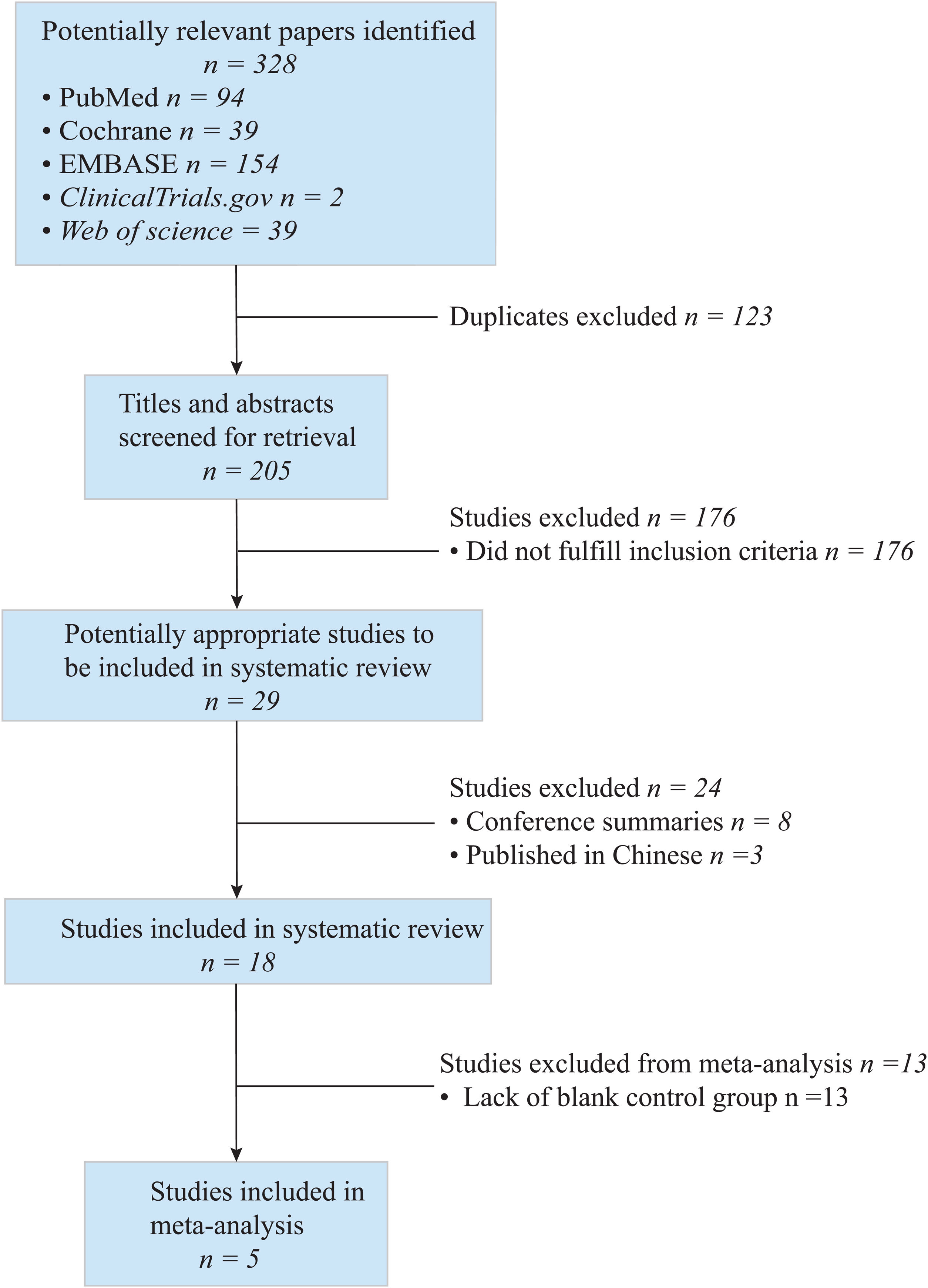
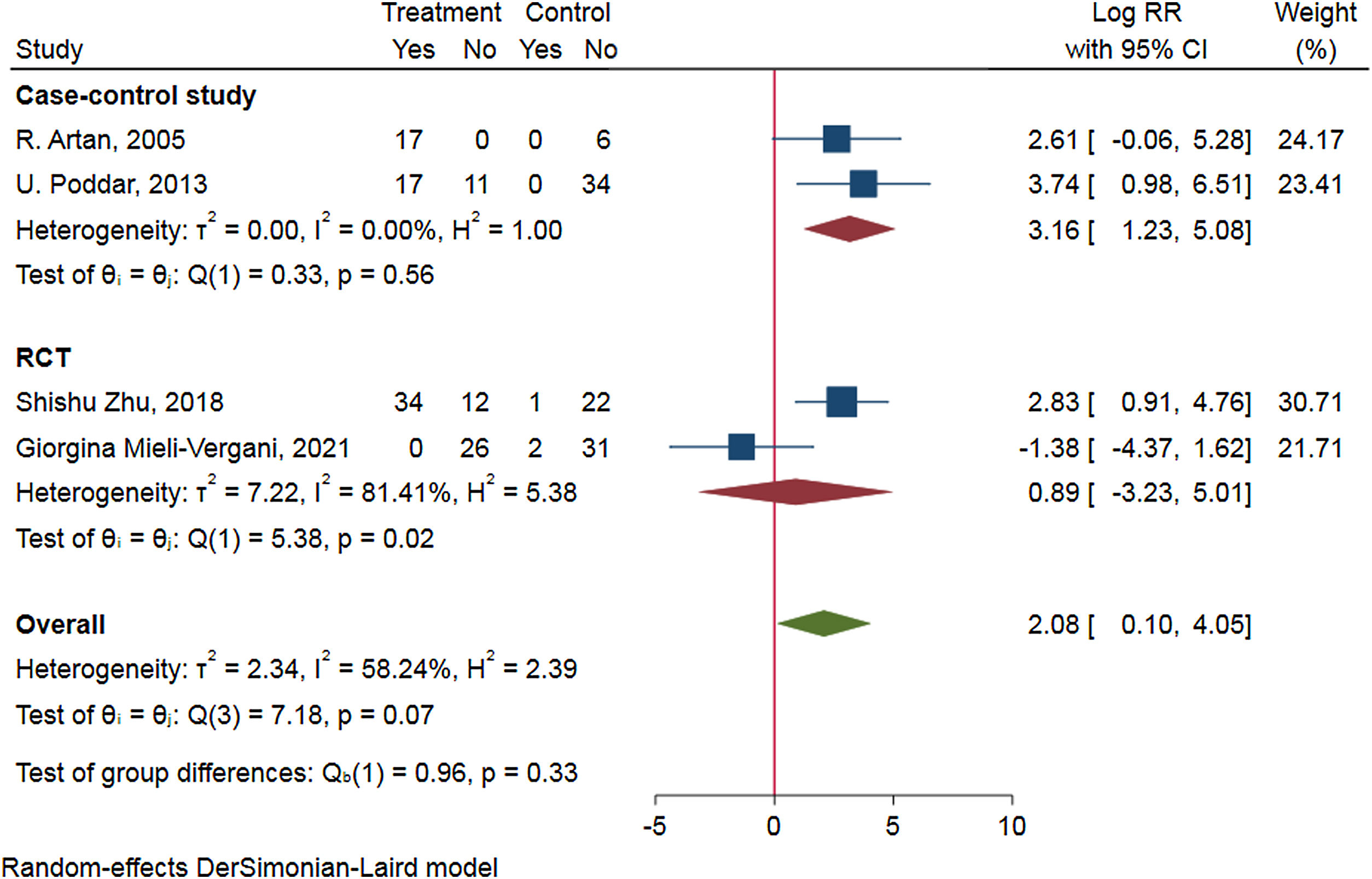
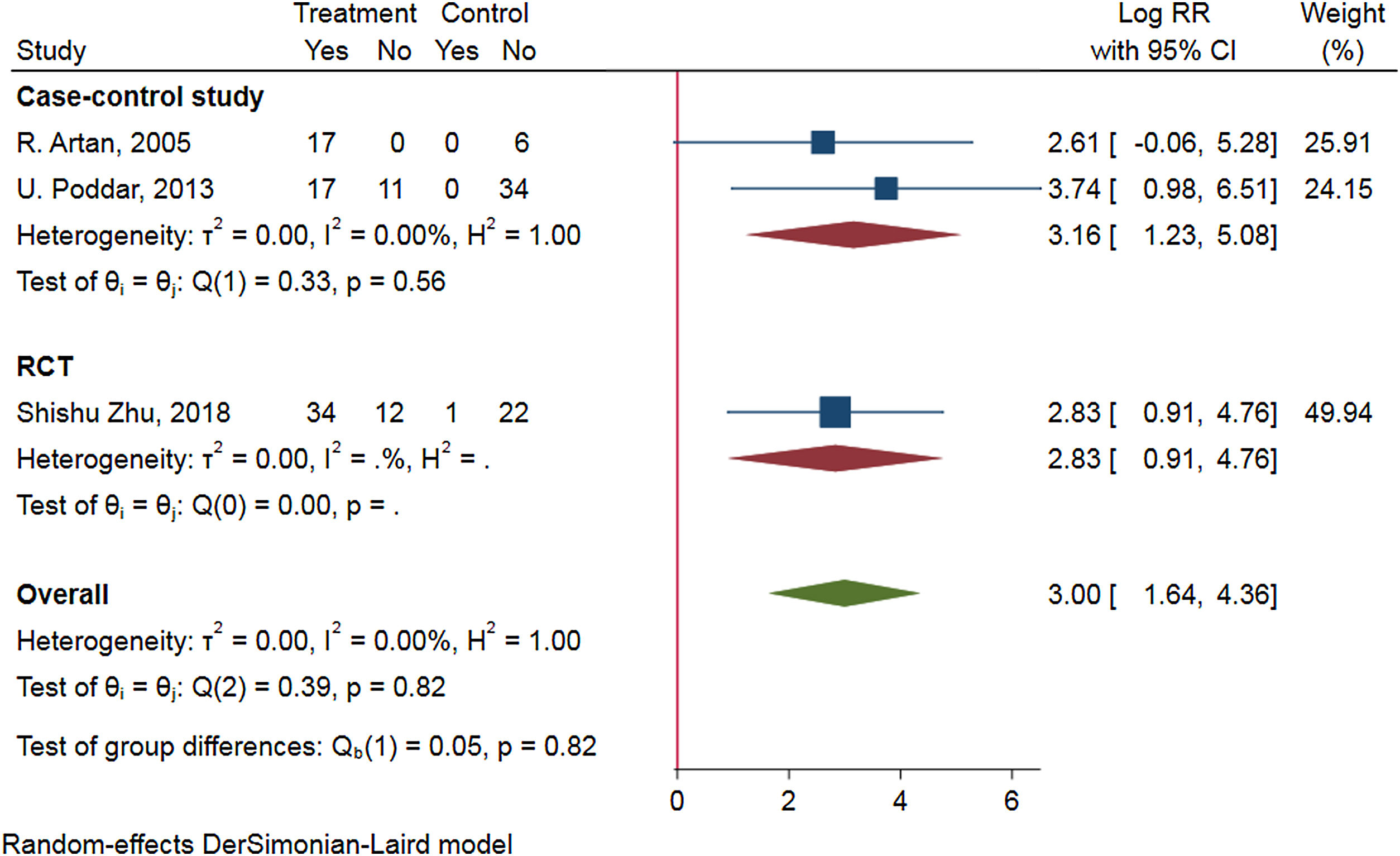
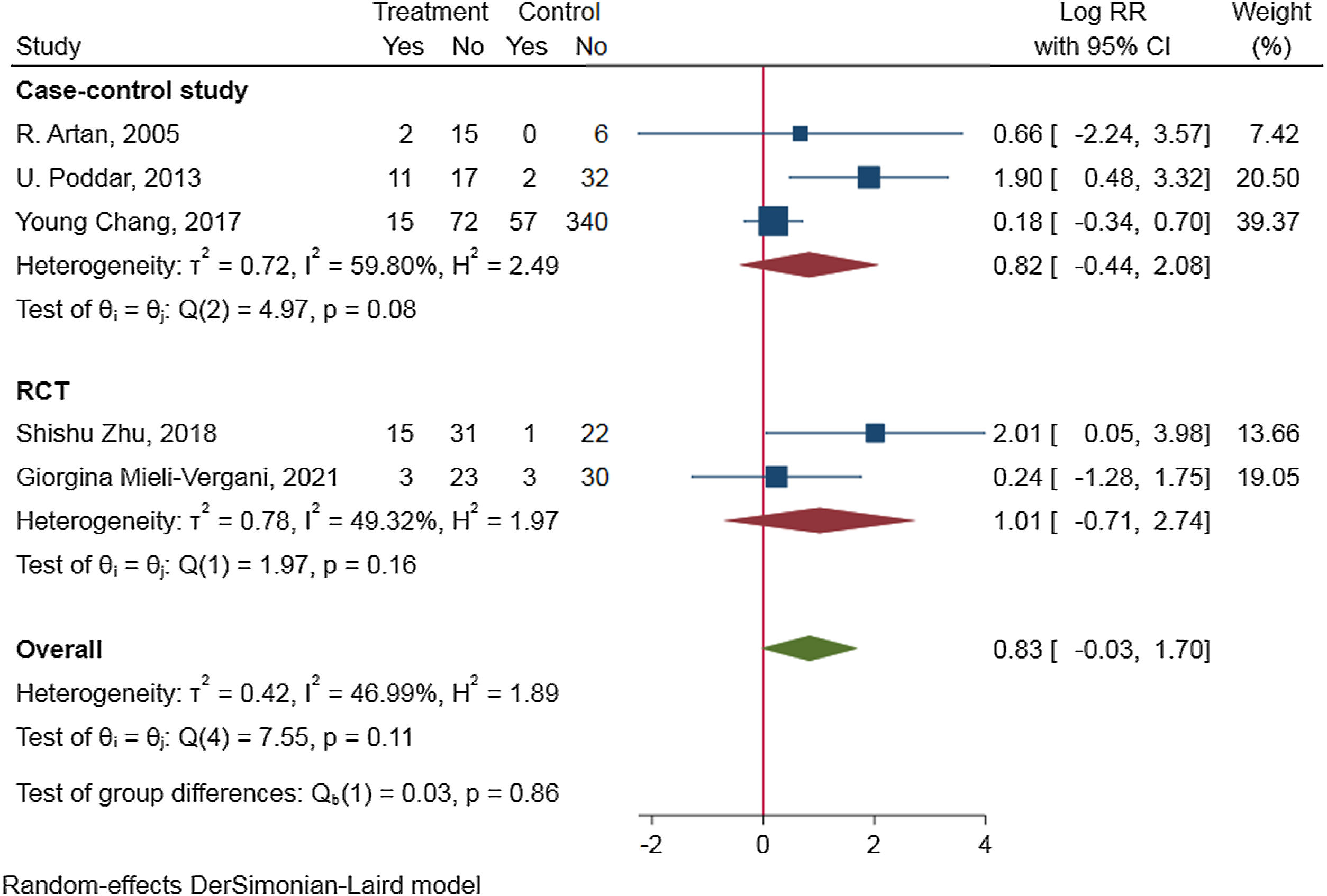
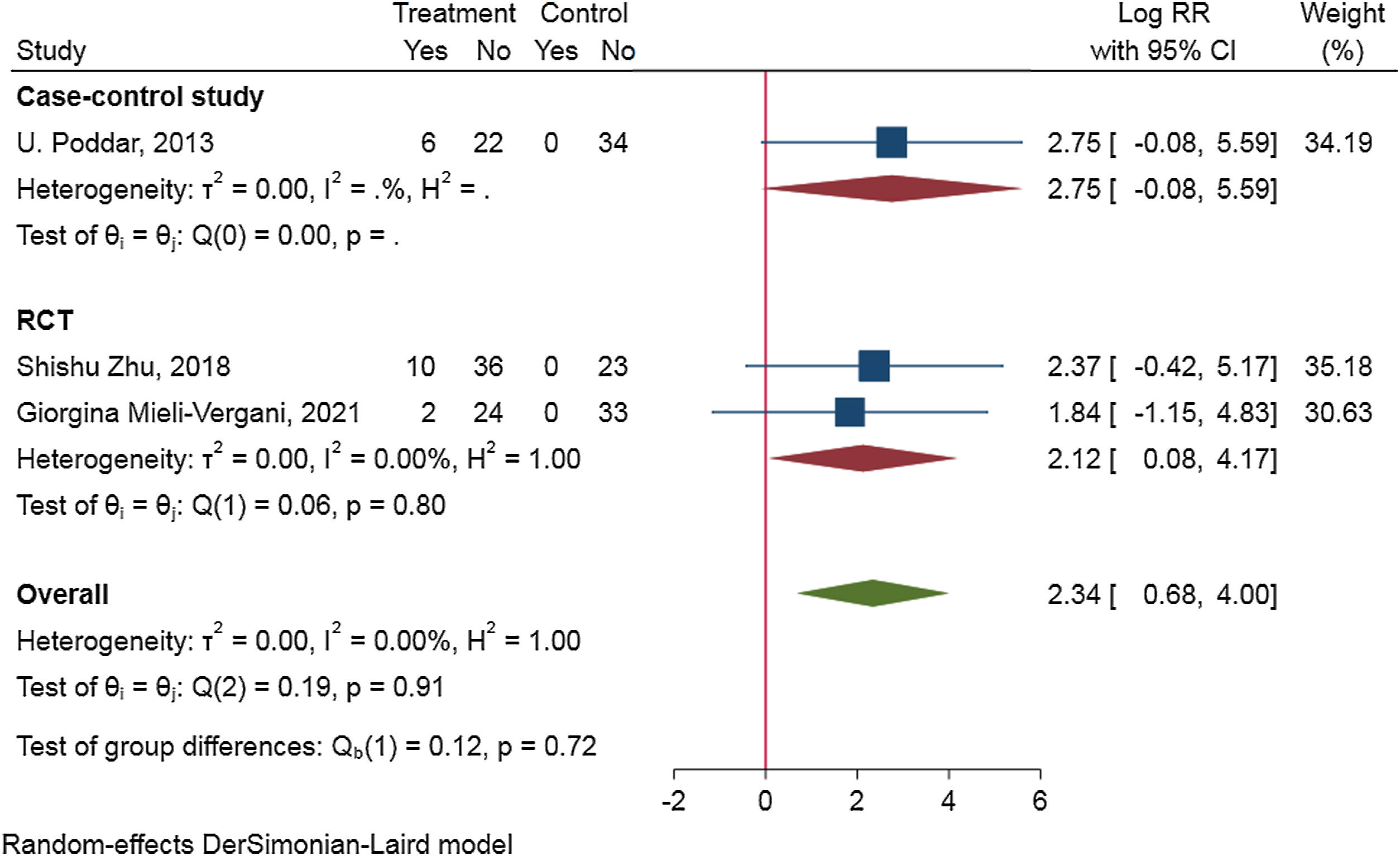
To access the efficacy of antiviral therapy in patients of HBV-infected with immune-tolerant. We conducted a meta-analysis search of the Cochrane Library, PubMed, ClinicalTrials.gov, Web of science, and EMBASE on through August 2021. We combined the data by means of a random-effect DrSimonian-Laird model and calculated risk ratios (RRs) for the outcomes of