This study employed the Delphi method to identify the ideal characteristics of a clozapine adverse drug reaction (ADR) scale, engaging 34 clozapine specialists at the 6th NEMEA meeting. Participants emphasized the integration of clinician-rated (CROM) and patient-rated (PROM) outcomes in the scale, aiming for a balance between comprehensiveness and practicality. Key consensus points included the inclusion of specific ADRs like somnolence, sialorrhea, and compulsivity, while metabolic complications were considered less relevant. This research highlights the need for a practical, patient-inclusive tool for monitoring clozapine ADRs, reflective of both clinical expertise and patient experiences in managing treatment-resistant schizophrenia.
Clozapine is the sole medication licensed for treatment-resistant schizophrenia (TRS), representing the disorder's most severe form. Between 20 % and 33 % of people with psychotic disorders are resistant to antipsychotic treatment,1 and so it is estimated that about a fifth of patients should be treated with clozapine.2 It is associated with various adverse drug reactions (ADRs), impacting quality of life and contributing to preventable deaths. Developing an effective monitoring tool for clozapine ADRs is essential to mitigate both fatal and non-fatal side effects.
Introduced in Europe in the early 1970s, clozapine was withdrawn following reports of agranulocytosis after its launch in Finland in 1975.3 It remained available off-label in the U.S., gaining recognition for its effectiveness in TRS, evidenced by a 1988 landmark study. Its re-introduction involved restricted indications and enhanced blood monitoring, substantially reducing mortality due to clozapine-related agranulocytosis. Unfortunately, it remains widely underused.
Clozapine, like other antipsychotics, is linked to ADRs that can affect medication adherence and lead to potentially fatal outcomes.4 These ADRs vary: acute vs. chronic, dose-dependent vs. idiosyncratic, and serious vs. non-serious. Systematic collection of ADRs in clinical settings is crucial to improve clozapine safety, as an expert report suggested.
Several scales exist for assessing antipsychotic-related ADRs, from specific symptoms to broader evaluations, like the Liverpool University Neuroleptic Side Effect Rating Scale (LUNSERS).5 However, critiques of LUNSERS' length (51 questions) and complexity led to alternative scales like the Glasgow Antipsychotic Side Effects Scale (GASS),6 which also failed to cover specific clozapine ADRs, resulting in the development of a clozapine-specific scale.
The GASS-Clozapine (GASS-C),6 a 16-item self-rated scale, was developed in the UK in 2015 and later validated in the Netherlands, Serbia, and Japan. It includes sections on smoking and caffeine use as potential pharmacokinetic modifiers but omits factors like compulsivity, prevalent among clozapine-treated patients. GASS-C needs to be validated in Spanish, limiting its use in Spanish-speaking countries. However, considering the needs of clinicians was necessary before embarking on its validation, as well as whether patient-reported outcome measures (PROM) would be useful too.
This project explores clinicians' requirements for a clozapine ADR scale, assessing GASS-C's alignment with these needs. A Delphi method involving 34 clozapine specialists was employed to identify the ideal scale characteristics.
Material and methodParticipants and role of fundingThe study involved 34 clozapine specialists who attended the 6th NEMEA meeting in Madrid on June 16–17, 2023. This annual gathering, sponsored by the clozapine manufacturer ADAMED, is a pivotal event for experts in clozapine treatment in Spain. It consistently attracts a stable group of active clinicians and researchers in the field of schizophrenia and clozapine therapy.
ADAMED provided funding for the meeting logistics, including accommodation and travel. However, the company was not involved in the conceptualization, design, or execution of this study, nor in analysing its results or preparing manuscripts. This was to ensure the integrity and impartiality of the research.
Delphi consensus and real-time feedbackThe Delphi method7 was employed, a systematic, interactive forecasting method relying on a panel of experts. Originating from the RAND Corporation in the 1950s, this methodology aims to achieve a consensus through a series of rounds involving questionnaires. Its key features include participant anonymity, iterative feedback, and the statistical aggregation of group responses. The anonymity aspect is crucial as it mitigates the influence of dominant individuals and promotes honest and uninhibited feedback from all participants.
For our study, we combined traditional Delphi methods with modern technology. Specifically, we utilized VEVOX (www.vevox.app), a real-time audience engagement tool, to enhance interaction and participation. VEVOX is designed for use in meetings, conferences, and educational settings, allowing for live polling, Q&A sessions, and surveys. Participants could engage through their smartphones or other devices, contributing to the discussion in real time. This integration of technology-facilitated a dynamic and interactive consensus process, with the added benefit of immediate compilation and analysis of responses.
