The Maastricht V Consensus recommends quadruple therapies as first-line Helicobacter pylori treatment in high clarithromycin (CLA) resistance areas.
AimsTo compare efficacy, side effects and compliance between quadruple concomitant non-bismuth vs bismuth quadruple therapy.
MethodProspective study enrolling H. pylori-positive patients. Omeprazol and a three-in-one formulation of bismuth–metronidazol–tetracycline (OBMT-3/1) for 10 days, or combination of omeprazol–clarithromycin–amoxicillin–metronidazol (OCAM) for 14 days, were prescribed. Eradication outcome was assessed by urea breath test or histology. Side effects and compliance were recorded during the treatment period with specific questionnaires.
Results404 patients were recruited (median age 53 years; 62.87% women). In 382 (183 with OCAM, 199 with OBMT-3/1) the post-treatment test result was available. The eradication rates were 85.94% (CI95%: 80.20–90.52) with OCAM and 88.21% (CI95%: 83.09–92.22) with OBMT-3/1 (p=0.595) in intention-to-treat analysis, whilst in per protocol analysis they were 91.12% (CI95%: 85.78–94.95) and 96.17% (CI95%: 92.28–98.45) respectively (p=0.083). Compliance over 90% was 91.35% with OCAM and 92.04% with OBMT-3/1 (p=0.951). Some side effect was present in 94.02% with OCAM and in 88.89% with OBMT-3/1 (p=0.109), being longer (12 vs 7 days, p<0.0001) and more severe (p<0.0001) with OCAM.
ConclusionsIn a high CLA-resistance area, there are no differences between OBMT-3/1 and OCAM in H. pylori eradication and compliance rates, but OBMT-3/1 achieves a higher safety profile.
El Consenso de Maastricht V recomienda tetraterapias como tratamiento de primera línea de Helicobacter pylori en áreas con elevada resistencia a claritromicina (CLA).
ObjetivosComparar la eficacia, los efectos secundarios y el cumplimiento terapéutico entre la tetraterapia sin bismuto y la tetraterapia con bismuto.
MétodoEstudio prospectivo que incluyó a pacientes con H. pylori. Se prescribió omeprazol y una formulación 3 en uno de bismuto-metronidazol-tetraciclina (OBMT-3/1) durante 10 días, o una combinación de omeprazol-claritromicina-amoxicilina-metronidazol (OCAM) durante 14 días. El resultado de la erradicación se evaluó mediante una prueba de aliento con urea o histología. Los efectos secundarios y el cumplimiento terapéutico se registraron durante el período de tratamiento empleando cuestionarios específicos.
ResultadosSe incluyeron 404 pacientes (mediana de edad de 53 años; un 62,87% de mujeres). El resultado de la prueba posterior al tratamiento estuvo disponible en 382 pacientes (183 con OCAM, 199 con OBMT-3/1). Las tasas de erradicación fueron del 85,94% (IC 95%: 80,20-90,52) con OCAM y del 88,21% (IC 95%: 83,09-92,22) con OBMT-3/1 (p=0,595) en el análisis por intención de tratar, mientras que en el análisis por protocolo fueron del 91,12% (IC 95%: 85,78-94,95) y del 96,17% (IC 95%: 92,28-98,45), respectivamente (p=0,083). El cumplimiento terapéutico superior al 90% fue del 91,35% con OCAM y del 92,04% con OBMT-3/1 (p=0,951). Se observaron efectos secundarios en el 94,02% de los pacientes tratados con OCAM y en el 88,89% de los tratados con OBMT-3/1 (p=0,109), y fueron más prolongados (12 frente a 7 días, p<0,0001) y más graves (p<0,0001) con OCAM.
ConclusionesEn un área con elevada resistencia a la CLA no se observan diferencias entre OBMT-3/1 y OCAM en la erradicación de H. pylori ni en las tasas de cumplimiento, pero OBMT-3/1 presenta un perfil de seguridad superior.
Helicobacter pylori (H. pylori) infects up to 40–50% of the world population, being more prevalent in developing countries.1 It has been linked to several gastroduodenal and extradigestive pathologies, such as gastritis, functional dyspepsia, peptic ulcer, gastric cancer, and iron or other micronutrient deficiency.2–4 The factors more often involved in the H. pylori eradication treatment efficacy are patient compliance of the antibiotic regime and the existence of bacterial resistance.5 In recent decades a progressive increase in these resistances has been described, in parallel to a drop in efficacy of the triple therapeutic combinations traditionally prescribed. Factors such as previous antibiotic consumption, migratory flows, bacterial genetic mutations and more resistant adaptive shapes could explain this phenomenon.6–11
The choice of pharmacological combination for first-line eradication treatment is made based on the described bacterial resistance in each geographical area. The American College of Gastroenterology Clinical Guideline,12 published in 2017, recommends the use in North America of triple therapies with proton pump inhibitor (PPI), clarithromycin (CLA) and metronidazole (MET) only in regions with resistance to the macrolide less than 15% and in patients without previous exposure to this antibiotic. The Maastricht V/Florence Consensus Report13 recommends bismuth quadruple or non-bismuth quadruple concomitant (PPI, amoxicillin, CLA and a nitroimidazole) therapies in areas of high (>15%) CLA-resistance. While in areas of high dual CLA-&-MET-resistance, bismuth quadruple therapy (BQT) is the recommended first-line treatment. The Central and South-European countries and USA have mostly demonstrated that they maintain high CLA and low or intermediate (<40%) MET-resistance pattern. A multicentric European study that prospectively evaluated patients between 2008 and 2009 found CLA-resistance in 17.5% and MET-resistance in 34.9% of cases.6 In a meta-analysis including studies published between 2006 and 2009, the highest CLA-resistance rate was reported in Spain (49.2%, 95% CI: 38.7–58.2).14 Two subsequent review articles and a recent meta-analysis showed very high resistance rates in countries such as Spain (17.6–51.2%), Italy (72.4%), Poland (9.0–55.2%) France (43%) and Ireland (51.9%).15–17
In this setting it has been documented that the combinations described in Maastricht V guideline achieve in clinical trials and meta-analysis an efficacy over 90%.18–20 Nevertheless, it is also known that treatment compliance affected the eradication rate, and it is a constant that the adherence to treatment achieved in a clinical trial substantially exceeds that observed in clinical practice. On the other hand we could expect that the rise of the consumption period, doses and number of drugs, regarding those used in traditional regimens, might make the treatment compliance difficult, and even increase the rate of side effects as well, which would constitute an added disadvantage to fulfilling the medical prescription in some patients. Trying to solve these problems concomitant probiotics use or written instructions to facilitate compliance 21 have rarely been tested with first-line quadruple therapies.