hepatitis B surface antigen (HBsAg) loss, hepatitis B e antigen (HBeAg) seroconversion, HBV deoxyribonucleic acid (DNA) negative conversion rate, and the risk for hepatocellular carcinoma (HCC) and cirrhosis. An extensive literature search identified 328 relevant publications, and five were included in the study. Antiviral therapy was in favor of HBsAg loss (RR=2.34, 95%CI 0.68–4.00, p=0.91, I2=0.00%), HBV DNA negative conversion (RR=2.08, 95%CI 0.10–4.05, p=0.07, I2=58.24%) and reduce the risk for HCC (HR=0.189, 95%CI 0.052–0.692, p=0.004) and cirrhosis (HR=0.347, 95%CI 0.095–1.270, p=0.036), but not beneficial to HBeAg seroconversion (RR=0.83, 95%CI −0.03 to 1.70, p=0.11, I2=46.99%). Subgroup-analyzed by the research type was similar results of HBsAg loss, HBV DNA negative conversion, and HBeAg seroconversion.Patients in HBV-infected with immune-tolerant responded well to antiviral therapy. The evidence from this meta-analysis supports antiviral therapy for patients with HBV in the immune tolerance stage. Well-designed, multi-center, larger sample sizes, and excellent quality prospective studies are needed to confirm our conclusion.