ProcedureA team expert (EFE) moderated the Delphi process and introduced each question to the participants. The consensus threshold was set at 75 %; if this was achieved in the initial round of voting, the decision was considered final. If the threshold was not met, a discussion period of 10 min was allowed, fostering debate and reconsideration of views. This was followed by a second round of voting, applying the same 75 % threshold for consensus. In cases where two consecutive rounds failed to reach this threshold, the question was tabled for future discussion and consideration.
The questionnaire was methodically divided into three distinct sections: (1) sociodemographic data of the participants, (2) general aspects and design considerations for a clozapine ADR scale, and (3) specific content and items that should be included in the scale. This structured approach ensured a comprehensive assessment of the participants' perspectives, covering both broad conceptual issues and detailed content-specific matters.
The questions were selected by the two authors (EFE, MB) from various sources including the other scales and clinical experience. A provision for extra questions arising from the discussions or the Delphi participants was also allocated.
This methodical and thorough process aimed to encapsulate the collective expertise and opinions of the participating clozapine specialists, leading to a well-rounded and consensus-driven outcome that would be beneficial in practical clinical settings.
ResultsThe final number of participants was 34, including the two moderators (EFE, MB).
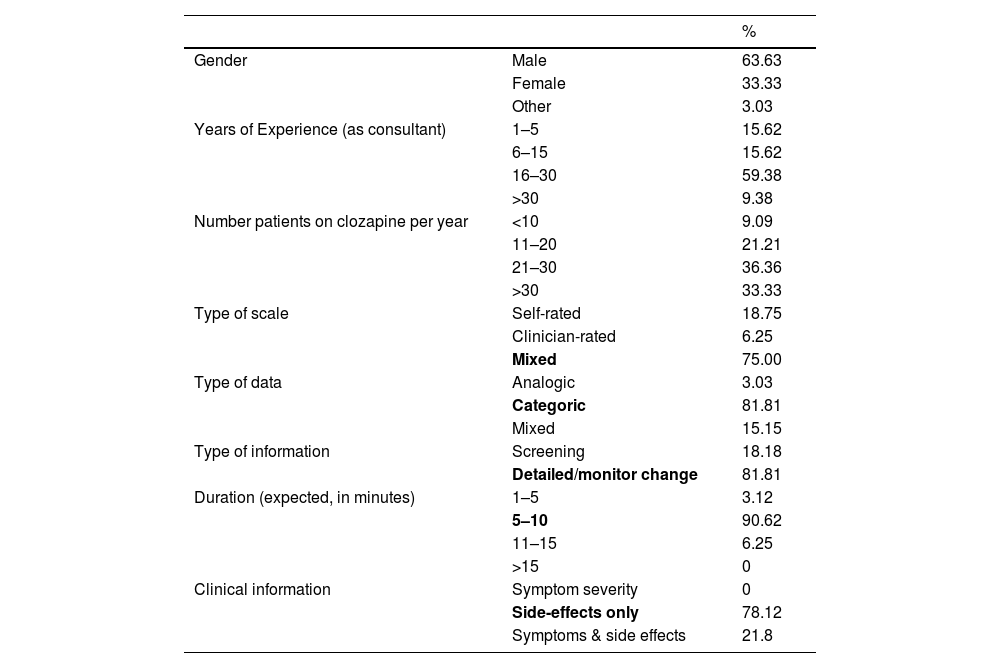
Table 1 shows the details, which included primarily males (63.63 %), with more than 16 years of experience (68.74 %), treating 20 or more patients on clozapine per year (69.69 %) from almost all autonomous communities in Spain, with the majority from Catalunya (24.24 %) and Andalusia (15.15 %). There were no representatives from Aragon, La Rioja or Melilla.
Dephi results of the main questions to the 34 participants.
| % | ||
|---|---|---|
| Gender | Male | 63.63 |
| Female | 33.33 | |
| Other | 3.03 | |
| Years of Experience (as consultant) | 1–5 | 15.62 |
| 6–15 | 15.62 | |
| 16–30 | 59.38 | |
| >30 | 9.38 | |
| Number patients on clozapine per year | <10 | 9.09 |
| 11–20 | 21.21 | |
| 21–30 | 36.36 | |
| >30 | 33.33 | |
| Type of scale | Self-rated | 18.75 |
| Clinician-rated | 6.25 | |
| Mixed | 75.00 | |
| Type of data | Analogic | 3.03 |
| Categoric | 81.81 | |
| Mixed | 15.15 | |
| Type of information | Screening | 18.18 |
| Detailed/monitor change | 81.81 | |
| Duration (expected, in minutes) | 1–5 | 3.12 |
| 5–10 | 90.62 | |
| 11–15 | 6.25 | |
| >15 | 0 | |
| Clinical information | Symptom severity | 0 |
| Side-effects only | 78.12 | |
| Symptoms & side effects | 21.8 |
Table 1 also details the scale's general aspects. The preferred scale should combine clinical and patient views (75 %), categorical over an analogic or mix (two rounds, 81.81 %), should take between 5 and 10 min to complete (90.62 %), not a screening tool but detailed enough to monitor change (81.81 %) and focused on side effects without including symptom severity (81.81 %). The scale should also include the date and results of the last available plasma levels (96.55 %). All agreed in the first round.
