Most studies concerning the relationship between depression and chronic physical disease focused on a single physical disease and did not consider multimorbidity or depression severity. We aimed to characterize this relationship considering chronic physical diseases’ type and number, and depression severity.
MethodsWe undertook a cross-sectional study, using data from a phone household panel, with “chronic physical disease” as the exposure and “depression” as the outcome. The sample is representative of the Portuguese population. Adjusted logistic and multinomial regression analyses were conducted between depression presence/severity and chronic physical disease presence/type/number. Odds ratio and 95% confidence intervals adjusted for possible confounders were calculated.
Results1027 individuals were included. Of the population, 8.9% had depression and 72.1% had at least one chronic physical disease. There was no statistically significant relationship between depression and physical disease in general (OR=1.68 [CI95%:0.55, 5.15]), but there was with allergy (OR=2.08 [CI95%:1.02, 4.25]) and COPD (OR=3.04 [CI95%:1.21, 7.61]). The risk of depression was smaller with two physical diseases (vs. three or more, OR=0.32 [CI95%:0.15, 0.68]).
ConclusionsA relationship between COPD, allergy and a higher number of physical diseases and depression was observed. Clinicians should be aware of these relationships. Evaluating the presence of depression in people with multimorbidity, COPD and allergy is recommended.
Depression is a common disorder and a major public health problem.1 In Europe, depression prevalence is estimated at 4.5%.2 In particular, in Portugal, the prevalence of depression is higher, estimated at 7.8%.3 Depression is usually recurrent and has numerous personal, social and economic consequences.4-6 Depression is a major contributor to the global burden of disease and a risk factor for premature mortality.5,7
Multimorbidity is usually defined as the co-occurrence of two or more chronic diseases in the same individual.8 It is common and it has been rising worldwide, with an estimated prevalence of 37.3% in Europe and 38.3% in Portugal.9-11
Previous studies have suggested high comorbidity between chronic physical disease and depression.12-16 People with chronic physical diseases seem to have a two to three times higher prevalence of depression in comparison to those without any chronic physical disease.12 The chronic physical diseases that most commonly have been related to depression include chronic pain, stroke, hypertension, cardiovascular disease, diabetes, cancer, arthritis, asthma, chronic obstructive pulmonary disease (COPD), neurological diseases and osteoporosis.12,14,16-19
Depression seems to be related to worse chronic physical disease outcomes, increasing the risk of treatment non-compliance, symptom burden, disability, work absenteeism and mortality.12,20 The presence of this comorbidity also seems to lead to a worse depression prognosis, with an increased tendency to relapse and re-occurrence, chronicity, lower quality of life and worse treatment responses.21 Some studies have also suggested an increase in healthcare utilization and longer hospital stays, although evidence is still scarce.21,22
Some authors have argued that there is a dose-dependent relationship between physical disease and depression, with a higher number of physical diseases associated with a higher prevalence of depression.20,23 In addition, it has been suggested that a higher chronic physical disease severity is also associated with a higher prevalence of depression.20 Furthermore, some authors suggest a relationship between certain types of physical disease and the number of physical diseases with depression severity.23-25
Nonetheless, most studies have disregarded the presence of multimorbidity of chronic physical diseases in patients with depression. The majority of studies have focused on one chronic physical disease and its relationship with depression, with only a few studying this relationship for an array of diseases in the same population. In addition, few have considered the number of physical diseases present in one individual and their impact on depression. Furthermore, only a few studies have considered the impact of this relationship on depression severity. In particular, there are few studies on the relationship between chronic physical disease and depression in Portugal. Amongst the few observational studies conducted in the country, most refer to one single physical disease, included small-sized samples and used hospital data.26-28
Given the high prevalence of depression in the Portuguese population, the growing prevalence of multimorbidity and the lack of knowledge regarding the relationship between multimorbidity and depression, the authors intended to explore the relationship between depression (including its severity) and chronic physical disease in general and according to specific characteristics such as the chronic disease type and chronic diseases’ number.
