Heterogeneity of depression in older adults is a challenge for the development of person-centered treatment. To address this, we studied glutamate and glutathione metabolism enzymes in blood cells in 53 older adult patients with depression and 20 controls. Patients with depression had decreased platelet glutathione-S-transferase and erythrocyte glutathione reductase. The biochemical and clinical data contributed to three clinical clusters that are not linked to the onset of depression or its duration but were related to anxiety, cerebrovascular and cardiovascular comorbidities, including parkinsonian features such as tremor, akathisia, and rigidity. These findings could aid person-centered diagnosis and outcomes and timely successful treatment(s) of depression in older adults.
Older patients with depressive and anxiety disorders are susceptible to the age-related acute and chronic brain and physical illnesses, including metabolic, endocrine and neurobiological disorders,1 and are at high risk for cardiovascular, and cerebrovascular comorbidities.2 The significant heterogeneity in geriatric depression results in difficulties in both comparing research findings and identifying biomarkers to establish homogeneous subgroups for depression personalized therapy.3 Since oxidative stress is associated with depression,4 it is reasonable to search for such biomarkers among antioxidant systems, i.e. glutathione system and glutathione-dependent enzymes.5
The balance of glutamate and GABA neurotransmitter systems’ activity, which provides general control and fine-tuning of the excitation, is, similarly, disturbed in depression.6 Activity of glutamate dehydrogenase (GDH) is of paramount importance in the brain glutamate system functioning7 with altered platelet GDH reported in mental disorders.8 Disturbances of the respiratory mitochondrial chain, complex IV (cytochrome c-oxidase - COX), can also contribute to depression.9The aim of this study was to both determine enzymatic activities, such as COX, GDH, glutathione reductase (GR) and glutathione-S-transferase (GST), in platelets and GR, and GST in erythrocytes in older patients with depression and determine their clinical relevance to aid person-centered treatment.
Materials and methodsThis cross-sectional study was performed from 2019-to 2021 and included 53 in-patients (41 women and 12 men, 60 - 86 years old) and a control group of 20 people (13 women and 7 men, 56 - 81 years old; Table 1), 34 patients (64.2%) had recurrent depression, 15 (28.3%) had bipolar disorder and 4 patients (7.5%) had a single depressive episode (DE). Most patients had comorbid pathology [hypertension, cerebrovascular diseases (CVDs), cardiovascular disease, and diabetes] and had been mentally ill for many years/decades (Table 1). Since for some, the current DE was not the first DE in their life, they had chronic extrapyramidal disorders (tremor or akinesia – rigidity syndrome) of both drug and subcortical vascular origin, preceding the exacerbation of depression. The DE type (apathetic, dreary, and complex) was determined as per Smulevich et al.10 criteria. The inclusion criteria for the patients’ group consisted of mild to severe DE in in-patients aged ≥60 years with recurrent depressive disorder (RDD, F33.0-F33.3), bipolar disorder (BD, F31.3, F31.4), or single DE (F32.0-F32.2) by ICD-10. The exclusion criteria were other mental illnesses, dementia of any etiology, brain trauma, drug addiction, severe somatic diseases in the stage of decompensation, a history of allergic reactions, or severe multiple hypersensitivities to medications.
Demographic and clinical data of studied groups.
Data are presented as Median and 25% and 75% Quartiles, M [Q25; Q75].
Psychometric assessment was carried out before starting the current treatment course using the Hamilton Depressive Scale (HAMD-17) and the Hamilton Anxiety Scale (HARS), MMSE was used for cognitive performance assessment. Duration of current DE was assessed until the moment of inclusion in the study. Many patients either already underwent pharmacotherapy being outpatient before the present hospitalization, or they had supportive pharmacotherapy and were monitored by psychiatrists. Duration of illness was recorded by psychiatrists in patients’ medical history. Blood cells were isolated and enzymatic activities were measured as described earlier.11 Activity of COX, GDH, GR, GST in platelets and GR, GST in erythrocytes (GRer, GSTer) was assessed once - both in patients (upon admission to the hospital), and in the control group.