With regards to the type of side effects that the scale should include, the participants agreed on somnolence/sedation (90.62 %), sialorrhea (100 %), constipation (90.32 %), compulsivity (100 %), sexual dysfunction (90.62 %), increased appetite (86.66 %, combined) and tachycardia (100 %).
Side effects that did not reach 80 % included dry mouth (76 %), miccional urgency (25.8 %), abnormal movements (17.24 %), nausea (34.37 %), and reflux (13.79 %). Metabolic complications received low support: diabetes (12.5 %) and dyslipidaemia (0 %) as they were part of the general medical history but not part of a scale.
Due to technical failures, enuresis, blurred vision, hypertension, and weight, results were not recorded.
Participants also offered views regarding the need for scales that combine information from clinicians (clinician-rated outcome measures or CROM) and patients (Patients-rated outcome measures or PROM), as sometimes insight or cognitive difficulties might reduce the reliability of the answer.
DiscussionThis is the first-time clozapine experts have used the Delphi methodology to define the characteristics of the ideal scale used in clinical practice. Compared to the newest gold standard, the GASS-C, the participants stressed the need to combine information from clinicians and service users.
Clinicians prioritised a dual-input scale combining CROM and PROM outcomes for practical implementation, acknowledging the influence of cognitive or insight issues on response reliability. Interestingly, there was minimal support (6 %) for a clinician-only scale, highlighting the growing emphasis on patient involvement in care decisions. This novel approach of merging CROM and PROM for antipsychotic side effects could improve symptom evaluation, serve as an effective monitoring tool, and facilitate side effect discussions during consultations.
The other essential part was a relatively quick-to-fill scale (5–10 min), so it can be used during regular clinical practice but detailed enough to monitor change (hence, not dichotomic) using categories rather than a severity score.
Regarding content, most side effects in the GASS-C were also agreed on in the ideal scale. These included somnolence and sedation, constipation, sialorrhea, sexual dysfunction, increased appetite, and tachycardia. As GASS-C, the ideal scale also excluded diagnoses associated with clozapine use, such as diabetes or hyperlipidaemia, in favour of a change in appetite or weight gain.
Interestingly, the clinicians disagreed on digestive symptoms, such as nausea, reflux, or dry mouth, reported in the GASS-C. There was an overwhelming request to include compulsivity as part of the assessment, which is not included in any other scale for antipsychotic side effects.
This study has some limitations, including the relatively reduced sample of clinicians and the inherent reduced options that a Delphi consensus method allows. We offered ways to reduce this limitation, including extra symptoms, but none were suggested. The sample size is small but representative of direct-care specialists. A strength is that it is the first time that direct-care clinicians with clozapine expertise can participate in developing a scale that could match their needs.
To sum up, clinicians are interested in categorical scales that help to identify and monitor most clozapine side effects, but that scale should have a limited duration (ten minutes max) to be effectively implemented in the clinical practice and contain both CROM and PROM. Academics and researchers should know the clinician's needs when developing novel scales.
CLUB NEMEA members (6th Meeting, Madrid 17 June 2023)Jose Angel Alcala Partera, Noelia Aller Gomez, Maria Pilar Andres Olivera, Antonio Arevalo Sanchez, Francisco Baron, Fernando Contreras Fernandez, Alberto Fuentes Garrido, Mateo Gallego Restrepo, Juan Antonio Garcia Mellado, Jose Manuel Gonzalez Moreno, Agopito Herrero, Olga Ibarra Uria, Milagro Lacruz Silvestres, Joan Latorre Soto, Marta Leonor del Pozo, Maria Luisa Lopez Lucas, Jose Lopez Moriñigo, Francisco Javier Manzanera Lopez, Pablo Moreno Flores, Leticia Muñoz Machado, Beatriz Oda, Albert Pendas, Marc Peraire, Eva Amanda Povedano Suarez, Josep Maria Ribes, Rodrigo Romero Jimenez, Luisa San Emeterio, Francisco Javier Sanz Fuentenebro, Juan Tortajada, Maria Isabel Vazquez Souza, Miguel Angel Vila Heredero, Jose Maria Villagran.
Ethical considerationsThis work did not involve human experimentation and was conducted as an anonymised survey. The meeting was funded by a pharmaceutical company that provided logistical support but had no input in the interpretation or communication of results.
FundingAdamed Laboratories have funded this project for the meeting organisation.