MethodsStudy design and selection of participantsWe conducted a cross-sectional study using data from Em Casa Observamos Saúde (ECOS). This is a household panel developed by the Portuguese National Institute of Health Doctor Ricardo Jorge to collect data on the health status of the Portuguese population, using a sample of families with mobile and landline phones.29 This panel results from a probabilistic sampling of household units stratified by region (NUTS II, 2002), with homogeneous allocation. The current panel was recruited in 2018 and included 1549 households (1284 were still active in 2020). In each unit, one participant, 18 years or older, provides information on the self. Each panel is maintained for three years. Data is collected through structured questionnaires phone/web administered. Sociodemographic data is collected upon the panel recruitment and further information is collected annually. In August 2019, information on chronic physical diseases was collected. In 2020, the project ECOS COVID-19 aimed to evaluate the impact of the COVID-19 pandemic on the Portuguese population and additional data was collected between April-May 2020, upon the first ‘State of Calamity’ in Portugal. This study combines data from ECOS 2020 on depression with data on chronic physical diseases collected in 2019. The final sample size consisted of 1027 participants.
Identification and measurement of variablesIn this study, “chronic physical disease” was considered the exposure and “depression” was the outcome. Data on depression was collected in two different sections: the mental health section and the chronic diseases section. In the mental health section, depression was identified and measured using the Patient Health Questionnaire-8 (PHQ-8).30 The PHQ-8 is an instrument that encompasses eight questions based on the DSM-IV and assesses the presence and severity of depression over the last two weeks.30 For each question a score based on the frequency of symptoms is applied, ranging from 0 (never) to 3 (almost every day); the total sum for each question is the final PHQ-8 score.30 A final score of ≥10 has a good sensibility and specificity for major depression or clinically significant depression.30 Depression severity is characterised as moderate (10–14 points); moderately severe (15–19 points); or, severe (20–24 points).30 Participants who failed to answer at least one of the PHQ-8 questions were excluded from the analysis. Due to the small number of participants in the depression severity categories, data on moderately severe and severe depression were aggregated. In the chronic disease section, depression was identified using a dichotomous question – “Over the last twelve months have you suffered from depression?”, which was used for the sensitivity analysis.
The occurrence of chronic physical disease was identified using dichotomous questions on the presence, over the last twelve months, of asthma, allergies, COPD, diabetes, obesity, hypertension, ischemic heart disease, stroke, cancer, osteoarticular disease, neurodegenerative disease, kidney disease, liver disease, back pain and immune disorders. Chronic physical disease was defined according to the presence of any physical disease, its type and to the total number of diseases in a given participant (0/ 1/ 2/ ≥3). Participants who failed to answer at least one of the dichotomous questions were excluded from the analysis.
Depression/chronic physical disease comorbidity was considered present if a participant had depression and at least one of the aforementioned chronic physical diseases. Due to the chronic nature of these conditions, it was assumed that the individuals had the condition at the time of the second data collection moment (2020).
Sociodemographic characteristics were also considered: gender (male/female); age group (15 to 44/ 45–64/ ≥65); education (0–3/ 4–9/ 10–12/ >12 years); employment status (employed/ domestic/ retired/ unemployed/ student); number of household members (1/ 2/ ≥3); and, region (North/ Center/ Lisbon Metropolitan Area/ Alentejo/ Algarve/ Azores and Madeira). Due to the small number of students, for analysis purposes, data on students and domestics were aggregated into one category.
To minimize bias, interviewers received training on the survey questionnaire. Data validation was performed through the identification of conflicting or non-possible values. When inconsistent values were identified, interviewers re-contacted participants to validate data. In addition, data on chronic physical disease was collected before data on depression as identified by the PHQ-8.
