Endoscopy units are considered to be at an increased risk of infection by SARS-CoV-2. Our aim is to assess the correlation between pre-endoscopic screening with reverse-transcription-polymerase-chain-reaction (RT-PCR) in asymptomatic individuals scheduled for elective endoscopy and the epidemiological data published by the local Health Administration.
Patients and methodsObservational retrospective study collecting the results of our screening strategy spanning June/2020–June/2021, the effective potential growth (EPG), an index measuring the outbreak risk, and the 7 and 14-day cumulative incidence (CI). Indication, delay and the findings of the endoscopic examinations were registered for RT-PCR positive patients.
ResultsA total of 5808 tests were performed, yielding 125 positive results (2.15%). All positive tests occurred in weeks of high/very high risk (EPG>100) with the highest monthly rate being 9.36%, recorded in January/2021. A significant correlation (rho=0.796; p<0.001) between weekly positive rates and EPG was observed, and a significantly lower weekly number of positive tests was recorded when EPG<100. Planning the screening strategy one week ahead according to EPG>100 would have avoided up to 826 tests with only one positive result to account for. One hundred and thirteen individuals tested positive and 89 endoscopies were delayed. The most common findings were colon polyps, colorectal cancer and gastric metaplasia. Oncological diagnosis was delayed 50±3 days.
ConclusionsNo positive RT-PCR test were registered out of high-risk periods. Epidemiological administrative data in the preceding two weeks showed a significant correlation with screening results and could be useful to plan pre-endoscopic screening and avoid unnecessary tests.
La actividad endoscópica comporta un riesgo elevado de transmisión de la infección por SARS-CoV-2. Nuestro objetivo es valorar la correlación entre el cribado pre-endoscópico con reverse-transcription-polymerase-chain-reaction (RT-PCR) en individuos asintomáticos programados para una endoscopia ambulatoria y los datos epidemiológicos publicados por el departamento de salud pública.
Pacientes y métodosEstudio observacional retrospectivo de los resultados del cribado realizado entre junio/2020 y junio/2021. Se registró el índice de crecimiento potencial (ICP) y la incidencia acumulada a 7 y 14 días. En los pacientes con RT-PCR positiva se registraron indicación, demora y hallazgos endoscópicos.
ResultadosSe identificaron un total de 5.808 test con 125 resultados positivos (2,15%). Todos los resultados positivos se registraron en semanas consideradas de alto/muy alto riesgo (ICP>100), con una tasa máxima de positivos del 9,26% en enero/2021. Se observó una correlación significativa (rho=0,796; p<0,001) entre la tasa de positivos y el ICP, así como una diferencia estadísticamente significativa con la tasa de positivos en las semanas con el ICP<100. Planificar el cribado acorde al ICP>100 en la semana previa, habría evitado hasta 876 test, con un único resultado positivo. Ciento trece pacientes resultaron positivos y se demoraron 89 endoscopias; los hallazgos más frecuentes fueron pólipos, cáncer colorrectal y metaplasia gástrica. El diagnóstico oncológico se demoró 50±3 días.
ConclusionesNo se registraron RT-PCR positivas fuera de los períodos de alto riesgo. Los datos epidemiológicos de las dos semanas anteriores mostraron una correlación significativa con los resultados del cribado y podrían ser útiles para planificar el mismo y evitar pruebas innecesarias.