So far studies evaluating the first-line quadruple therapies under real practice conditions are limited. The aim of this study was prospectively to analyse the compliance of H. pylori eradication treatment, its efficacy and side effects with the regimens: quadruple concomitant non bismuth therapy for 14 days vs bismuth quadruple for 10 days, in patients with de novo diagnosis, and living in a high CLA-resistance geographical area. The potential influence of the variables compliance and side effects on the eradication outcome was analysed too.
MethodsPatientsAdults (over 18 years) diagnosed with de novo H. pylori infection in whom eradication treatment was indicated according to current clinical practice guidelines.5,13,20 The exclusion criteria were severe comorbidity – groups IV and V of the American Society of Anesthesiologists,22 – contraindications for study drugs, partial gastrectomy, pregnant or nursing women, drug or alcohol abuse, severe psychiatric or neurological illness, and patient refusal to participate in the study.
DesignObservational, prospective, single-centre study (Miguel Servet University Hospital, Zaragoza, Spain) performed under routine clinical practice conditions, including consecutively H. pylori-positive patients.
The infection diagnosis in patients who underwent endoscopy was performed by means of ultra-fast urease test (Biohit Helicobacter pylori UFT300®; Biohit Health Care Helsinky, Finland) or after histological exam with haematoxylin–eosin stain in gastric biopsies. In patients without endoscopy indication the diagnosis was carried out through 13C-urea breath test (UBT) (TAU-KIT®100mg; Isomed Pharma, Madrid, Spain).
The first-line eradication treatment was prescribed at the discretion of the physician, choosing between one of the two following regimens: 1. Omeprazol 40mg, Clarithromycin 500mg, Amoxicillin 1g, and Metronidazole 500mg, all b.i.d., for 14 days (OCAM). 2. Omeprazol 20mg b.i.d and 3 three-in-one capsules (Pylera®) q.i.d., each containing bismuth subcitrate potassium 140mg, metronidazole 125mg and tetracycline hydrochloride 125mg), for 10 days (OBMT-3/1).
Demographic information, indication to treat, smoking habit, diagnostic method, eradication treatment and its outcome, were collected. A therapeutic schedule with the chosen regimen was given to the patients and they were asked to mark the drugs consumed each time, when taken. Adherence to the treatment was defined by the number of consumed pills in comparison to the prescribed ones. Consumption of over 90% was considered good compliance, and intake over 80% was analysed too. A side effects questionnaire to obtain information about duration, subjective severity (mild/moderate/severe) and use of adjuvant therapies to alleviate them was completed by the patient during the treatment period.
To evaluate the treatment result a UBT was performed not earlier than 4 weeks after the end of treatment and 2 weeks after PPI cessation. Under these same conditions when an upper endoscopy was justified for any other reason, the histological examination of gastric biopsies was considered as valid to assess the treatment outcome if at minimum samples from incisura and major curvature of antrum and corpus were obtained.
StatisticsA minimum sample size of 183 subjects with full follow-up in each arm was estimated, assuming 10% of clinically relevant difference in the main study variables, confidence level of 95% and statistical power of 90%.
A descriptive analysis was performed. The efficacy and compliance rates were expressed with their 95% confidence intervals (CI). The efficacy was assessed per protocol (PP) and by intention-to-treat (ITT) analysis, considering for the latter the patients with positive post-treatment checking test and those missed for follow-up as treatment failures. The Shapiro–Wilk test was applied to evaluate normality for quantitative variables. Chi square, Fisher test, T-Student for parametric, and U-Mann–Whitney for non-parametric variables were also used. Statistical significance was set at p<0.05.
EthicsThe study data were confidential as is required by the Spanish Organic Law 15/99 concerning Personal Data Protection. All subjects signed informed consent before enrolment. Our study was done in accordance with the principles of the 1975 Declaration of Helsinki (6th revision, 2008). The protocol was approved by the Regional Ethics Committee (protocol code: C.I. PI16/0232).