Statistical analysisSociodemographic characteristics were described using absolute and relative frequencies, both at the sample and population level (correcting for the sample design). These were also considered potential confounders. Prevalence and corresponding 95% confidence intervals (95%CI) of the presence/ severity of depression and the presence/ type/ number of chronic diseases were calculated. A Chi-square test with Rao-Scott adjustment was performed between each sociodemographic variable and depression and chronic physical disease. Those with statistically significant associations with both dependent and independent variables were deemed confounders. The relationship between chronic physical disease and depression was studied through: i) logistic regression between the presence/ type/ number of chronic diseases and the presence of depression; and, ii) multinomial regression between the presence/ type/ number of chronic disease and severity of depression. We conducted a sensitivity analysis using depression as measured in the chronic diseases section; this included all the above analyses, except for the type of physical disease. Crude (OR) and adjusted odds ratios (aOR) and correspondent 95%CI were calculated for each analysis. All analyses were weighted to account for the complex sample design. R software (version 3.6.2) was used for statistical analysis, namely survey and svrepmisc packages.31
ResultsDescriptive analysisThe total sample size included 1027 participants (Table 1). Most participants were women (61.5%), 45–65 years old (41.0%), had 4 to 9 complete years of school (52.0%), were employed (49.8%), belonged to households with 3 or more members (47.0%) and were from the North of Portugal (16.6%).
Sample and population description.
N.A. – Not available; A.R. – Autonomous Region.
Depression according to the PHQ-8 was identified in 130 participants, corresponding to 8.9% (CI95%: 6.6, 12.0) of the population. Of the population, 5.8% (CI95%: 4.0, 8.0) had moderate depression and 3.2% (CI95%: 1.8, 5.0) had moderately severe to severe depression. Of the participants, 790 had at least one chronic physical disease, corresponding to 72.1% (CI95%: 67.0, 77.0) of the population. Of the population, 26.2% (CI95%: 22.3, 30.0) had a total of 3 or more diseases, 26.2% (CI95%: 21.4, 31.0) one disease, and 19.7% (CI95%: 15.8, 24.0) had two diseases. The most common physical disorders were back pain (43.4%; CI95%: 38.5, 48.0), hypertension (25.9%; CI95%: 22.1, 30.0), osteoarticular disease (22.1%; CI95%: 18.6, 26.0), allergy (23.2%; CI95%: 18.7, 28.0), obesity (11.0%; CI95%: 8.3, 14.0), diabetes (10.2%; CI95%: 8.0, 13.0) and ischemic heart disease (8.4%; CI95%: 6.2, 11.0).
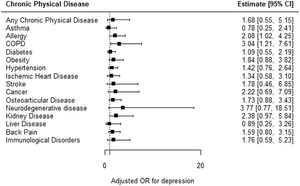
Relationship between chronic physical disease and depressionDepression was more frequent among those with any chronic physical disease but this was not statistically significant (aOR: 1.68; CI95%: 0.55, 5.15) (Fig. 1, Table 2). Regarding the type of chronic physical disease, depression was more frequent among all types except liver disease and asthma, but the results were not significant. The exceptions were allergy (aOR: 2.08; CI95%: 1.02, 4.25) and COPD (aOR: 3.04; CI95%: 1.21, 7.61) (Fig. 1, Table 2).
Relationship between chronic physical disease and depression.