Statistical analysis was performed using nonparametric (Mann-Whitney U-test, Kruskal-Wallis test, Chi-square test (χ²)), multivariate exploratory and cluster analyses (Statistica 8.0, StatSoft).
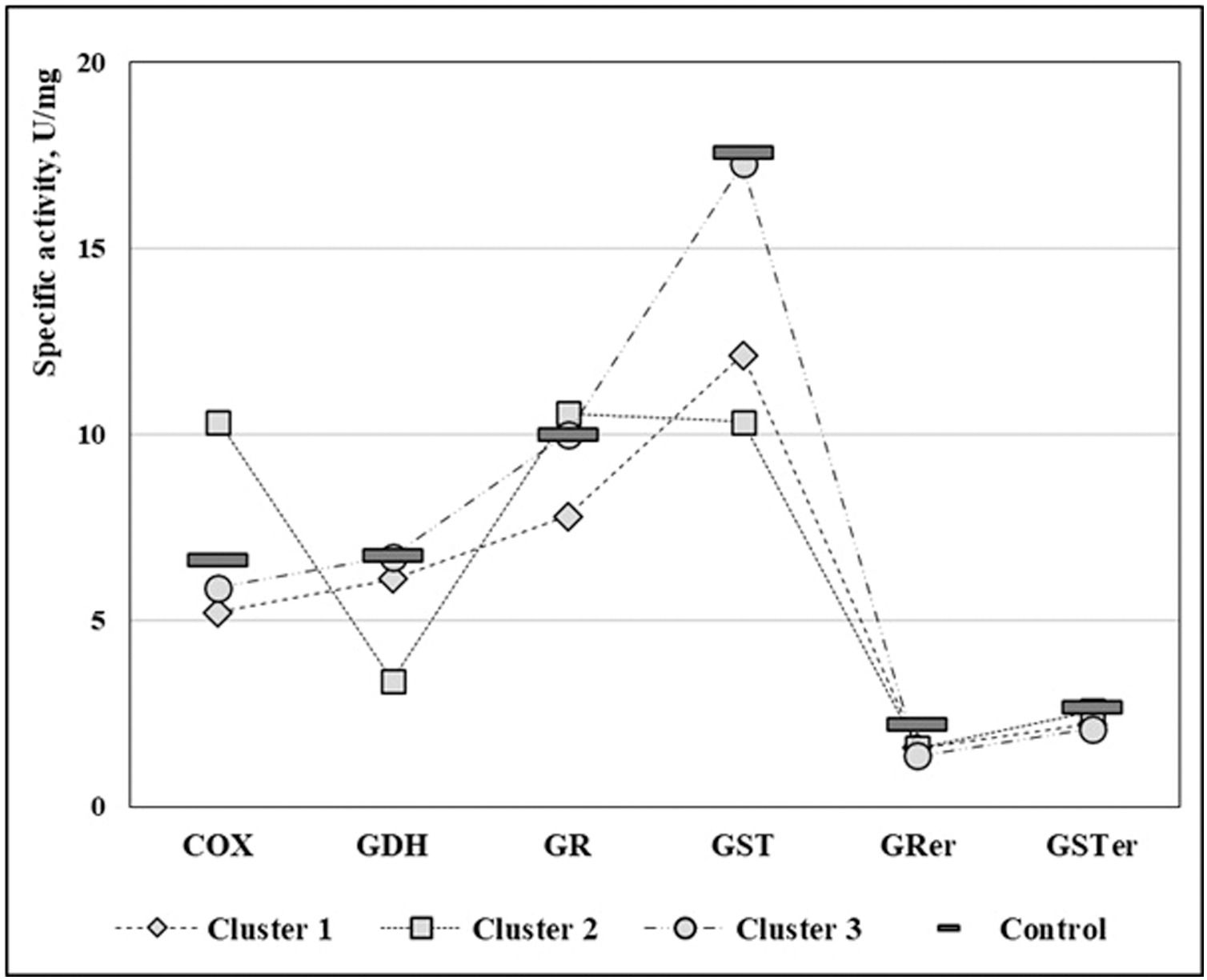
ResultsPatients with DE had a significant decrease in platelet GST and GRer compared to their control counterparts (p < 0.0008 and p < 0.0002, respectively; Table 1), while no significant differences in other enzymatic activities were found (Figs. 1). Since patients’ enzymatic activities were variable, cluster analysis was carried out to identify more homogeneous subgroups of patients. Clustering of the 53 patients by 6 biochemical measures by k-means method gave 3 clusters after two iterations (K1, K2, K3, Table 1 and Fig. 2). Enzymatic activities differed significantly from control values in the majority of patients (K1 and K2 comprising 66% of the surveyed group):
- •
K1 – significant decrease in COX, GR, GST, GRer (p < 0.001);
- •
K2 – COX was significantly increased, whereas GDH, GST and GRer were significantly reduced (p < 0.001, p < 0.02, respectively); and
- •
K3 – GRer was significantly reduced (p < 0.02) and lowest among the clusters.
K1 was composed mostly of women (21/24) and had a tendency toward later DE age at onset (≥60 years) (Table 2). Compared with K3, K1 was characterized by more frequent apathetic depression and cerebrovascular diseases (CVDs) accompanied by significantly more frequent manifestations of vascular parkinsonism (i.e. akinesia - rigidity syndrome and tremor). K1 significantly differed from K2 and K3 in the seasonality of depression development and had a higher frequency of recurrence. The DE seasonality in late autumn- wintertime was most likely associated with seasonal worsening of patients underlying CVDs.
Clinical and biochemical features of patients comprised in K1, K2, and K3 clusters.
| Parameters | K1 (N = 24; 3m/21f) | K2 (N = 11; 5m/6f) | K3 (N = 18; 4m/14f) | Significance | |
|---|---|---|---|---|---|