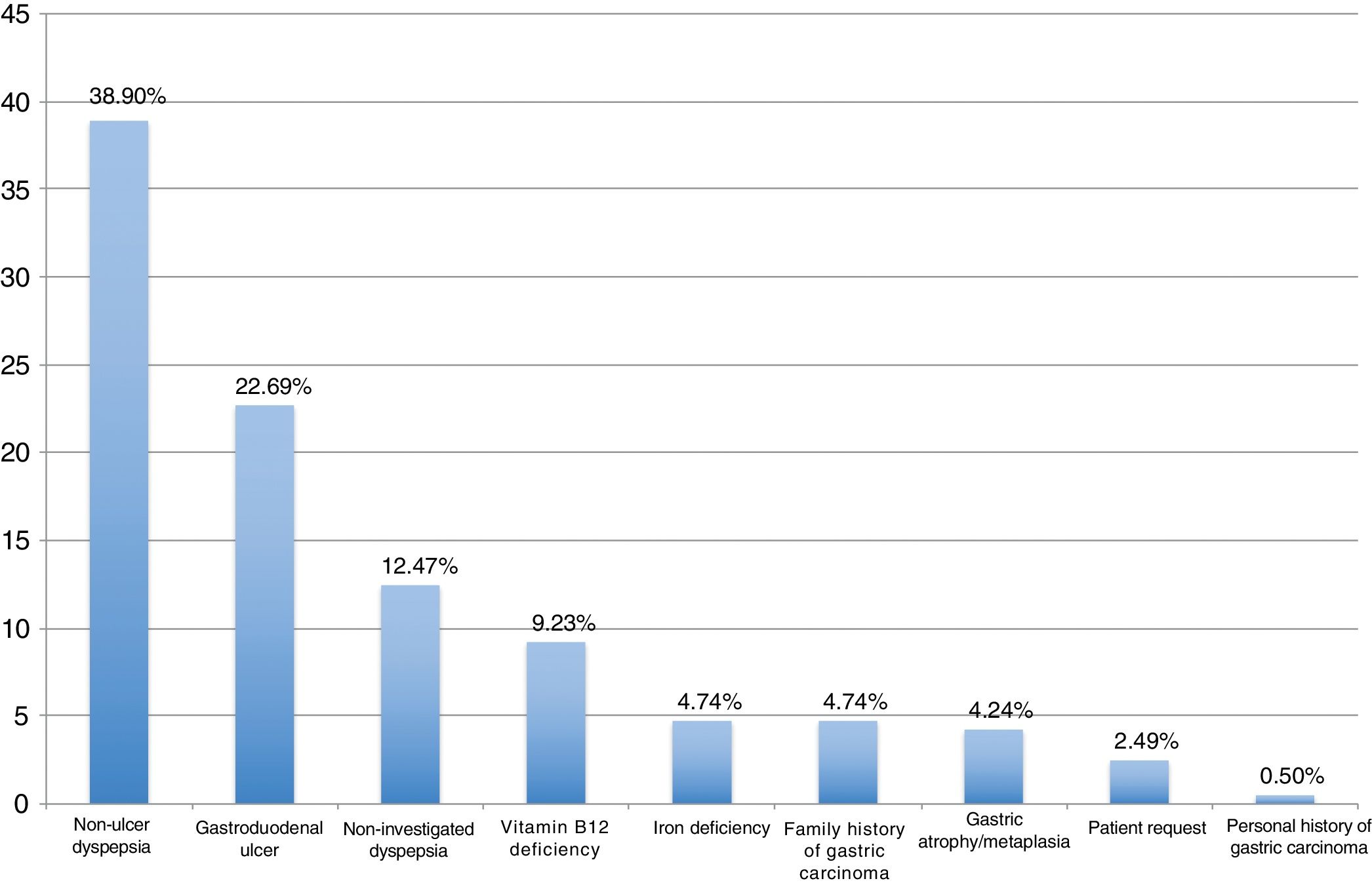
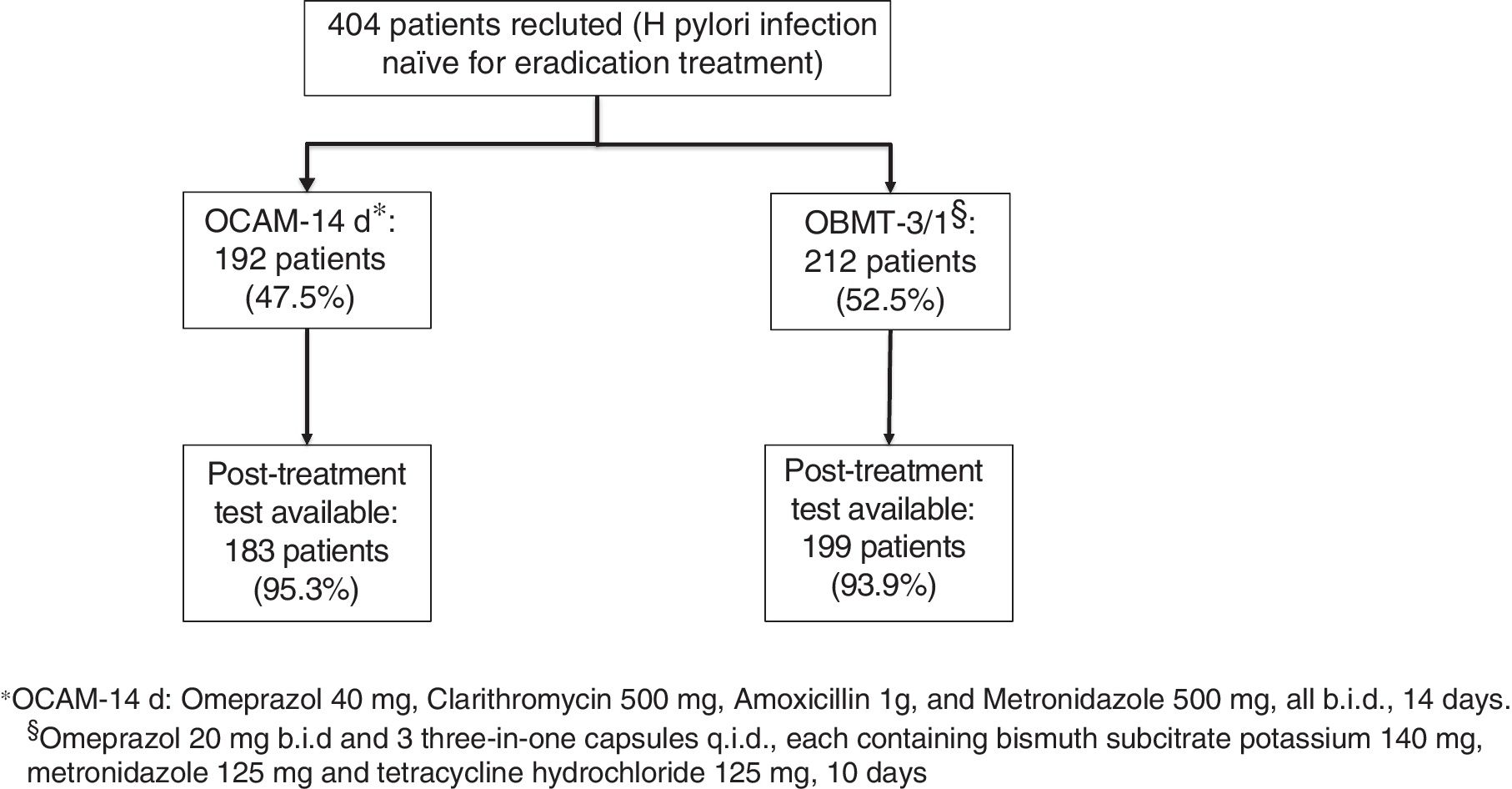
ResultsBetween September 2016 and April 2018, 404 patients were recruited, all of them naïve for H. pylori eradication treatment. The median age was 53 years (range 18–84), 62.87% were women and 24.60% were smokers. Among eradication indications, the most frequent were non-ulcer dyspepsia associated with H. pylori (38.90%), gastroduodenal ulcer (22.69%), and non-investigated dyspepsia (12.47%) (Fig. 1).