| OR [IC95%] | p-value | Adjusted OR [CI95%] | Adjusted p-value* | |
|---|---|---|---|---|
| Chronic physical disease in general (n = 990) | 2.5 [0.83,7.51] | 0.102 | 1.68 [0.55,5.15] | 0.366 |
| Type of chronic physical disease | ||||
| Asthma (n = 1024) | 0.78 [0.25,2.41] | 0.662 | 0.78 [0.25,2.41] | 0.662 |
| Allergy (n = 1015) | 2.08 [1.02,4.25] | 0.044 | 2.08 [1.02,4.25] | 0.044* |
| COPD (n = 1022) | 1.93 [0.66,5.66] | 0.232 | 3.04 [1.21,7.61] | 0.018* |
| Diabetes (n = 1024) | 1.70 [0.90,3.20] | 0.102 | 1.09 [0.55,2.19] | 0.800 |
| Obesity (n = 1023) | 2.06 [1.02,4.14] | 0.044 | 1.84 [0.88,3.82] | 0.104 |
| Hypertension (n = 1022) | 2.20 [1.20,4.02] | 0.011 | 1.42 [0.76,2.64] | 0.272 |
| Ischemic heart disease (n = 1015) | 2.14 [1.02,4.48] | 0.045 | 1.34 [0.58,3.10] | 0.487 |
| Stroke (n = 1024) | 1.78 [0.46,6.85] | 0.401 | 1.78 [0.46,6.85] | 0.401 |
| Cancer (n = 1022) | 2.36 [0.82,6.77] | 0.110 | 2.22 [0.69,7.09] | 0.180 |
| Osteoarticular disease (n = 1016) | 3.15 [1.71,5.80] | 0.000 | 1.73 [0.88,3.43] | 0.114 |
| Neurodegenerative disease (n = 1023) | 3.77 [0.77,18.51] | 0.102 | 3.77 [0.77,18.51] | 0.102 |
| Kidney disease (n = 1021) | 2.38 [0.97,5.84] | 0.059 | 2.38 [0.97,5.84] | 0.059 |
| Liver disease (n = 1022) | 0.89 [0.25,3.26] | 0.865 | 0.89 [0.25,3.26] | 0.865 |
| Back pain (n = 1023) | 2.27 [1.12,4.58] | 0.023 | 1.59 [0.80,3.15] | 0.187 |
| Immunological disorders (n = 1022) | 2.48 [0.79,7.78] | 0.120 | 1.76 [0.59,5.23] | 0.310 |
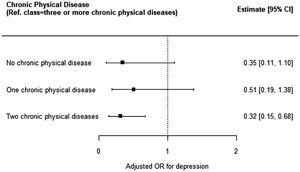
| Number of Chronic physical diseases (n = 990) | ||||
| None | 0.22 [0.07,0 0.67] | 0.008 | 0.35 [0.11,1.10] | 0.074 |
| One | 0.37 [0.14,0 0.96] | 0.041 | 0.51 [0.19,1.38] | 0.184 |
| Two | 0.27 [0.13,0.56] | 0.001 | 0.32 [0.15,0.68] | 0.003* |
Statistically significant values. Chronic physical disease, obesity, hypertension, ischemic heart disease, cancer, osteoarticular disease, back pain and depression – adjusted for sex and employment status; COPD and depression – adjusted for sex; diabetes, immunological disorders and depression – adjusted for employment status. Referent class for the relationship between number of diseases and depression: three or more physical diseases. CI – Confidence Interval, OR – Odds Ratio.
Depression was less frequent in those with two physical diseases in comparison with those with three or more diseases (aOR: 0.32; CI95%: 0.15, 0.68), which was statistically significant, but a dose-dependent relationship was not observed (Fig. 2, Table 2).
There was no statistically significant relationship between chronic physical disease in general and moderate depression (aOR: 8.01; CI95%: 0.48, 134.37) or moderately severe to severe depression (aOR: 0.63: CI95%: 0.03, 15.35). Allergy and DPCO had a statistically significant relationship with moderate depression in comparison with no depression (aOR: 3.31; CI95%: 1.38, 7.97; and aOR: 4.66; CI95%: 1.46, 14.87, respectively) but not with moderately severe to severe depression (aOR: 0.73; CI95%: 0.19, 2.85; and aOR: 0.53; CI95%: 0.0, 42,300.0, respectively). Both moderate and moderately severe to severe depression decreased when the number of chronic physical diseases decreased from three or more to two diseases, but this was only statistically significant for moderate depression (aOR: 0.26; CI95%: 0.09, 0.77) (Table 3). There was no dose-dependent relationship observed between chronic physical disease and depression severity (Table 3).