As of November 2021, infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causing coronavirus disease 2019 (COVID-19) has occurred in more than 290,000,000 people and caused over 5,400,000 deaths globally.1 Although the most commonly described symptoms are fever and cough, a recent meta-analysis found that gastrointestinal symptoms were reported in up to 18% of patients with COVID-19.2 Although the transmission of SARS-CoV-2 occurs most commonly through the inhalation of aerosols or droplets, some studies have described that angiotensin converting enzyme 2 receptor (a membrane receptor supporting the entry of SARS-CoV-2 into the cell) is expressed in the glandular digestive epithelia, leading to the possibility of fecal-oral transmission.3,4
Endoscopy units are considered to be at an increased risk of infection by SARS-CoV-2, not only because of the inhalation of airborne droplets or conjunctival contact but also because of potential fecal-oral transmission.5 For this reason, most of the endoscopy units worldwide decreased (or even stopped) their activity during the first wave of the pandemia, delaying many elective examinations and the diagnosis of potentially life-threatening conditions.6,7 In fact, a substantial reduction in the diagnosis of new gastrointestinal cancers has already been reported.8 National and international endoscopy societies have proposed recommendations regarding the reopening of endoscopy units. These recommendations include pre-procedure testing, health-professional personal protective equipment (PPE) and prioritization rules for endoscopic examinations. Nevertheless, there is currently no consensus on the use of routine SARS-CoV-2 testing with reverse transcription polymerase chain reaction (RT-PCR) or antigen-tests for asymptomatic individuals scheduled for endoscopic procedures. The European Society of Gastrointestinal Endoscopy and the European Society of Gastroenterology and Endoscopy Nurses and Associates proposed that all patients be required to provide a negative viral test (RT-PCR) performed within 48h before endoscopy or either documentation of full vaccination status or recovery from COVID-19 infection within the past 6 months.9 The Spanish Gastroenterology societies proposed checking every patient for fever and asking about symptoms such as fever or cough before endoscopy.10 Finally, the American Gastroenterological Association recommended against routine testing irrespective of the vaccination status of patients.11
Nasopharyngeal and throat swabs are widely used to perform SARS-CoV-2 RT-PCR tests on asymptomatic patients as it identifies viral RNA rather than viral infection status. A meta-analysis including seven studies observed a sensitivity of 73.3% and a specificity of 95% for this test.12 Recent studies have demonstrated that SARS-CoV-2 transmission is feasible from people who suffered asymptomatic infection (SARS-CoV-2 detected but symptoms not developed) or during the pre-symptomatic phase (SARS-CoV-2 detected before symptom onset).13 However, more studies evaluating selective screening strategies to optimize the resources are needed, considering that universal screening with RT-PCR may not be available in all centers.
The primary aim of this study is to describe the results of routine pre-endoscopic screening in our endoscopy unit and to correlate them with the administrative epidemiological data of our background population.
Patients and methodsWe conducted an observational, retrospective study of routine pre-endoscopic SARS-CoV-2 RT-PCR screening carried out at our center from June 2020 to June 2021 (comprising from the second to the fourth pandemic wave in Spain).
All adult individuals scheduled for an outpatient elective endoscopic procedure underwent mandatory SARS-CoV-2 RT-PCR testing (Panther Fusion, Hologic and Procleix Panther, Grifols, Spain) performed at our center by nasopharyngeal swab in the 48–72h prior to endoscopy. In order to ensure all individuals were asymptomatic, they were contacted by telephone at least 7 days before the endoscopy and they were specifically advised not to assist to the mandatory RT-PCR if they presented cough, fever, dyspnea, asthenia or anosmia from the day of the calling until the day of the RT-PCR. In case they presented any of these symptoms, the procedure were adjourned 30 days.
All endoscopic procedures were performed using PPE that included full body gown, FFP (filtering face pieces) type 2 face mask, gloves, face shield and cap. Patients who tested positive were individually evaluated to reschedule the procedure at least 30 days later and only high-priority indications were performed regardless of the test result.
Individuals with a negative test answered a survey on the day of the endoscopy regarding COVID-19 related-symptoms and close contacts within the previous week.
RT-PCR results and time between the screening test and the endoscopic procedure were registered. Demographic data, SARS-CoV-2 vaccination status, SARS-CoV-2 related symptoms (fever, cough, dyspnea, asthenia and anosmia), indication for endoscopy, delay (if needed) and endoscopic findings were all recorded in the case of a positive RT-PCR result. Epidemiological data in our healthcare area (northern metropolitan area of Barcelona) were obtained from the Catalan Health Department website.14 During the study period, 7 and 14-day cumulative incidence (CI) and the weekly effective potential growth index (EPG) were retrieved.