The first infection's diagnosis was carried out by means of 13C-urea breath test in 252 (62.38%) patients, through histological examination of gastric biopsies in 139 (34.11%), and urease test was used in 13 (3.22%). OCAM for 14 days to 192 patients (47.5%) and OBMT-3/1 for 10 days to another 212 patients (52.5%) were prescribed as eradication therapy (Fig. 2). There were no statistically significant differences between the two groups of treatment in sex, age, tobacco consumption and indication to eradicate distribution (Table 1). The method used to check the eradication was 13C-urea breath test in 375 (98.18%) cases; only in 7 (1.83%) cases in which the protocol described in Methods has been followed, was the histological examination of gastric biopsies considered valid for this target.
Distribution of demographic characteristics and indications to H. pylori eradication, depending on the treatment regimen.
| OCAMn (%) | OBMT-3/1n (%) | p-value | |
|---|---|---|---|
| Gender | 1.0000 | ||
| Male | 121 (63.02%) | 133 (62.74%) | |
| Female | 71 (36.98%) | 79 (37.26%) | |
| Age (mean; range) | 52 [20–78] | 54 [18–84] | 0.8006 |
| Smoking | 0.1373 | ||
| No or ex | 129 (71.67%) | 156 (78.79%) | |
| Current | 51 (28.33%) | 42 (21.21%) | |
| Indication to H. pylori treatment | |||
| Non-ulcer dyspepsia | 77 (40.74%) | 79 (37.26%) | 0.5417 |
| Gastroduodenal ulcer | 42 (22.22%) | 49 (23.11%) | 0.9257 |
| Non-investigated dyspepsia | 29 (15.34%) | 21 (9.91%) | 0.1352 |
| Vitamin B12 deficiency | 14 (7.41%) | 23 (10.85%) | 0.3097 |
| Iron deficiency | 8 (4.23%) | 11 (5.19%) | 0.8303 |
| Family history of gastric carcinoma | 5 (2.65%) | 14 (6.60%) | 0.1037 |
| Gastric atrophy/metaplasia | 6 (3.17%) | 11 (5.19%) | 0.4527 |
| Patient request | 6 (3.17%) | 4 (1.89%) | 0.5264 |
| Personal history of gastric carcinoma | 2 (1.06%) | 0 (0.00%) | 0.2215 |
OCAM: omeprazol, claritromycin, amoxicillin and metronidazole for 14 days.
OBMT-3/1: omeprazol, bismuth, metronidazole and tetracycline, 3-in-1 capsules, for 10 days.
Regarding treatment efficacy, in 382 patients (183 with OCAM and 199 with OBMT-3/1) the result of the post-treatment test was available, being negative in 165 cases (90.16%; CI95%: 84.90–94.07) in OCAM group, and in 189 (95.45%; CI95%: 91.55–97.90) in OBMT-3/1 group, with a statistically significant difference (p=0.0479). In ITT analysis the eradication percentages were 85.94% (CI95%: 80.20–90.52) with OCAM and 88.21% (CI95%: 83.09–92.22) with OBMT-3/1 (p=0.5950), whilst in PP analysis they were 91.12% (CI95%: 85.78–94.95) and 96.17% (CI95%: 92.28–98.45), respectively (p=0.0827).
The assessment of adherence to the treatment could be performed in 386 patients (185 with OCAM and 201 with OBMT-3/1), observing very minor differences between the two lines in compliance over 80% and 90%. The adherence to treatment considered as optimum (≥90%) was reached in 91.35% with OCAM and in 92.04% with (OBMT-3/1) (p=0.9519), whilst compliance more than 80% was achieved in 94.59% and 95.02% (p=1.0000), respectively.