| Age at onset (years)Onset ≥60 years (numbers of patients) | 51 [40; 60.5] 7 | 32 [19; 59] 2 | 45 [35; 55] 2 | K1/K2: p = 0.052 | |
| HAMD-17 total score | 22.5 [21; 24] | 24.0 [23; 25] | 24.0 [22; 25] | K1/K2: p = 0.08 | |
| HARS-1 total score | 20.0 [15; 23] | 23.0 [21; 26] | 22.5 [19; 24] | K1/K2: p = 0.047 | |
| Diagnoses (numbers of patients) | |||||
| RDD | 17 | 6 | 12 | K1/K2/K3: χ²= 4.08, p = 0.395 | |
| BD | 4 | 4 | 6 | ||
| DE | 3 | 1 | 0 | ||
| Depression type 10 (numbers of patients) | |||||
| apathetic | 8 (33.3%) | 1 (9.1%) | 1 (5.6%) | K1/K3: χ²=6.15, p = 0.047 | |
| dreary | 2 (8.3%) | 1 (9.1%) | 5 (27.8%) | ||
| complex | 14 (58.4%) | 9 (81.8%) | 12 (66.7%) | ||
| Seasonality (numbers of patients) | 21 (87.5%) | 4 (36.4%) | 9 (50%) | K1/K2: χ²=9.66, p = 0.002K1/K3: χ²=7.09, p = 0.008 | |
| Cardiovascular disorders (numbers of patients) | 10 (41.7%) | 8 (72.8%) | 8 (44.4%) | K1/K2: χ²=2.91, p = 0.088 | |
| Cerebrovascular diseases of II-III degree (numbers of patients) | 17 (70.9%) | 6 (54.5%) | 3 (16.7%) | K1/K3: χ²=12.10, p = 0.0005K2/K3: χ²=4.58, p = 0.032 | |
| Chronical tremor (numbers of patients) | 9 (37.5%) | 4 (36.4%) | 1 (5.5%) | K1/K3: χ²=5.79, p = 0.016K2/K3: χ²=4.54, p = 0.033 | |
| Chronical akinesia – rigidity syndrome (numbers of patients) | 11 (45.8%) | 4 (36.4%) | 2 (11.1%) | K1/K3: χ²=7.43, p = 0.006 | |
| Platelet COX activity (U/mg) | 5.5 [4.3; 6.1] | 9.7 [7.4; 12.7] | 5.2 [4.4; 7.0] | K1/Ctr p < 0.001 (Decreased)K2/Ctr p < 0.001 (Increased) | |
| Platelet GDH activity (U/mg) | 6.4 [5.0; 7.5] | 3.4 [2.8; 4.1] | 6.9 [5.8; 7.5] | K2/Ctr p < 0.02 (Decreased) | |
| Platelet GR activity (U/mg) | 7.9 [6.4; 9.0] | 10.9 [9.1; 11.6] | 10.2 [8.6; 11.1] | K1/Ctr p < 0.001 (Decreased) | |
| Platelet GST activity (U/mg) | 11.8 [10.8; 13.7] | 11.4 [6.2; 13.3] | 17.4 [16.1; 18.2] | K1/Ctr p < 0.001 (Decreased)K2/Ctr p < 0.02 (Decreased) | |
| Erythrocyte GRer activity (U/mg) | 1.8 [1.2; 2.0] | 1.5 [1.0; 2.1] | 1.3 [1.0; 1.6] | K1/Ctr p < 0.001 (Decreased)K2/Ctr p < 0.02 (Decreased)K3/Ctr p < 0.02 (Decreased) | |