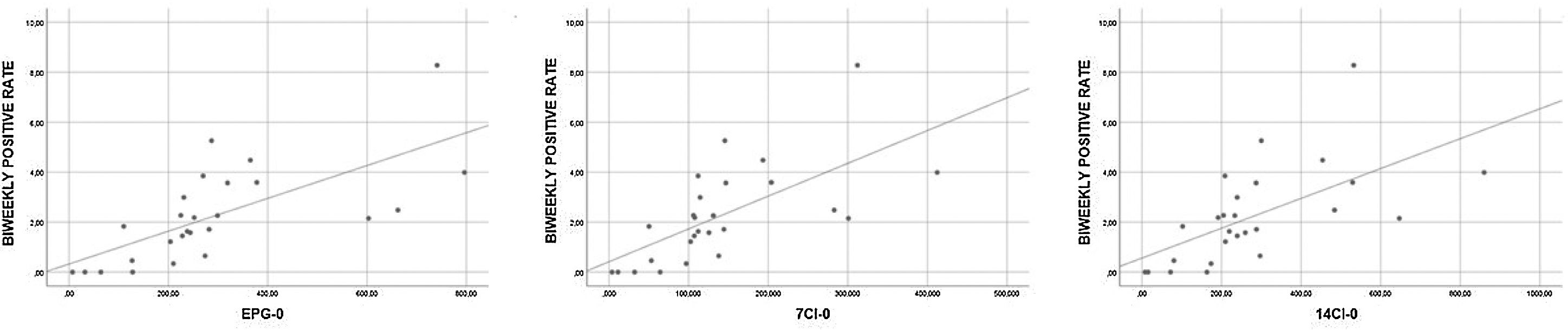
The EPG was developed by a multidisciplinary team to measure the probability of new disease outbreaks based on the 14-day CI and disease propagation rate (R0) which was used to define local restrictions.15 The risk of disease outbreak is stratified into five main stages: low risk (EPG<30), low-medium risk (EPG 30–70), medium-high risk (EPG 70–100), high risk (EPG 100–200) and very high risk (EPG>200). We grouped the results of RT-PCR tests in a weekly and biweekly manner. In relation to the scheduled endoscopic procedure, the EPG was retrieved for three different time points: the first day of the same week (EPG-0) of the scheduled procedure, the first day of the previous week (EPG-1) and the first day of the week two weeks before (EPG-2). CI values for seven (7CI) and 14 days (14CI) were retrieved in the same way (7CI-0, 7CI-1, 7CI-2, 14CI-0, 14CI-1, 14CI-2).
Quantitative variables are presented as mean and standard deviation or median and interquartile range (IQR), depending on their distribution. Categorical variables are presented as raw numbers and proportions. Categorical variables were compared using the Chi2-squared test. The Saphiro–Wilk test was performed to examine the normality of continuous variables. To evaluate the correlation between continuous variables, Spearman tests were performed. All statistical analyses were performed using SPSS statistics software (Version 25.0, IBM).
