous response to treatment. Without explicit reporting of key characteristics, it is not possible to understand the complexity of a study sample, and hence how representative it may be of a clinical population. These findings have significant implications for the interpretation of trial results and associated systematic reviews. Although some reviews have attempted to examine the effects of particular characteristics, these conclusions are necessarily limited if it is unknown whether a specific characteristic was included or excluded from a study sample.
Cipriani et al.,12 analysed “suicidal behaviour or ideation” in their network meta-analysis of efficacy and tolerability of antidepressants for major depressive disorder in children and adolescents but stated that “due to the absence of reliable data on suicidality for many antidepressants, it was not possible to comprehensively assess the risk of suicidality for all drugs”. The authors stated that sub-group analysis of comorbidity did not produce different results but given that this review found high rates of exclusion of comorbidity in medication trials, this data is likely to have been lacking in many of the included RCTs. In a network meta-analysis of 54 RCTs of psychotherapies for depression in children and adolescents, Zhou et al.,17 reported that they could not analyse data on suicidality because the information was “lacking in almost all studies”. The authors reported that IPT and CBT had less significant effects in studies in which patients had comorbid psychiatric disorders. However, the number of RCTs which provided data on comorbidity was not reported and given that many of the RCTs are the same as the psychotherapy RCTs included in this review, that conclusion is likely drawn from limited data. In a multilevel meta-analysis of youth psychological therapy, Weisz et al.,18 commented that “To the extent that studies of a target problem exclude comorbidities, this may be a concern because the evidence indicates that co-occurring problems are pervasive in clinically referred youths”.
Reporting details of self-harm selection criteria was particularly low across trials. Yet one in four (25.5%) 11-16 year olds with a mental health disorder, and nearly half of 17-19 year olds (46.8%) with a mental health disorder, in the UK, have self-harmed or attempted suicide.49 Although suicidal ideation was described in the majority of trials, reporting was low in a substantial minority. Self-harm may indicate a different treatment pathway,50 and a poorer response to depression treatments, including antidepressants. In some adolescents, repeated self-harm may also be an indication of an emerging personality disorder. One study of adolescents with repeated self-harm reported that 60% of referred adolescents showed one or more forms of personality disorder, which was associated with significantly greater psychopathology and impairment, with worse outcomes, including depressive symptoms.51
Other notable absences included reporting around experiences of trauma or of being a looked after child despite evidence that adverse childhood experiences increase the risk of developing depression.52 Reporting of clinical characteristics such as age of onset and duration of MDD were also notably absent, particularly in the psychotherapy trials despite both being important predictors of treatment outcome.37,38,53
When comorbidity was reported, rates of inclusion were low across all three trial types. Medication trials had the most explicit exclusion criteria, confirming the conclusion by Blanco that the majority of young people with MDD would be excluded from these trials.21 The combination trials were more inclusive and included more information on imminent risk of suicide. These trials therefore appear to be more inclusive of the spectrum of emotional difficulties and associated risk seen in clinical practice.
The IMPACT trial is an example of a more recent psychological treatment trial which recruited adolescents with a diagnosis of major depressive disorder from Child and Adolescent Mental Health Service (CAMHS), and had relatively broad inclusion criteria with high rates of comorbidity, complexity and risk.54 For example, 34% reported lifetime suicide attempts, 56% reported lifetime self-harm, 18% reported recent self-harm and 46% had more than one comorbid disorder.
Clinicians see young people and their families with more comorbidity than in many trials. For example, Orchard et al.,55 describes a consecutive series of 100 adolescents, who were referred to CAMHS in the UK with moderate to severely impairing anxiety, depressive or obsessive-compulsive symptoms. Even after excluding those adolescents presenting with a high risk of suicide, psychosis, safeguarding concerns or “very significant psychosocial complexity”, 25% of participants met the criteria for a comorbid disorder such as generalised anxiety disorder, and 86% of adolescents diagnosed with a depressive disorder presented with suicidal ideation. Those young people with comorbid anxiety would have been included in just 39% of psychotherapy trials, 14% of medication trials and 57% of combination trials and those with suicidal ideation would have been included in 35% of psychotherapy trials, 14% of medication trials and 71% of combination trials.
