Granulocyte–monocyte apheresis (GMA) has shown to be safe and effective in treating ulcerative colitis (UC), also in combination with biologics. The objective of this study is to evaluate the efficacy and safety of combining GMA after primary non-response (PNR) or loss of response (LOR) to tofacitinib (TOFA) in patients with UC.
Patients and methodsRetrospective study including all patients with refractory UC who received GMA plus TOFA. Efficacy was assessed 1 and 6 months after finishing GMA by partial Mayo score, C-reactive protein (CRP) and fecal calprotectin (FC). Descriptive statistics and non-parametric tests were used in the statistical analysis.
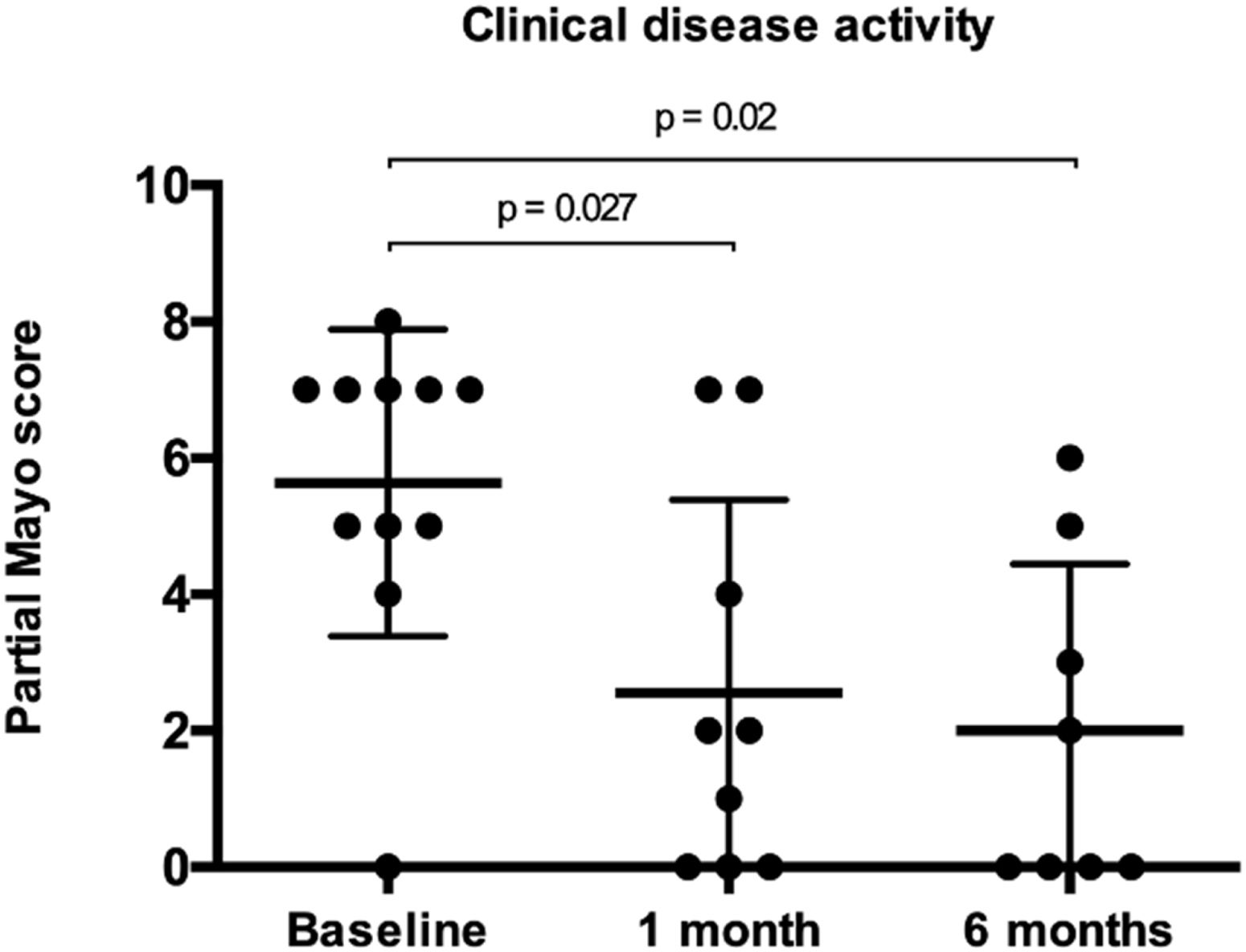
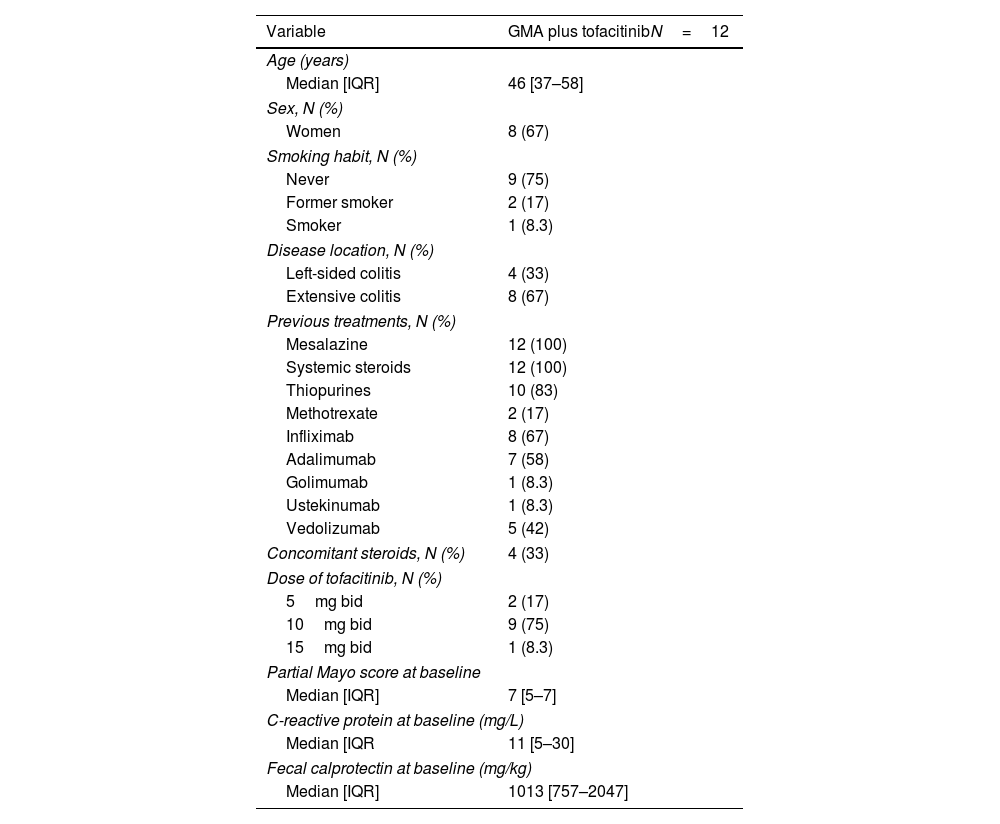
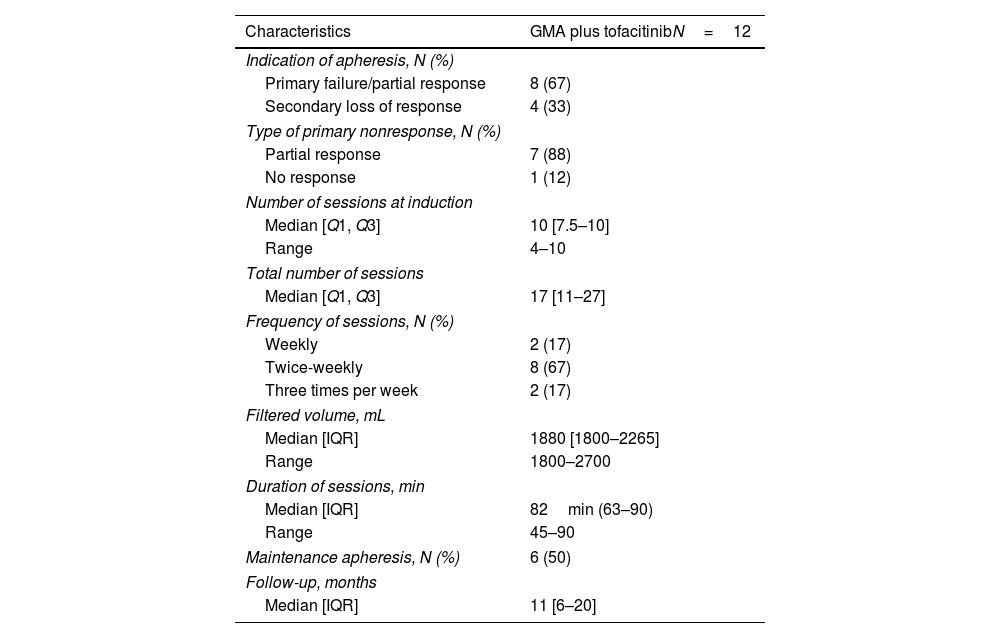
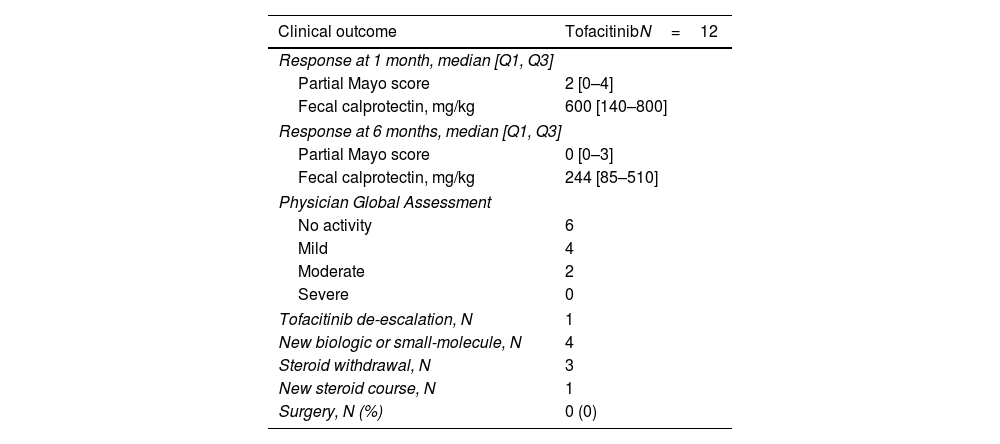
ResultsTwelve patients were included (median 46 years [IQR, 37–58]; 67% female; 67% E3). Patients were mostly receiving TOFA 10mg bid (75%), and 33% also concomitant steroids at baseline. Median partial Mayo score at baseline was 7 (IQR, 5–7), and it decreased to a median of 2 (IQR, 0–3) and 0 (IQR, 0–3) after 1 and 6 months (p=0.027 and 0.020, respectively), while no differences were found in CRP and FC. Clinical remission was achieved by 6 patients both at 1 (50%) and 6 months (67%). CF values<250mg/kg were achieved by 2 and 4 patients at 1 and 6 months (data available in 5 and 7 patients, respectively). No patient required dose-escalation of TOFA, and one patient was able to de-escalate the drug. No patient required colectomy and all patients under steroids were able to stop them.
ConclusionThe combination of GMA and TOFA can be effective in selected cases of UC after PNR or LOR to this drug.