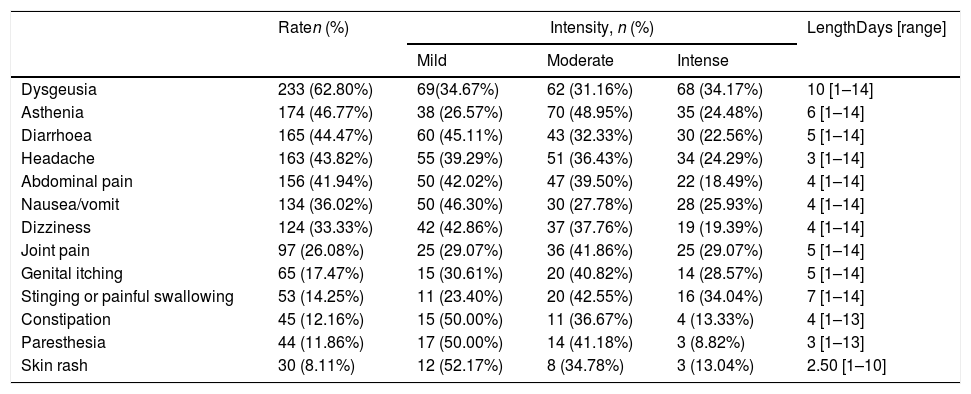
More than 90% (91.36%) of patients reported adverse effects (AE) during treatment period (data available in 382 patients), and more than 50% of cases displayed 4 or more AE (range: 0–13), with dysgeusia (62.80%), asthenia (4677%) and diarrhoea (44.47%) being the most common. The median of maximum duration of all AE was 9 days (range: 1–14). Dysgeusia had a median duration of 10 days, and the second longest AE (7 days) was stinging or painful swallowing (Table 2). Almost half of patients (48.39%) marked some of their AE as intense, 32.90% reported only moderate AE, and for 18.71% were mild.
Total rate, intensity and length of side effects.
| Raten (%) | Intensity, n (%) | LengthDays [range] | |||
|---|---|---|---|---|---|
| Mild | Moderate | Intense | |||
| Dysgeusia | 233 (62.80%) | 69(34.67%) | 62 (31.16%) | 68 (34.17%) | 10 [1–14] |
| Asthenia | 174 (46.77%) | 38 (26.57%) | 70 (48.95%) | 35 (24.48%) | 6 [1–14] |
| Diarrhoea | 165 (44.47%) | 60 (45.11%) | 43 (32.33%) | 30 (22.56%) | 5 [1–14] |
| Headache | 163 (43.82%) | 55 (39.29%) | 51 (36.43%) | 34 (24.29%) | 3 [1–14] |
| Abdominal pain | 156 (41.94%) | 50 (42.02%) | 47 (39.50%) | 22 (18.49%) | 4 [1–14] |
| Nausea/vomit | 134 (36.02%) | 50 (46.30%) | 30 (27.78%) | 28 (25.93%) | 4 [1–14] |
| Dizziness | 124 (33.33%) | 42 (42.86%) | 37 (37.76%) | 19 (19.39%) | 4 [1–14] |
| Joint pain | 97 (26.08%) | 25 (29.07%) | 36 (41.86%) | 25 (29.07%) | 5 [1–14] |
| Genital itching | 65 (17.47%) | 15 (30.61%) | 20 (40.82%) | 14 (28.57%) | 5 [1–14] |
| Stinging or painful swallowing | 53 (14.25%) | 11 (23.40%) | 20 (42.55%) | 16 (34.04%) | 7 [1–14] |
| Constipation | 45 (12.16%) | 15 (50.00%) | 11 (36.67%) | 4 (13.33%) | 4 [1–13] |
| Paresthesia | 44 (11.86%) | 17 (50.00%) | 14 (41.18%) | 3 (8.82%) | 3 [1–13] |
| Skin rash | 30 (8.11%) | 12 (52.17%) | 8 (34.78%) | 3 (13.04%) | 2.50 [1–10] |
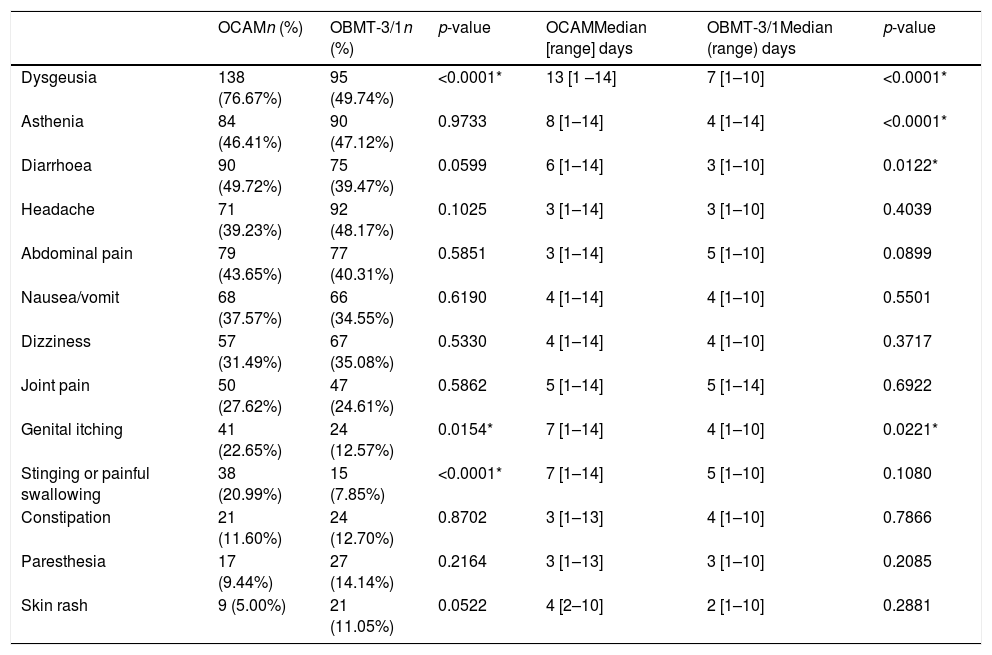