| Erythrocyte GSTer activity (U/mg) | 2.0 [1.7; 2.9] | 2.6 [2.2; 3.3] | 1.8 [1.3; 2.8] | ||
Data are presented as Median and 25% and 75% Quartiles, M [Q25; Q75]. Percentages (%) are calculated based on the total size of every group (N).
Only significant differences are given in the table (p < 0.05). Comparisons were done with the Mann-Whitney U-test for age of onset, HARS scores, enzymatic activities. Other parameters were compared by Chi-square test (χ²).
In K2, DEs were of an early manifestation and tended to a protracted course without obvious seasonality of their development. K2 patients had often pronounced signs of CVDs with some symptoms of parkinsonism and cardiac pathology. K2 differed from K1 in respect to greater severity of anxiety symptoms (as measured via total HARS score) with a predominance of complex anxious, hypochondriacal cenesthopathy and delusional depression. The frequency of severe CVDs and chronic tremor was similar to that of K1 but significantly exceeded that in K3 (Table 2).
Patients in the K3 cluster had enzymatic activities similar to control subjects, except for the GPer measures. Although they had a similar age of DE onset and severity of anxiety-like K1 and K2 clusters, their clinical phenotype significantly differed. Thus, there was the absence of pronounced CVDs in a majority of K3 patients with a significantly lower frequency of parkinsonism (chronical tremor and akinesia – rigidity syndrome). Whereas apathetic depression in K3 was the least common, with statistically significant differences from K1, it was the classic dreary and dreary-inhibited depression, typical of middle-aged patients, was more often noted, albeit not significantly different from K1 and K2 clusters. Most interestingly, the ‘seasonality of phases’ (which is typical for Moscow latitude and is associated with fluctuations in atmospheric pressure and climatic changes) in K3 was found in half of the patients, and the portion was significantly lower in comparison with K1.