La aféresis de granulocitos-monocitos (GMA) ha demostrado ser segura y eficaz en el tratamiento de la colitis ulcerosa (CU), incluso en combinación con fármacos biológicos. El objetivo de este estudio es evaluar la eficacia y la seguridad de combinar GMA con tofacitinib (TOFA) tras falta de respuesta primaria o pérdida de respuesta a este fármaco en pacientes con CU.
Pacientes y métodosEstudio retrospectivo que incluyó a todos los pacientes con CU refractaria que recibieron GMA más TOFA. La eficacia se evaluó 1 y 6 meses después de finalizar la GMA mediante la puntuación de Mayo parcial, la proteína C reactiva (PCR) y la calprotectina fecal (CF). El análisis estadístico se realizó mediante parámetros descriptivos y pruebas no paramétricas.
ResultadosSe incluyeron doce pacientes (mediana 46 años [RIQ, 37-58]; 67% mujeres; 67% E3). En el momento basal, la mayoría de los pacientes estaban recibiendo TOFA 10mg dos veces al día (75%), y el 33% también recibían esteroides concomitantes. La mediana de la puntuación de Mayo parcial basal fue de 7 (RIC, 5-7), y disminuyó a una mediana de 2 (RIC, 0-3) y 0 (RIC, 0-3) tras 1 y 6 meses (p=0,027 y 0,020, respectivamente), mientras que no se encontraron diferencias en la PCR y la CF. Se logró remisión clínica en 6 pacientes tanto al mes (50%) como a los 6 meses (67%). Dos y 4 pacientes alcanzaron valores de CF <250mg/kg el mes 1 y el 6 (datos disponibles en 5 y 7 pacientes, respectivamente). Ningún paciente requirió aumentar la dosis de TOFA y un paciente pudo reducirla. Ningún paciente necesitó colectomía y todos los pacientes tratados con esteroides pudieron suspenderlos.
ConclusiónLa combinación de GMA y TOFA puede ser eficaz en casos seleccionados de CU tras la falta de respuesta primaria o pérdida de respuesta a este fármaco.
Granulocyte–monocyte apheresis (GMA) can selectively deplete activated neutrophils/monocytes/macrophages by adsorption and has been associated with significant clinical efficacy in patients with inflammatory bowel disease (IBD).1,2 The mechanism of action of GMA relies on the interaction between cellular and humoral blood components and cellulose acetate beads immersed in physiological saline solution.3 After passing through the device, the blood is further reinfused to the patient.4–7
Several studies have shown the efficacy and safety of tofacitinib (TOFA), an oral, small-molecule Janus kinase inhibitor in the treatment of moderate to severe ulcerative colitis (UC) patients who failed to achieve clinical remission, did not respond to conventional treatments, or lost response to tumor necrosis factor (TNF)-α antagonists.8,9 However, despite the efficacy of small-molecules in UC, a significant proportion of patients receiving TOFA do not respond or lose response over time.8 In contrast, the concomitant use of GMA plus TOFA has been described with up to 71% of patients achieving clinical remission at 10 weeks in seven patients with refractory UC.10 Different studies and case series have also shown promising results when combining GMA with different biologics, as well as with vedolizumab or ustekinumab.5,7,16,11–13
The aim of our study was to evaluate the efficacy and safety of combining GMA and TOFA after primary non-response (PNR) or loss of response (LOR) to this drug in patients with refractory UC.
Patients and methodsStudy populationA retrospective, multicentre study was performed in 7 IBD Units in Spain. We included all patients with refractory UC who received the combination of GMA and TOFA between September 2019 and October 2022.
Treatment and assessmentsWe collected data on age at diagnosis, disease location according to Montreal classification,14 previous UC-related medications, concomitant medical therapy, past relevant disease history, smoking habits, body mass index, disease duration, and extraintestinal manifestations. All IBD Units involved in this study follow the current clinical practices guidelines from the European Crohn's and Colitis Organisation (ECCO)15 and the Spanish Working Group on Crohn's Disease and Ulcerative Colitis (GETECCU).16
The number of GMA sessions, its frequency, the filtered blood volume and the length of each session were compiled, along with the clinical data described above. The mean volume per session was calculated by dividing the sum of the total blood volume filtered in all these sessions by the number of sessions. Efficacy was assessed 1 and 6 months after finishing GMA by partial Mayo score, including also C-reactive protein (CRP) and fecal calprotectin (FC) at the same time points. The proportion of patients with FC values<250mg/kg was calculated. Data regarding the need of TOFA dose escalation, use of new immunomodulators or biologics and colectomy were also registered during follow-up.