Fitzpatrick et al.,56 carried out a detailed standardized initial research assessment with 100 young people aged 12–15 years newly referred to CAMHS in Ireland. These were not specifically referrals for depressive disorders so included a range of presentations. Despite this, 31% of participants presented with suicidal ideation and 26% presented with suicidal acts. Essau et al.,57 examined the psychiatric comorbidity rates of adolescents with depressive disorder in community and clinical settings in Germany. They found that 58% of adolescents with depressive disorder in the community setting and 63.5% in the clinical setting had an additional disorder. The most common comorbidities found were anxiety disorders.
Psychosis was commonly reported as an exclusion criteria, yet the ADAPT trial found that 8% of the included participants presented with psychosis.3 There is evidence to suggest that many children do have experiences, which may be considered unusual. In a study of 6455 twelve year olds born in the UK, 7.6% self-reported auditory hallucinations, 6.1% self-reported visual hallucinations and 16.3% self-reported beliefs that they were being spied on.58 Whilst most of these children are not presenting to mental health services it does suggest that a proportion of young people with depression will be experiencing symptoms that could be described as psychosis within the context of their depression and they are not likely to have been represented in clinical trials.
Although not specific to depression, the recent national Mental Health of Children and Young People Survey (MHCYP) in England49 also reported a high rate of comorbidity with emotional disorders. The survey found that 29.6% of 5-19 year olds with an emotional disorder met the criteria for two disorders, and 18.6% of 5-19 year olds with an emotional disorder met the criteria for three disorders. Many of these participants would have been excluded in the majority of trials included in this review. The MHCYP also showed that young people with emotional disorders can present with comorbid physical health problems (75.9% of 5-19 year olds with an emotional disorder had a comorbid physical or developmental problem), and substance misuse (16.7% of 11-16 year olds with an emotional disorder had taken illicit drugs), the latter contributing to risk, and potentially affecting treatment outcomes.59
Similar results were reported by Kashani et al.,60 who analysed a community sample of adolescents in the United States of America and reported that those who met criteria for a depressive disorder had at least one comorbid diagnosis, and anxiety disorders were the most common comorbidities. Adult population studies also report high levels of comorbidity in depressed adults.61,62
Angold et al.,63 reviewed epidemiological studies of children and adolescents with depressive disorder. They found rates of comorbid conduct disorder/oppositional defiant disorder between 21% to 83% and rates of comorbid anxiety disorder from 30% to 75%. Meinzer et al.64 performed a meta-analysis of cross-sectional and longitudinal studies including both clinical and epidemiological samples, looking at comorbidity of ADHD and depressive disorder and found that the two conditions were positively related (rbar = 0.22).
In summary, this heterogeneity of depression, associated co-morbidity and contextual problems shown in both clinical and epidemiological samples is likely to affect the generalisability of the research findings discussed in this study and used to inform meta-analytical findings and clinical guidelines.
Strengths and limitationsThis review remains one of only a few papers to look at the reporting of inclusion criteria in RCTs for adolescent MDD. It is therefore a valuable addition to the literature given the impact of comorbidity and risk on RCT outcomes. This review only included RCTs with a minimum of 20 participants in each arm in order to focus on trials with the largest impact on clinical practice. However, this will have excluded a number of small trials included by systematic reviews focusing on treatment efficacy. Trials of complimentary or alternative medicine were excluded in order to focus on the most commonly recommended treatment in guidelines for MDD, however these trials may offer additional findings. As a result of the absence of reporting of participant characteristics in many of the included RCTs, some important clinical characteristics such a history of trauma or looked after child status are absent in this review.
ConclusionDepression in adolescents is difficult to treat and a great deal of research effort has led to systematic reviews to inform clinical practice. Whilst efficacy trials are needed, this study highlights the significant limitations of our current data, and the need for future pragmatic RCTs and sophisticated approaches to effectiveness studies to recruit participants that better reflect the clinical population seen in mental health services. RCTs should report inclusion and exclusion criteria more fully; psychotherapy trials in particular have not always been given the degree of scrutiny seen in medication trials. Guidance should be developed on best practice for reporting of inclusion criteria at the time of publication so that the impact of the sample population's characteristics on the trial results can be properly analysed. Meanwhile, any conclusions from systematic reviews are necessarily tentative when applied to clinical populations.
Ethical considerationsNo human or animal subjects were involved in this study.
Conflict of interestThe authors have no conflict of interest to declare.
FundingThere was no funding for this work.