Relationship between the number of chronic physical diseases and depression severity.
| Depression severity | Number of physical diseases (n = 990) | OR [CI95%] | p-value | Adjusted OR [CI95%] | Adjustedp-value* |
|---|---|---|---|---|---|
| Moderate | None | 0.06 [0.00,1.06] | 0.058 | 0.08 [0.00,1.55] | 0.099 |
| One | 0.56 [0.17,1.84] | 0.343 | 0.71 [0.17,2.96] | 0.639 | |
| Two | 0.22 [0.08,0.61] | 0.005 | 0.26 [0.09,0.77] | 0.017* | |
| Moderately severe to severe | None | 0.50 [0.01,18.29] | 0.705 | 0.74 [0.00,1777.00] | 0.939 |
| One | 0.02 [0.00,9.42] | 0.222 | 0.03 [0.00,10,180,720.00] | 0.731 | |
| Two | 0.35 [0.10,1.18] | 0.095 | 0.44 [0.13,1.53] | 0.199 |
The odds for depression (as assessed in the chronic diseases section) in those with any chronic physical disease was 2.59 (CI95%: 0.78, 8.62) times that of those without disease, but it was also not statistically significant (Table 4). Depression was also associated with a higher number of chronic physical diseases, but no dose-dependent relationship was observed (Table 4).
Sensitivity analysis.
| OR [CI95%] | p-value | Adjusted OR [CI95%] | Adjusted p-value* | |
|---|---|---|---|---|
| Chronic physical disease in general (n = 1024) | 3.40 [1.04;11.13] | 0.043 | 2.59 [0.78;8.62] | 0.120 |
| Number of chronic physical diseases (n = 1024) | ||||
| No disease | 0.14 [0.04;0.47] | 0.002 | 0.18 [0.05;0.59] | 0.005* |
| One disease | 0.13 [0.06;0.27] | <0.001 | 0.18 [0.07;0.43] | 0.000* |
| Two diseases | 0.40 [0.19;0.87] | 0.020 | 0.40 [0.18;0.88] | 0.022* |
Earlier studies suggest that the prevalence of depression is 2 to 3 times higher in people with any chronic physical disease.12,13 In this study, the odds of depression were 1.68 times higher in people with any chronic physical disease, but this was not statistically significant. Considering the specific type of chronic physical disease, the study has shown a statistically significant relationship between depression with both allergy and COPD. This contrasts with previous studies which have shown a relationship between depression and all the other considered chronic diseases in this study.12,13,32,33 The differences between this study and the previous ones may be explained by different sample sociodemographic characteristics, different types or severity of chronic physical disease, the prevalence of other medical comorbidities or different data origin or collection times. For example, some studies used samples from elderly or hospital databases, which may have overestimated the prevalence of chronic diseases.32,33 Other studies were conducted in low and middle-income countries, where the prevalence of chronic physical diseases may be different, such as the case of stroke, which has a higher prevalence in the aforementioned countries.13,34 Furthermore, in some cases, depression was identified using other instruments, such as the Composite International Diagnostic Interview (CIDI), which has only a moderate concordance with the PHQ-8 and which refers to the last 12 months, and, therefore, may lead to the detection of more cases of depression.13,35 The identification of more cases of chronic physical disease and/or depression may lead to higher estimates for the relationship between depression and chronic physical disease. The sensitivity analysis showed higher odds of depression in people with physical diseases when compared with the main analysis, but it was also not statistically significant. Albeit not statistically significant, the higher odds may be because depression identified in the chronic diseases section regards the last 12 months. Other reasons may also include low comparability between the two depression measures and different data collection periods.
In this study, the prevalence of depression was lower in those with less than three physical diseases in comparison with those with three or more diseases. This is consistent with previous studies which have shown a relationship between depression and a higher number of medical comorbidities.13,20,23 However, contrary to a few previous studies, a dose-dependent response was not observed, which may be explained by the fact that these studies analysed specific populations (such as nursing homes residents or primary care users) and used different methods for collecting data (such as face-to-face interviews and medical records).13,20,23 This may have overestimated the prevalence of chronic diseases and, thus, identified people with more comorbidities or with more severe physical diseases.13,20 Since chronic physical diseases in the general population are possibly less severe and depression is possibly more evident in those with more severe diseases and more functional limitations, using populations and collection methods more prone to find severe diseases or more comorbidities may lead to an evident dose-response relationship between depression and chronic physical disease. The sensitivity analysis has similarly shown an association between depression and a higher number of chronic physical diseases, which was also not dose-dependent.