The decision to start GMA combination therapy and its regimen were made on a case-by case basis at each hospital based on the individual characteristics of the patient and the clinical situation, as the use of GMA plus TOFA is not an established treatment for UC. All patients were treated with the same GMA device (Adacolumn®, JIMRO, Takasaki, Japan) receiving weekly, twice-weekly or three times per week sessions through a peripheral venous access. The filtered volume and the duration of the sessions were chosen at the physician's discretion (the recommended volume is 1800mL per session of 60min, though this schedule can be adjusted according to the clinical and individual characteristics of each patient).3 An intravenous bolus of weight-based low molecular-weight heparin was administered to all patients before starting GMA.
All adverse events (AEs) occurring during follow-up were recorded, and serious AEs were defined as any AE leading to treatment discontinuation, hospitalization, disability, colectomy, or death.
DefinitionsThe definition of PNR included the persistence of symptoms with a partial or complete absence of improvement in the partial Mayo score during the first 8–16 weeks of TOFA therapy. Secondary LOR was defined as a clinical relapse with a partial Mayo score≥2 and a bleeding subscore>1 after 16 weeks of starting TOFA and after a period of clinical remission achieved during the induction period. Clinical remission was defined as a partial Mayo score≤2 with no individual subscore>1 and a rectal bleeding score of 0.
Statistical analysisDescriptive statistics of the sample were examined, using frequencies and percentages for categorical variables and means and SD for continuous variables. Variables with biased distributions were expressed as median and interquartile ranges (IQRs). The Wilcoxon test was used to assess differences in continuous variables between the different time points. Statistical significance was defined as p<.05.
ResultsA total of twelve patients were treated with this combination therapy. Patient characteristics are summarized in Table 1. The median age at the moment of starting GMA was 46 years (IQR, 37–58) and 67% of patients were women, with 75% being non-smokers. UC was classified as extensive (E3) in eight cases (67%) and left-sided in four (33%). The median disease duration was 27 months (IQR, 11–112). No patient reported extraintestinal manifestations.
Patient characteristics.
| Variable | GMA plus tofacitinibN=12 |
|---|---|
| Age (years) | |
| Median [IQR] | 46 [37–58] |
| Sex, N (%) | |
| Women | 8 (67) |
| Smoking habit, N (%) | |
| Never | 9 (75) |
| Former smoker | 2 (17) |
| Smoker | 1 (8.3) |
| Disease location, N (%) | |
| Left-sided colitis | 4 (33) |
| Extensive colitis | 8 (67) |
| Previous treatments, N (%) | |
| Mesalazine | 12 (100) |
| Systemic steroids | 12 (100) |
| Thiopurines | 10 (83) |
| Methotrexate | 2 (17) |
| Infliximab | 8 (67) |
| Adalimumab | 7 (58) |
| Golimumab | 1 (8.3) |
| Ustekinumab | 1 (8.3) |
| Vedolizumab | 5 (42) |
| Concomitant steroids, N (%) | 4 (33) |
| Dose of tofacitinib, N (%) | |
| 5mg bid | 2 (17) |
| 10mg bid | 9 (75) |
| 15mg bid | 1 (8.3) |
| Partial Mayo score at baseline | |
| Median [IQR] | 7 [5–7] |
| C-reactive protein at baseline (mg/L) | |
| Median [IQR | 11 [5–30] |
| Fecal calprotectin at baseline (mg/kg) | |
| Median [IQR] | 1013 [757–2047] |
Patients were receiving TOFA 10mg bid (75%), 5mg bid (17%), or 15mg bid (8%), and 33% also received systemic steroids at baseline. TOFA was maintained at stable doses while combined with GMA. All patients had prior exposure to anti-TNF agents, 67% to infliximab and 58% to adalimumab. Median partial Mayo score at baseline was 7 (IQR, 5–7). Median CRP at baseline was 11mg/L (IQR, 5–30; N=8) and FC 1013mg/kg (IQR, 757–2047; N=8).