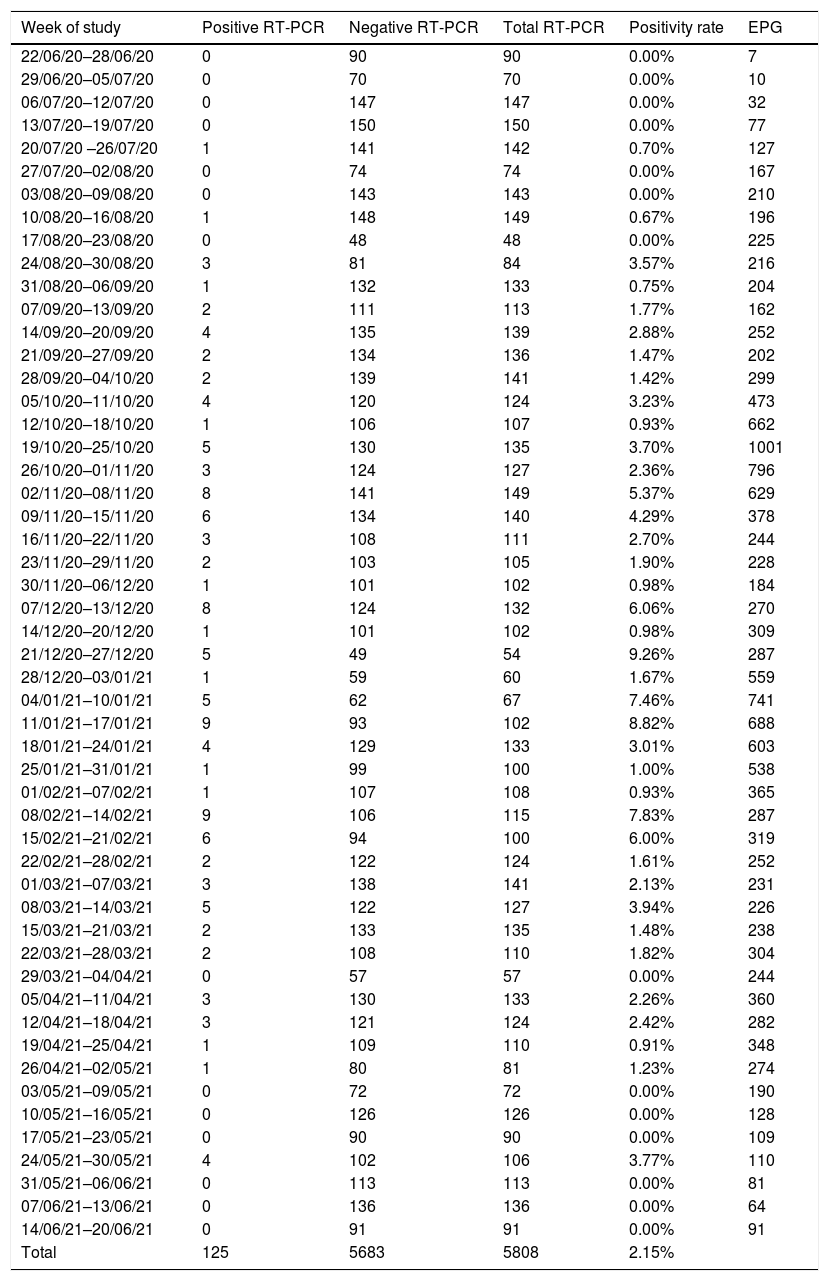
ResultsResults of the routine screening and correlation with epidemiological indexesDuring the study period (52 weeks), 5808 RT-PCR tests were performed on 5112 individuals as screening for a planned endoscopic procedure. Of these, 125 tests (0.15%) on 113 individuals yielded positives. A mean number of 113±27.78 tests were performed per week and the mean time interval from RT-PCR to endoscopy was 2.46±0.76 days. The rate of positive tests was 1.71% (49/2859) during the second pandemic wave (from the end of June 2020 to the beginning of December 2020), 4.10% (60/1456) during the third wave (December 2020 to March 2021) and 1.08% (16/1484) during the fourth (March 2021 to June 2021). The highest weekly rate of positive tests was observed in January 2021, reaching 9.36% (5/54). All retrieved data is exposed in Table 1.
RT-PCR results and EPG value.