Finally, no relationship between chronic physical disease in general and depression severity was observed, but there was a statistically significant relationship between COPD and allergy and moderate depression. Both moderate and moderately severe to severe depression prevalence decreased with the reduction from three or more to less than three physical diseases, but no dose-dependent relationship was observed. In general, pathologies associated with more severe depression are those that involve more immunological activation or more depression-related biological pathways.24,25 Assuming the physical diseases present in the general population are less severe may explain the predominant association with moderate depression and the lack of a dose-dependent response. However, this analysis lacked power as suggested by the width of the confidence intervals.
This study has been conducted in a nationally representative sample and reinforces the association between depression and chronic physical disease, namely between depression and COPD and allergy, whose prevalence was twice that of the general population. In addition, we considered the presence of multimorbidity and its relationship with depression in the general population, and observed a higher prevalence of depression with multimorbidity, especially in people with three or more chronic physical diseases. Clinicians should be aware of the relationship between chronic physical disease and depression and its potentially negative consequences for the involved pathologies, especially between depression and allergy and COPD. Evaluating the presence of depression in people with multimorbidity, COPD and allergy is thus recommended. In this study, the lack of a dose-dependent relationship between depression in general and the number of chronic physical diseases and the lack of a relationship between depression severity and chronic physical disease suggest that other factors may be involved in this association, which warrants further investigation.
Some limitations must be considered. Due to the low frequencies in several categories, especially regarding moderately severe to severe depression, some results showed low precision. Although questionnaires are useful in screening certain conditions, namely depression or chronic physical disease, a careful evaluation by a trained clinician is necessary to confirm the diagnosis, which was not the case in this study. For example, although the PHQ-8 includes the DSM-IV diagnostic criteria for depression, it does not allow for the evaluation of functioning or for differential diagnosis, which is fundamental to confirm the diagnosis of depression or, similarly, of depression severity. In addition, due to the self-report nature of the questionnaires used to identify depression and chronic physical disease, information bias cannot be excluded, such as selective recall and social desirability bias. Given that the PHQ-8 refers to the last two weeks, cases of depression occurring outside that timeframe may have not been identified. Similarly, cases of people with depression being treated effectively may not have been identified. Data used in this study was originally collected for different purposes other than studying the relationship between depression and chronic physical disease. As a consequence, it was not possible to collect important additional information known to be related to depression, such as health behaviours, genetic factors, or adverse life events. In addition, even though depression was measured after chronic physical illness, it is not possible to exclude that depression was present at the same time or even before the measurement of physical disease. Therefore, the direction of the relationship between depression and chronic disease cannot be determined, and reverse causality cannot be excluded. For example, it is known that COPD may lead to the development of depression owing to the limitations and changes it causes. However, on the other hand, depression may lead to adverse health behaviours, such as smoking, which may contribute to the development of COPD. Given this situation, and given what is known, it is likely that two-way mechanisms between chronic physical illness and depression have occurred. Selection bias might have led to the exclusion of people with more severe chronic diseases, whether by excluding those in hospitals, nursing homes and rehabilitation units or by healthy user bias. The generalisability of results is limited due to the period in which data was collected, namely the period of the first lockdown in Portugal due to the COVID-19 pandemic.
Authors’ contributionsBP designed the study, analysed the data and drafted the initial version of the manuscript. MN supervised the study design and data collection. SN was involved in data collection. AL supervised the study design and data analysis. All authors contributed to the interpretation of results and revision of the manuscript. All authors read and approved the final manuscript.
FundingThis study is included in the project ECOS COVID-19, which is funded by Portuguese national funds through the FCT - Foundation for Science and Technology, I.P., within the scope of project n°130 of the 1st edition of the special support Research 4 COVID-19.