The characteristics of GMA therapy are summarized in Table 2. GMA was started mostly after PNR (73%), and the median number of GMA sessions was 17 (IQR, 11–27). Sessions were twice-weekly in most patients (58%), with 17% undergoing three-weekly or weekly sessions. The treatment protocols consisted in filtering 1800–2700mL per session with a 30–50mL/min rate for 45–90min. In most of the patients, the filtered volume was 1800mL with sessions of 45–70min. Half of the patients received maintenance therapy with GMA. The median clinical follow-up was 11 months (IQR, 6–20).
Characteristics of the granulocyte and monocyte apheresis (GMA) treatment (n=12) and duration of follow-up.
| Characteristics | GMA plus tofacitinibN=12 |
|---|---|
| Indication of apheresis, N (%) | |
| Primary failure/partial response | 8 (67) |
| Secondary loss of response | 4 (33) |
| Type of primary nonresponse, N (%) | |
| Partial response | 7 (88) |
| No response | 1 (12) |
| Number of sessions at induction | |
| Median [Q1, Q3] | 10 [7.5–10] |
| Range | 4–10 |
| Total number of sessions | |
| Median [Q1, Q3] | 17 [11–27] |
| Frequency of sessions, N (%) | |
| Weekly | 2 (17) |
| Twice-weekly | 8 (67) |
| Three times per week | 2 (17) |
| Filtered volume, mL | |
| Median [IQR] | 1880 [1800–2265] |
| Range | 1800–2700 |
| Duration of sessions, min | |
| Median [IQR] | 82min (63–90) |
| Range | 45–90 |
| Maintenance apheresis, N (%) | 6 (50) |
| Follow-up, months | |
| Median [IQR] | 11 [6–20] |
Follow-up data at 1 month was available for all patients and for 9 patients (75%) after 6 months. Partial Mayo score significantly decreased to a median of 2 (IQR, 0–3; p=0.027) 1 month after the last GMA session, and to a median of 0 (IQR, 0–3; p=0.062) at 6 months (Fig. 1 and Table 3). Clinical remission was achieved by 6 patients after 1 (50%) and 6 months (67%). CF values<250mg/kg were obtained by 2 and 4 patients at 1 and 6 months (data available in 5 and 7 patients, respectively). There were no statistically significant changes in CRP or FC levels after 1 (p=0.39 and 0.23, respectively) or 6 months (p=0.11 and 0.08, respectively).
Clinical outcomes (n=12).
| Clinical outcome | TofacitinibN=12 |
|---|---|
| Response at 1 month, median [Q1, Q3] | |
| Partial Mayo score | 2 [0–4] |
| Fecal calprotectin, mg/kg | 600 [140–800] |
| Response at 6 months, median [Q1, Q3] | |
| Partial Mayo score | 0 [0–3] |
| Fecal calprotectin, mg/kg | 244 [85–510] |
| Physician Global Assessment | |
| No activity | 6 |
| Mild | 4 |
| Moderate | 2 |
| Severe | 0 |
| Tofacitinib de-escalation, N | 1 |
| New biologic or small-molecule, N | 4 |
| Steroid withdrawal, N | 3 |
| New steroid course, N | 1 |
| Surgery, N (%) | 0 (0) |
No patients receiving TOFA 5mg bid (n=2) required dose escalation and one patient under 10mg bid was able to de-escalate to 5mg bid. All patients under steroids at baseline were able to stop them. During follow-up, one patient required a new course of steroids. Four patients (36%) stopped TOFA and started a new therapy (ustekinumab [75%] and upadacitinib [25%]) and no patient required colectomy. No patients reported AEs related to this combination therapy.