Discussion and conclusionIn this study, we demonstrate that depression in older adults can be classified into three clusters based on significant changes in their blood enzymatic activities relatively matched healthy controls: decrease in most enzymatic measures (K1); decrease in GDH, GST, and GRer, but increase in COX activity (K2) and decrease in GRer alone (K3). These three clusters are not influenced by age at first DE onset, cumulative duration, or types of depression, but it is cerebrovascular and cardiovascular diseases and physical symptoms associated with them, i.e. parkinsonism in the form of rigidity/akathisia and tremor, that discriminates the clusters. The seasonal relapse featured in all three clusters though was highly expressed in K1 and K3. Since this feature appears to be typical for Central regions of the European part of Russia and is associated with fluctuations in atmospheric pressure and climatic changes, this will require further validation in other samples from other latitudes to determine the validity of this measure in the general population. From clinical measures, it was only anxiety, as measured with HARS, that was significantly elevated in K2 relatively to K1.
Our study extends the current understanding of DE neurobiology. Although previous studies have largely assigned depressive symptoms to the three monoamine neurotransmitters (dopamine, norepinephrine, or serotonin), we describe a specific peripheral glutamatergic dysfunction (decreased GDH activity in K2 relatively control) that accompanies distinct clinical DE phenotypes with a predominance of parkinsonian neurological symptoms. Namely, it was the extrapyramidal symptoms that were prominent physical symptoms seen in the K2 cluster. Lesions of dopamine nigral neurons not only result in depression-like behavior (in the substantia nigra pars compacta) but also lead to a cascade of functional modifications on the activity of basal ganglia circuits, with the dysregulation of the glutamate system.12,13 This pathophysiological mechanism is also shared with Parkinson's disease, with the destabilization of the brain reward mechanism being linked to an increased risk of DE relapse.14 Note, the higher frequency of cardiovascular disorders than in other patients is also associated with the decrease in the activity of GDH, the glutamate-metabolizing enzyme.
We note also that the decrease in the activity of the glutathione-related enzymatic antioxidant system (GST, GR) may play a role in the pathophysiology of seasonal DE, as demonstrated by high seasonal DE in all 3 clusters, especially K1. It is interesting to speculate that the increase in COX activity may underlie anxiety, as demonstrated in K2. Bipolar disorder is characterised with elevated peripheral blood expression of electron transport chain encoding genes, including COX (complex IV). However, we have to bear in mind that all three clusters had mild to moderate severity of anxiety, and, thus, further studies are required to determine this clinical relevance.15
Before arriving at the MHRC hospital all the elderly in-patients studied in our research were treated with different medicines, accordingly to with their mental state. MHRC psychiatrists recorded the data on the preceding treatments from the interview with the patients and their relatives before starting the in-patient treatment course (at the point of blood taken for biochemical analyses). The prior pharmacotherapy might have an influence on the levels of the measured individual baseline enzymatic activities and, hence, the patient clustering, but presently we can only note this fact to analyze larger groups in the future.
In the current study, we identified three DE clusters with distinct phenotypes that can potentially be used to identify the course and treatment targets of DE (dopamine and glutamate neurotransmitter system targeted drugs for K1 and K2, and glutamatergic drugs for K2, and antioxidants, especially for K1 and K2). The prevention and/or early treatment of accompanying physical comorbidities may, per sé, may also modify the DE course. Our findings, therefore, could aid person-centered diagnosis and outcomes, and timely successful treatment(s) of depression in older adults.
Ethical considerationsThe study was done with the approval of the local Ethics Committee of the FSBI “MHRC” (Protocol No. 8 of 02.26.2019, the Protocol complies with contemporary ethical standards and rules for biomedical research approved by the Helsinki Agreement (amended in 1975/2000)).
Funding informationThe work was carried out within the framework of the planned topics of the MHRC and was not supported by grants.
Data availability statementThe anonymized database is available on demand.
The authors are deeply indebted to Professor Elizabeta Mukaetova-Ladinska, University of Leicester, UK, for her invaluable help in discussion of results, writing and editing the manuscript. The authors thank all participants and their families.