Para conocer la eficacia de la terapia antiviral en pacientes infectados con el virus de la hepatitis B (VHB) inmunotoler ante, se realizó una búsqueda de meta-análisis de la Cochrane Library, PubMed, ClinicalTrials.gov, Web of Science y EMBASE hasta agosto de 2021. Se combinaron los datos mediante un modelo DrSimonian-Laird y se calcularon los coeficientes de riesgo (RR) para los resultados de la pérdida del antígeno de superficie de la hepatitis B (HBsAg), la seroconversión del antígeno de superficie de la hepatitis B (HBeAg), la tasa de conversión negativa del ácido desoxirribonucleico (ADN) del VHB y el riesgo de carcinoma hepatocelular y de cirrosis. Una extensa búsqueda bibliográfica identificó 328 publicaciones relevantes, 5 de las cuales fueron incluidas en el estudio. El tratamiento antiviral favoreció la reducción de HBsAg (RR=2,34; IC95%: 0,68–4,00; p=0,91; I2=0,00%), la conversión negativa del ADN del VHB (RR=2,08; IC95%: 0,10–4,05; p=0,07;I2=58,24%) y redujo el riesgo de carcinoma hepatocelular (HR=0,189, ic95% 0,052 a 0,692, p=0,004) y de cirrosis (HR=0,347; IC95%: 0,095–1,270; p=0,036). Sin embargo, no fue beneficioso para la seroconversión a HBeAg (RR=0,83; IC95%: −0,03–1,70; p=0,11; I2=46,99%). El subgrupo analizado por el tipo de investigación mostró resultados similares de pérdida de HBsAg, conversión negativa del ADN del VHB y seroconversión del HBeAg. Los pacientes infectados con VHB inmunotolerantes respondieron bien a la terapia antiviral. La evidencia de este metaanálisis apoya el tratamiento antiviral para pacientes con VHB en estadio de inmunotolerancia. Se necesitan estudios prospectivos de excelente calidad para confirmar nuestra conclusión.