| Week of study | Positive RT-PCR | Negative RT-PCR | Total RT-PCR | Positivity rate | EPG |
|---|---|---|---|---|---|
| 22/06/20–28/06/20 | 0 | 90 | 90 | 0.00% | 7 |
| 29/06/20–05/07/20 | 0 | 70 | 70 | 0.00% | 10 |
| 06/07/20–12/07/20 | 0 | 147 | 147 | 0.00% | 32 |
| 13/07/20–19/07/20 | 0 | 150 | 150 | 0.00% | 77 |
| 20/07/20 –26/07/20 | 1 | 141 | 142 | 0.70% | 127 |
| 27/07/20–02/08/20 | 0 | 74 | 74 | 0.00% | 167 |
| 03/08/20–09/08/20 | 0 | 143 | 143 | 0.00% | 210 |
| 10/08/20–16/08/20 | 1 | 148 | 149 | 0.67% | 196 |
| 17/08/20–23/08/20 | 0 | 48 | 48 | 0.00% | 225 |
| 24/08/20–30/08/20 | 3 | 81 | 84 | 3.57% | 216 |
| 31/08/20–06/09/20 | 1 | 132 | 133 | 0.75% | 204 |
| 07/09/20–13/09/20 | 2 | 111 | 113 | 1.77% | 162 |
| 14/09/20–20/09/20 | 4 | 135 | 139 | 2.88% | 252 |
| 21/09/20–27/09/20 | 2 | 134 | 136 | 1.47% | 202 |
| 28/09/20–04/10/20 | 2 | 139 | 141 | 1.42% | 299 |
| 05/10/20–11/10/20 | 4 | 120 | 124 | 3.23% | 473 |
| 12/10/20–18/10/20 | 1 | 106 | 107 | 0.93% | 662 |
| 19/10/20–25/10/20 | 5 | 130 | 135 | 3.70% | 1001 |
| 26/10/20–01/11/20 | 3 | 124 | 127 | 2.36% | 796 |
| 02/11/20–08/11/20 | 8 | 141 | 149 | 5.37% | 629 |
| 09/11/20–15/11/20 | 6 | 134 | 140 | 4.29% | 378 |
| 16/11/20–22/11/20 | 3 | 108 | 111 | 2.70% | 244 |
| 23/11/20–29/11/20 | 2 | 103 | 105 | 1.90% | 228 |
| 30/11/20–06/12/20 | 1 | 101 | 102 | 0.98% | 184 |
| 07/12/20–13/12/20 | 8 | 124 | 132 | 6.06% | 270 |
| 14/12/20–20/12/20 | 1 | 101 | 102 | 0.98% | 309 |
| 21/12/20–27/12/20 | 5 | 49 | 54 | 9.26% | 287 |
| 28/12/20–03/01/21 | 1 | 59 | 60 | 1.67% | 559 |
| 04/01/21–10/01/21 | 5 | 62 | 67 | 7.46% | 741 |
| 11/01/21–17/01/21 | 9 | 93 | 102 | 8.82% | 688 |
| 18/01/21–24/01/21 | 4 | 129 | 133 | 3.01% | 603 |
| 25/01/21–31/01/21 | 1 | 99 | 100 | 1.00% | 538 |
| 01/02/21–07/02/21 | 1 | 107 | 108 | 0.93% | 365 |
| 08/02/21–14/02/21 | 9 | 106 | 115 | 7.83% | 287 |
| 15/02/21–21/02/21 | 6 | 94 | 100 | 6.00% | 319 |
| 22/02/21–28/02/21 | 2 | 122 | 124 | 1.61% | 252 |
| 01/03/21–07/03/21 | 3 | 138 | 141 | 2.13% | 231 |
| 08/03/21–14/03/21 | 5 | 122 | 127 | 3.94% | 226 |
| 15/03/21–21/03/21 | 2 | 133 | 135 | 1.48% | 238 |
| 22/03/21–28/03/21 | 2 | 108 | 110 | 1.82% | 304 |
| 29/03/21–04/04/21 | 0 | 57 | 57 | 0.00% | 244 |
| 05/04/21–11/04/21 | 3 | 130 | 133 | 2.26% | 360 |
| 12/04/21–18/04/21 | 3 | 121 | 124 | 2.42% | 282 |
| 19/04/21–25/04/21 | 1 | 109 | 110 | 0.91% | 348 |
| 26/04/21–02/05/21 | 1 | 80 | 81 | 1.23% | 274 |
| 03/05/21–09/05/21 | 0 | 72 | 72 | 0.00% | 190 |
| 10/05/21–16/05/21 | 0 | 126 | 126 | 0.00% | 128 |
| 17/05/21–23/05/21 | 0 | 90 | 90 | 0.00% | 109 |
| 24/05/21–30/05/21 | 4 | 102 | 106 | 3.77% | 110 |