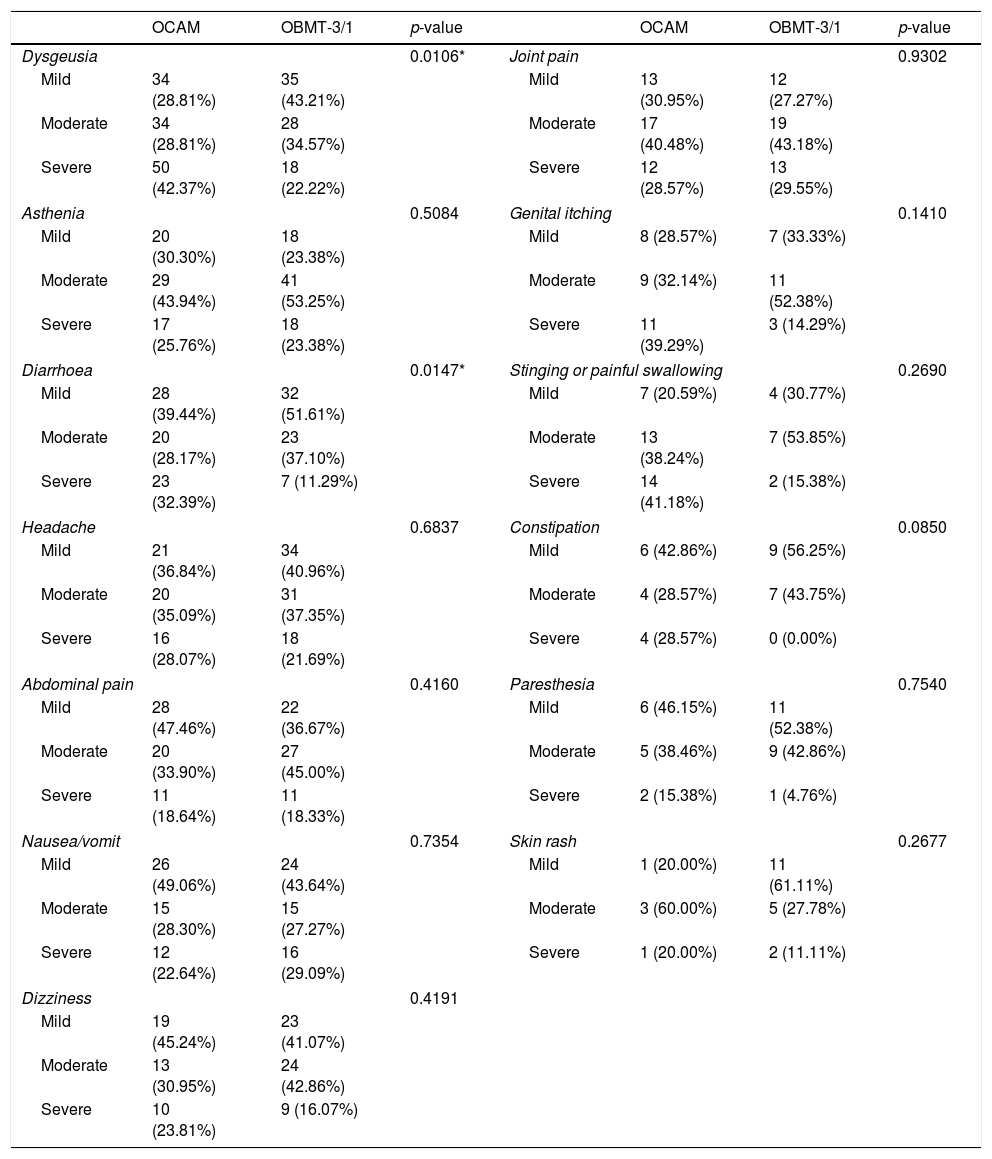
The group with OCAM showed a higher median of AE duration than that with OBMT-3/1 (12 vs 7 days; p<0.0001), and a higher rate of subjects with an intense AE (59.33% with OCAM vs 38.12% with OBMT-3/1; p<0.0001). Neither in median number (4 with OCAM vs 3 with OBMT-3/1; p=0.0809) nor in global presence of AE (94.02% vs 88.89%; p=0.1091) were any differences observed between the groups. In addition, Tables 3 and 4, and Fig. 3 describe the differences in presence, duration and intensity of each AE, depending on the regimen used.
Rate and length distribution of side effects depending on the treatment chosen.
| OCAMn (%) | OBMT-3/1n (%) | p-value | OCAMMedian [range] days | OBMT-3/1Median (range) days | p-value | |
|---|---|---|---|---|---|---|
| Dysgeusia | 138 (76.67%) | 95 (49.74%) | <0.0001* | 13 [1 –14] | 7 [1–10] | <0.0001* |
| Asthenia | 84 (46.41%) | 90 (47.12%) | 0.9733 | 8 [1–14] | 4 [1–14] | <0.0001* |
| Diarrhoea | 90 (49.72%) | 75 (39.47%) | 0.0599 | 6 [1–14] | 3 [1–10] | 0.0122* |
| Headache | 71 (39.23%) | 92 (48.17%) | 0.1025 | 3 [1–14] | 3 [1–10] | 0.4039 |
| Abdominal pain | 79 (43.65%) | 77 (40.31%) | 0.5851 | 3 [1–14] | 5 [1–10] | 0.0899 |
| Nausea/vomit | 68 (37.57%) | 66 (34.55%) | 0.6190 | 4 [1–14] | 4 [1–10] | 0.5501 |
| Dizziness | 57 (31.49%) | 67 (35.08%) | 0.5330 | 4 [1–14] | 4 [1–10] | 0.3717 |
| Joint pain | 50 (27.62%) | 47 (24.61%) | 0.5862 | 5 [1–14] | 5 [1–14] | 0.6922 |
| Genital itching | 41 (22.65%) | 24 (12.57%) | 0.0154* | 7 [1–14] | 4 [1–10] | 0.0221* |
| Stinging or painful swallowing | 38 (20.99%) | 15 (7.85%) | <0.0001* | 7 [1–14] | 5 [1–10] | 0.1080 |
| Constipation | 21 (11.60%) | 24 (12.70%) | 0.8702 | 3 [1–13] | 4 [1–10] | 0.7866 |
| Paresthesia | 17 (9.44%) | 27 (14.14%) | 0.2164 | 3 [1–13] | 3 [1–10] | 0.2085 |
| Skin rash | 9 (5.00%) | 21 (11.05%) | 0.0522 | 4 [2–10] | 2 [1–10] | 0.2881 |
* Statistical significance (p<0.05).