DiscussionHere we report the feasibility of combining GMA with TOFA after PNR or LOR to this agent in a cohort of patients with multi-refractory UC. All of these patients had prior exposure to anti-TNF agents, mainly infliximab and adalimumab, and one third were receiving steroids at baseline. GMA was mostly started after PNR, and our data show how this strategy could potentially reduce the inflammatory burden and even avoid switching to another therapy in a cohort of patients with limited therapeutic options.
In recent years, anti-TNF, anti-interleukin and anti-integrin monoclonal antibodies have demonstrated their ability to achieve high rates of remission in the management of UC. Unfortunately, in a significant number of patients subsequent lines of therapy are required due to an inadequate response or a relapse after an initial response.17 Among the most recent therapeutic options, TOFA has also shown its efficacy in UC patients who do not respond or lose response to TNF-α antagonists achieving clinical remission rates of 18.5% and 16.6% after 8 weeks in the Oral Clinical Trials for Tofacitinib in Ulcerative Colitis (OCTAVE) induction 1 and 2 trials, respectively.8
In addition to its use in other inflammatory conditions as rheumatoid arthritis, ocular Behçet's disease, systemic lupus erythematosus or pustular psoriasis, GMA is approved in Europe for inducing remission in patients with active UC and Crohn's disease.18 The usual GMA protocol generally consists in 1–2 sessions per week usually up to a total of 10 sessions,11 with a length of 60–90min per session.4–7,19 Intensive GMA of bi-weekly sessions has been shown to be superior to weekly GMA in patients with refractory UC, both in terms of remission rate and time to remission.20,21 In our study, almost 60% of the patients received twice-weekly GMA sessions, with a median number of 10 sessions and a median duration of 82min per session. In addition, half of the patients received maintenance GMA, reflecting the use of more intensive therapy in this highly refractory subset of patients. However, maintenance GMA still remains controversial.11
GMA has shown long-term benefit in patients with active UC and insufficient response or intolerance to immunosuppressants or biologics, a difficult-to-treat patient subgroup.22 Its unique mechanism of action provides immunological effects that are expected to act synergistically when combined with more conventional agents for UC. There are several studies and case series already showing promising results when combining GMA with different biologics like anti-TNF agents, as well as with vedolizumab or ustekinumab.5,7,11–13 Most of the evidence with this combination therapy has been described with biologics, and its use with small molecules is still very limited.10 In our cohort, partial Mayo score significantly decreased 1 and 6 months after the last GMA session, although we did not observe significant changes in biomarkers. Notably, no patient required colectomy during follow-up; all patients under steroids at baseline were able to stop them and only one patient required a new course of steroids. This is in line with previous studies that have demonstrated the steroid-sparing effect of adsorptive GMA.23 Thus, increasing evidence supports that GMA might be an attractive alternative in the setting of steroid-dependency even in patients receiving biologics and small molecules.11
Only one previous case series has described the feasibility of combining GMA with TOFA during the induction in patients with refractory UC.10 In seven patients who received twice-weekly concomitant sessions of GMA plus TOFA during the induction period, 71.4% achieved clinical remission at 10 weeks. The percentages of patients with mucosal healing and complete mucosal healing at 10 weeks were 100% and 43%, respectively. There were statistically significant changes in the full Mayo score and endoscopic subscore at 10 weeks compared to baseline. Here, Tanida et al. concluded that combining intensive GMA plus TOFA was well tolerated and may be useful for inducing clinical remission in patients with refractory UC.10 It should be noted that, in our study, the strategy consisted in combining GMA with TOFA after PNR or LOR to this agent and not during the induction. Moreover, it could be expected that a more intensive GMA regimen might have improved the results observed in our cohort. Despite these considerations, both reports highlight the safety of this combination therapy and the possibility of improving outcomes in different scenarios of patients treated with small molecules.