| 31/05/21–06/06/21 | 0 | 113 | 113 | 0.00% | 81 |
| 07/06/21–13/06/21 | 0 | 136 | 136 | 0.00% | 64 |
| 14/06/21–20/06/21 | 0 | 91 | 91 | 0.00% | 91 |
| Total | 125 | 5683 | 5808 | 2.15% |
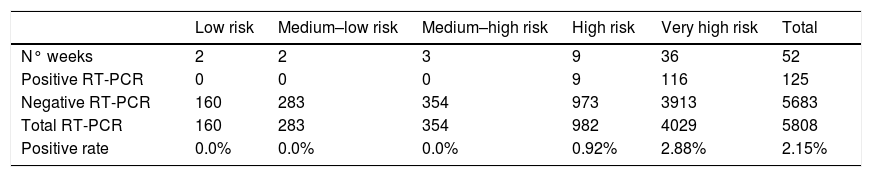
Thirty-six weeks of the study period were considered to be very high risk, nine weeks high risk, three medium-high risk, two medium-low risk and two low risk. All positive RT-PCR tests were registered during high or very high-risk weeks. The cumulative rate of positive tests in high-risk weeks was 0.92% (9/982), and 2.88% (116/3913) in very high-risk weeks (a summary of the stratified RT-PCR results is provided in Table 2). The cumulative proportion of positive RT-PCR tests was significantly lower when screening was performed during low, medium-low and medium-high risk weeks compared to high and very high-risk weeks (0% vs. 2.50%, p<0.001).
RT-PCR results according to EPG risk index.
| Low risk | Medium–low risk | Medium–high risk | High risk | Very high risk | Total | |
|---|---|---|---|---|---|---|
| N° weeks | 2 | 2 | 3 | 9 | 36 | 52 |
| Positive RT-PCR | 0 | 0 | 0 | 9 | 116 | 125 |
| Negative RT-PCR | 160 | 283 | 354 | 973 | 3913 | 5683 |
| Total RT-PCR | 160 | 283 | 354 | 982 | 4029 | 5808 |
| Positive rate | 0.0% | 0.0% | 0.0% | 0.92% | 2.88% | 2.15% |
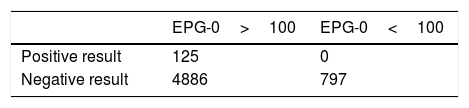
According to EPG-1 (one week before the planned endoscopy), seven weeks had an EPG-1<100 (low, medium-low or medium-high risk) with 1/826 positive tests (0.12%). The remaining 45 weeks had an EPG-1>100 (high or very high risk) with 124/4982 positive tests (2.49%). Using the same approach, according to EPG-2 (two weeks before the planned endoscopy), seven weeks had an EPG-2<100 with 1/764 positive tests (0.13%) and 45 weeks had an EPG-2>100 with 124/5044 (2.46%). The cumulative rate of positive tests in high or very-high risk weeks was significantly higher taking into account both EPG-1 and EPG-2 (2.49% vs. 0.02%, p<0.001 and 2.45 vs. 0.13%, p<0.001 respectively). Table 3 shows the Chi2-squared test contingency tables of the three groups.
Chi2-squared contingency tables of risk-stratified weeks.
| EPG-0>100 | EPG-0<100 | |
|---|---|---|
| Positive result | 125 | 0 |
| Negative result | 4886 | 797 |
| EPG-1>100 | EPG-1<100 | |
|---|---|---|
| Positive result | 124 | 1 |
| Negative result | 4858 | 825 |
| EPG-2>100 | EPG-2<100 | |
|---|---|---|
| Positive result | 124 | 1 |
| Negative result | 4920 | 763 |
EPG-0: Effective potential growth of the same week. EPG-1: Effective potential growth from one week before. EPG-2: Effective potential growth from two weeks before.