Intensity of side effects depending on the treatment chosen.
| OCAM | OBMT-3/1 | p-value | OCAM | OBMT-3/1 | p-value | ||
|---|---|---|---|---|---|---|---|
| Dysgeusia | 0.0106* | Joint pain | 0.9302 | ||||
| Mild | 34 (28.81%) | 35 (43.21%) | Mild | 13 (30.95%) | 12 (27.27%) | ||
| Moderate | 34 (28.81%) | 28 (34.57%) | Moderate | 17 (40.48%) | 19 (43.18%) | ||
| Severe | 50 (42.37%) | 18 (22.22%) | Severe | 12 (28.57%) | 13 (29.55%) | ||
| Asthenia | 0.5084 | Genital itching | 0.1410 | ||||
| Mild | 20 (30.30%) | 18 (23.38%) | Mild | 8 (28.57%) | 7 (33.33%) | ||
| Moderate | 29 (43.94%) | 41 (53.25%) | Moderate | 9 (32.14%) | 11 (52.38%) | ||
| Severe | 17 (25.76%) | 18 (23.38%) | Severe | 11 (39.29%) | 3 (14.29%) | ||
| Diarrhoea | 0.0147* | Stinging or painful swallowing | 0.2690 | ||||
| Mild | 28 (39.44%) | 32 (51.61%) | Mild | 7 (20.59%) | 4 (30.77%) | ||
| Moderate | 20 (28.17%) | 23 (37.10%) | Moderate | 13 (38.24%) | 7 (53.85%) | ||
| Severe | 23 (32.39%) | 7 (11.29%) | Severe | 14 (41.18%) | 2 (15.38%) | ||
| Headache | 0.6837 | Constipation | 0.0850 | ||||
| Mild | 21 (36.84%) | 34 (40.96%) | Mild | 6 (42.86%) | 9 (56.25%) | ||
| Moderate | 20 (35.09%) | 31 (37.35%) | Moderate | 4 (28.57%) | 7 (43.75%) | ||
| Severe | 16 (28.07%) | 18 (21.69%) | Severe | 4 (28.57%) | 0 (0.00%) | ||
| Abdominal pain | 0.4160 | Paresthesia | 0.7540 | ||||
| Mild | 28 (47.46%) | 22 (36.67%) | Mild | 6 (46.15%) | 11 (52.38%) | ||
| Moderate | 20 (33.90%) | 27 (45.00%) | Moderate | 5 (38.46%) | 9 (42.86%) | ||
| Severe | 11 (18.64%) | 11 (18.33%) | Severe | 2 (15.38%) | 1 (4.76%) | ||
| Nausea/vomit | 0.7354 | Skin rash | 0.2677 | ||||
| Mild | 26 (49.06%) | 24 (43.64%) | Mild | 1 (20.00%) | 11 (61.11%) | ||
| Moderate | 15 (28.30%) | 15 (27.27%) | Moderate | 3 (60.00%) | 5 (27.78%) | ||
| Severe | 12 (22.64%) | 16 (29.09%) | Severe | 1 (20.00%) | 2 (11.11%) | ||
| Dizziness | 0.4191 | ||||||
| Mild | 19 (45.24%) | 23 (41.07%) | |||||
| Moderate | 13 (30.95%) | 24 (42.86%) | |||||
| Severe | 10 (23.81%) | 9 (16.07%) | |||||
Finally, only in 19 patients (4.93% of 386 patients with known data) did the AE cause partial or total treatment discontinuation, happening in 9 patients with OCAM (4.86%) and 10 with OBMT-3/1 (4.98%) (p=1.000). In 315 patients the information about drug intake to alleviate the AE was available, and it occurred in 90 patients (23.38%), without differences between eradication treatments (p=0.43). The most used were analgesics (37 patients), probiotics (16 patients) and prokinetics (12 patients).
DiscussionThe current study compares the efficacy, therapeutic adherence and appearance of adverse effects in real practice, in two first-line therapies (OCAM for 14 days and OBMT-3/1 for 10 days) recommended for H. pylori eradication by the last European Consensus for geographical areas with CLA-resistance over 15% and MET-resistance under 40%.13 The available research about antibiotic resistance in Europe confirms that these conditions are fulfilled in Central and Southern European countries, and also in Spain.6,14–16
One main factor for the H. pylori rate of resistance to antibiotics is their prior use, specially macrolides,6,7 increasing eradication failure rate with regimens containing CLA. In a large Asian cohort who underwent eradication therapy, 6.5% had a macrolide intake history,23 while in a Spanish series the number reached 17.94%, with OBMT-3/1 eradication failure rates very close to those presented in our study.24 The prior use of macrolide in our series was not determined, and therefore not considered in the choice of regimen.