GMA selectively depletes activated leukocytes of the myeloid lineage, mainly by removing neutrophils, the most prominent cellular population during disease flares.11 Besides, it has several secondary immunomodulatory effects, including release of anti-inflammatory mediators, decreased production of proinflammatory cytokines, reduced neutrophil chemotaxis and endothelial cell adhesion, down-regulation of circulating leukocyte cell surface proteins, and induction of regulatory T and B-cells (Tregs and Bregs).11,24 Furthermore, in the event of secondary LOR to anti-TNFs, recent evidence suggests that GMA may contribute to re-establishing a treatment effect in a proportion of patients,11,25 probably by reducing anti-drug antibodies and without an influence on trough levels.13,26 The exact mechanism of action of the combination of GMA with TOFA is still unknown and should be further explored.
GMA is safe and well tolerated in the treatment of IBD in steroid-dependent cases and also in combination with biologics.11,27,28 In our population, no patient reported any AE related to the combination therapy. The low rate of surgery and the ability to reduce steroids with few AEs may allow to control the disease and the inflammatory process without switching the therapy in refractory patients with limited treatment options once they have already used TOFA. Our results regarding the safety profile appear to be even better than the ones described by Tanida et al., where three patients showed an AE mainly attributed to TOFA (an orolabial herpes and temporary increase in creatinine phosphokinase and triglyceride).10
Our study has also some limitations that should be addressed. The retrospective design and the variability of TOFA and GMA regimens may limit our ability to obtain more definite conclusions. Though, this study could serve as a basis for future clinical trials exploring the potential of combining GMA with TOFA in refractory UC patients with an inadequate response to this agent. Further large prospective studies are expected to clarify the exact mechanisms underlying the beneficial effect of this strategy and define the characteristics of patients with the highest probability of responding to this strategy.
In conclusion, our results suggest the potential effectiveness, safety and tolerability of combining GMA with TOFA in selected UC patients with an insufficient or inadequate response to this drug. This approach could expand the number of therapeutic options available for some patients with refractory UC and deserves more research.
Authors’ contributionsIR-L and JLC: study design, data analysis and drafting the manuscript.
FC, EG, CH, EI, EL, YZ, MB-A, DG: data collection.
DG and JLC: revised the manuscript for important intellectual content.
Ethical considerationsInformed consent was obtained from all patients included in the study. The study was approved by the local Ethics Committee of the coordinating center (EPA2017050).
FundingIR-L is funded by a research grant from Gobierno Vasco – Eusko Jaurlaritza [grant number 2020111061] and by a research grant from Biobizkaia Health Research Institute [Grant No BCB/I/LIB/22/008].
Conflict of interestIR-L has received financial support for traveling and educational activities from or has served as an advisory board member for Abbvie, Adacyte, Celltrion, Chiesi, Danone, Ferring, Faes Farma, Janssen, Galapagos, MSD, Pfizer, Roche, Takeda, and Tillotts Pharma. Financial support for research: Tillotts Pharma.
YZ has received support for conference attendance, speaker fees, research support and consulting fees from AbbVie, Adacyte, Almirall, Amgen, Dr. Falk Pharma, FAES Pharma, Ferring, Janssen, MSD, Otsuka, Pfizer, Shire, Takeda, Galapagos, Boehringer Ingelheim and Tillotts Pharma.
MB-A has received financial support for traveling and educational activities from or has served as an advisory board member for Pfizer, MSD, Takeda, AbbVie, Kern, Janssen, Fresenius Kabi, Galapagos, Lilly, BMS, Faes Farma, Chiesi and Adacyte.
JLC has received financial support for traveling and educational activities from or has served as an advisory board member for Abbvie, Adacyte, Chiesi, Ferring, Janssen, MSD, Pfizer, Takeda, and Tillotts Pharma.
The remaining authors have no conflicts of interest to declare.
We want to thank Adacyte for supporting the development of the database for this study. The company did not have an influence on the study design, interpretation of the results or drafting the manuscript.