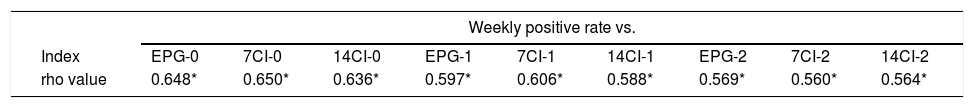
The correlation between positive rates (grouped weekly and biweekly) and epidemiological data (7CI, 14CI and EPG) in three different moments (current week, previous week and two weeks prior) was statistically significant in all the Spearman correlation tests (results of all Spearman tests can be seen in Table 4). The biweekly positive rate and the epidemiological data of the corresponding week (7CI-0, 14CI-0 and EPG-0) showed a strong correlation (rho>0.7, p<0.001), whereas the remaining tests showed moderate correlations (rho 0.5–0.7, p<0.001). Fig. 1 showing scatterplots with strong correlation.
Spearman correlation test results.
| Weekly positive rate vs. | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Index | EPG-0 | 7CI-0 | 14CI-0 | EPG-1 | 7CI-1 | 14CI-1 | EPG-2 | 7CI-2 | 14CI-2 |
| rho value | 0.648* | 0.650* | 0.636* | 0.597* | 0.606* | 0.588* | 0.569* | 0.560* | 0.564* |
As mentioned above, 113 individuals tested positive during the study period, only one of whom (0.88%) was fully vaccinated, while 20 (17.7%) developed COVID-19 symptoms following the test (cough 50%; dyspnea, asthenia and anosmia 25% each). Endoscopy was adjourned in 83/113 (73.5%) individuals with a median of 50 (IQR 41–68) days; in 6/113 (5.3%) endoscopy was performed with PPE, and postponed and not yet performed in 24/113 (21.2%). Pathological findings were observed in 24/89 (27%) of the endoscopic examinations, 21/83 (25.3%) in delayed explorations and 3/6 (50%) in non-delayed ones. The most common diagnostics among delayed examinations were colonic polyps (nine patients), colorectal cancer (CRC) (three patients) and gastric metaplasia (three patients). In non-delayed examinations, the pathological findings were a cytomegalovirus colitis in a patient diagnosed with ulcerative colitis, a new diagnosis of ulcerative colitis and non-specific ileitis. Oncological diagnosis was delayed in three patients (renamed as patients A, B, C), with a mean delay of 57±32.6 days. Patient A was a 43-year-old male with no past medical history. A colonoscopy was requested because of constipation and was delayed for 31 days. The diagnosis was a stage IV CRC (pT4N1M1) and urgent placement of an endoprosthesis was required. Patient B was a 77-year-old male with metabolic syndrome, coronary heart disease and skin squamous cell carcinoma undergoing oral antiplatelet treatment. The indication for the endoscopic examination was iron-deficiency anemia, which was delayed 94 days. The final diagnoses were stage II CRC (pT2N0M0) and synchronic stage III pancreatic carcinoma (pT2N1M0). Although colonic and pancreatic surgeries, as well as adjuvant chemotherapy, were performed, the patient passed away nine months later. Patient C was a 77-year-old female with arterial hypertension and congenital cognitive impairment. The endoscopic indication was the scheduled resection of a large polyp in the rectum which was delayed 48 days; ultimately only biopsies were taken according to optical diagnostic criteria. The final diagnosis was stage IV CRC (pT1N0M1) for which the patient underwent surgical treatment.
DiscussionNational and international gastrointestinal endoscopy societies mainly recommended against universal RT-PCR screening SARS-CoV-2 in asymptomatic individuals before endoscopy based on the scarce available evidence, the heterogeneity of the published studies and some additional drawbacks such as cost, delays in care, impact on cancer burden and the consequences of false positive and negative results.9–11
Even though the adoption of protective measures substantially diminishes the risk of transmission during the endoscopic procedure, the identification of asymptomatic carriers allows for the protection from infection of not only health professionals but also of health care users who share common facilities.16 Beyond the immediate consequences of disease transmission, sick leaves by health professionals usually lead to increased delays in the provision of health care services. Similar studies evaluating the prevalence of pre-endoscopic positive RT-PCR among asymptomatic individuals in different periods of the pandemic exhibited low rates ranging from 0% to 1.5%,17–21 contrasting with our study in which a total prevalence of 2.15% was observed. This difference may be explained by the markedly higher number of tests and longer study period of our study.