The eradication efficacy observed in our study in tested therapies was 85.94% with OCAM and 88.21% with OBMT-3/1 by ITT, whilst in PP analysis they were 91.20% and 96.17%, respectively, tending to be higher for OBMT-3/1 but not reaching statistically significant differences. These percentages were very close to those previously reported. Molina-Infante et al., 25 in a randomised trial performed in Spain and Italy, treated 170 naive patients for H. pylori therapy with OCAM for 14 days. They found cure rates of 91.7% (95% CI 88%–95%) and 96.1% (95% CI 93%–99%) in ITT and PP analysis respectively; 6% discontinued therapy because of AE, mainly abdominal pain, vomiting and diarrhoea. A South Korean trial,26 however, achieved with first line regimens success rates by ITT less than 80%, using non-bismuth concomitant therapy, with 43.7% of patients reporting AE, mainly epigastric pain, diarrhoea and dysgeusia.
In a retrospective multicenter study Miehlke et al.27 assessed the efficacy in first or second/salvage lines, of the three-in-one capsule bismuth quadruple therapy, in daily routine practice, in 322 patients from a high CLA-resistance European area, reaching H. pylori eradication rate of 96.7% (95% CI 94.6%–98.8%) in PP analysis. By modified-ITT analysis the rate was as high as 95.0% (95% CI 94.92%–95.08%), but considering the patients lost for follow-up as treatment failures (as we considered in our study) the rate was only 83%. In a similar way, Tursi et al.28 in another European multicenter study, included for the ITT analysis all subjects who took at least one dose of study medication, obtaining a rate of efficacy of 90.5% (95% CI 80.8–1.0).
Malfertheiner et al.19 led a multicentric trial using OBMT for 10 days in one of its arms. They reported AE in 47% of patients, what was similar to that observed with classical triple therapy (OCA: Omeprazol, Clarithromycin and Amoxicillin, for 7 days). The most common AE were dyspepsia, abdominal pain, diarrhoea and dysgeusia. These AE were not classified by their duration, and regarding severity, 5% of cases with OBMT and 7% with OCA were considered as severe, with less than 2% causing treatment termination. Tursi et al.28 retrospectively reviewed the AE with Pylera®, notifying their presence in 15.8% of patients (the most common being abdominal pain, nausea, dysgeusia and asthenia), but only in 1.5% did they lead to stopping of the treatment. Nevertheless, in another retrospective study by Zagari et al.,29 using the same antibiotic regimen, discontinuation of treatment for AE was reported in 6.1% of subjects, uninfluenced for concomitant probiotics intake.
Recently, two European studies comparing OBMT-3/1 and OCAM as first-line treatments have been published.30,31 One Spanish non-randomised study, including 50 patients treated with OCAM and 54 with OBMT-3/1 did not find significant differences in efficacy, compliance and side effect rates (AE with OCAM 56.0% vs 46.3% with OBMT-3/1, p=0.323), with a percentage of discontinuation of treatment due to AE close to 2% in both groups. In the second study, randomised and carried out in Italy, a few more than 60 patients by arm were included in ITT analysis, with similar results in side effects and efficacy rates, although with OBMT-3/1 the cure rate tended to be lower than that with OCAM (85.2% vs 95.2%; p=0.07) and the early interruption of treatment due to AE was higher (9.8% vs 1.6%; p=0.046). In both studies the low number of patients enrolled in each arm could justify the lack of significant differences in the side effect rate, and even the variability in the early treatment interruption rate with OBMT-3/1 as well, which in the Italian study is almost double those found in our study, where 199 patients were treated with the same regime. In neither of these two studies was the duration of AE reported nor was the intensity of them categorised in each treatment group. These aspects are critical in our analysis in order to find differences in the safety profile.
We collected the AE in a prospective way by means of a specific questionnaire filled out during the treatment intake period, which made the acknowledgement and note of symptoms by the patient easier. Under these conditions we found at least one AE in 91.36% of subjects, but the majority were mild. Less than 50% were classified as intense by the patients and in less than 5% did a total or partial discontinuation of prescribed treatment occur. Dysgeusia, asthenia and diarrhoea were the most frequent AE, which is in agreement with that observed in the studies mentioned above. There were no differences in the presence/absence of AE between the two therapeutic lines, but the average length and the frequency of more severe AE were both higher in the OCAM group. We could expect that this regime, being longer (14 vs 10 days), provoked an increased duration of the AE, since, as was reported by Gisbert et al.,32 these appear only during the drug intake period in the majority of cases. Despite the high number of AE and the differences shown between the therapies, compliance must be considered optimal and similar for the two groups.
One strength of our study is to be a large comparative and prospective head-to-head analysis between two first-line eradication treatments proposed to be applied in high CLA-resistance areas. Other strengths are its performance under real-life conditions, its analysis of the efficacy, adherence to treatment and AE in detail, and consideration of less assessed issues such as length and severity classification of symptoms. Among the limitations of the study may be considered the non-randomised design, although the lack of significant differences in the patients’ basal characteristics do make the existence of bias that could change the sense of the results unlikely.
In conclusion, our study shows that in real practice the two first-line therapies proposed by the last European Consensus for H. pylori eradication in geographical areas with high CLA-resistance,13 achieve similar efficacy and optimal compliances (both over 90%). However the OBMT-3/1 regime reaches a higher safety profile, reporting a lower mean duration of side effects and lower rate of intense ones.
Disclosure statementJavier Alcedo reports that he has received support for providing speeches from Astra-Zeneca, Abbvie, Allergan, Casen-Fleet, Janssen, Norgine, Ferring Pharmaceuticals, and has participated in advisory boards of Abbvie, Allergan, Norgine, Janssen, and Ferring Pharmaceuticals. The remaining authors disclosed no financial relationship relevant to this publication.
Allergan Inc. funded the article charges related to statistical analysis, while not having access to the results. Neither honoraria nor payments were made for authorship. All authors met the ICMJE authorship criteria.
Conflict of interestThe authors declare that they have no conflict of interest.
We wish to acknowledge the help provided by Allergan Inc. by funding the article charges related to statistical analysis.