From an economic point of view, Corral et al.22 conducted a study evaluating different screening strategies and concluded that RT-PCR screening before endoscopy is an effective strategy to restart endoscopic activity, underlining that disease prevalence is the most determining factor to support its effectiveness.
In our cohort, no positive RT-PCR results were registered out of high-risk periods. We found a significant correlation between the rate of positive tests and local epidemiological data, not only from the collected data on the same week, as expected, but also from the data of one and two weeks before the screening test. These findings may allow us to plan the screening based on local epidemiological indexes, particularly EPG since it takes into account not only 14CI but also R0.
Hypothetically, if the screening had been planned according to EPG>100 took from 1 week before and 2 weeks before (EPG-1 and EPG-2, respectively), up to 826 and 763 tests would have been avoided, which would have accounted for only one positive result during the weeks considered of lower risk. Even though it is a non-negligible number of avoidable tests, identifying one positive result meant testing over 46 individuals.
We also aimed to assess the impact of our screening strategy on oncological diagnostic delays, one of the most feared consequences of the pandemic. Despite the potential negative impact of any screening strategy for SARS-CoV-2, the individual evaluation of patients with positive RT-PCR and the implementation of prioritization strategies resulted in oncological diagnostic delay in three cases among almost 6000 planned endoscopies. Some authors suggest that delays as short as three months in cancer diagnosis might significantly impact on patients’ long-term survival.23,24 These statements are based on a computational model simulation and take into account only advanced and aggressive cancers. In contrast, cancers with overall good prognosis, such as colorectal, kidney and breast cancers, would not suffer such an impact on short delays. Similarly, Zorzi et al. described in a recent study that delays shorter than 270 days on CRC diagnosis after a positive result of fecal immunochemical test screening did not have a significant impact on staging nor on long-term survival. Even though study populations are not comparable, these remarks suggest that potential COVID-19 screening-related diagnostic delays might not have such a significant impact if strict prioritization strategies are implemented. In our study population, oncological diagnoses were delayed a maximum of 94 days and deeper evaluation of the individual medical record might even have resulted in even shorter delays.
Our study has several limitations. First, our screening strategy did not take into account whether individuals were fully or partially vaccinated, previous history of COVID-19 infection or recent negative antigen or RT-PCR tests, factors that may well decrease pre-test probability and should probably be taken into account in an optimal screening strategy design. Second, the epidemiological data from the Catalan Health Department are calculated based on a population tested mainly under a consistent suspicion of SARS-CoV-2 infection and, therefore, it is not strictly equivalent to our asymptomatic cohort. Finally, several health professionals in our endoscopy unit were diagnosed with COVID-19 or asymptomatic SARS-CoV-2 infection. Although most of these infections seemed to be related to contacts outside our unit, no particular investigations beyond testing co-workers were performed to rule out the unit as the origin of the infection. In contrast, the length and sample size of our study, together with the availability of a symptomatic and epidemiological survey of all the individuals with negative screening, are factors that strengthen our results.
In conclusion, to the best of our knowledge, this study constitutes the largest retrospective study to date of universal RT-PCR SARS-CoV-2 pre-endoscopic screening in asymptomatic patients spanning one year and three waves of the pandemic. Planning tests based on the EPG index, due to its correlation with screening results, may be a cost-effective strategy to optimize material and human resources and avoid unnecessary tests. Nonetheless, two full years have passed since the COVID-19 was described and the scenario we currently face is far different from the one on which the screening programs were designed and thus they should be periodically updated.
Ethics standard statementThe study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. The data was anonymized from the authors according to data protection regulation.
FundingNone to declare.
Conflicts of interestNone to declare.