Fistulizing perianal Crohn's disease (CD) is a phenotype with a poor prognosis. There are no studies in our country. Our objective is to determine the clinical, sociodemographic and treatment characteristics of perianal fistulizing CD in a Colombian multicenter registry.
Materials and methodsA retrospective, multicenter observational study was carried out, with prospective data collection, in the main reference centers for Inflammatory Bowel Disease (IBD) in the country. Continuous variables were expressed as medians and interquartile ranges. The categorical outcome variables were compared by the chi-square test.
Results65 patients with perianal fistulizing CD were documented, with a median age of appearance of perianal fistula of 31.0 years (Range: 24–42), Predominantly in men (61.5%, H: M ratio, 1.4: 1). Complex perianal fistulas were more frequent than simple ones (75.35% vs 24.6%). Regarding medical treatment, 66.2% of the patients received antibiotics, 64.6% steroids, 78.5% biological therapy, 47.7% non-cutting setons, and 46.2% required surgical management, other than seton placement. Only 29.2% achieved complete remission of the fistula, and 9.2% of the patients ended up in a definitive colostomy. CD patients with complex fistulas received more biological therapy, compared to CD patients with simple fistulas (84.8% vs 56.3%, P: 0.038).
ConclusionsPerianal fistulizing CD has a poor prognosis in our setting, only 3 out of 10 patients achieve complete remission despite treatment. A multidisciplinary management is essential for the comprehensive management of this difficult pathology.
La Enfermedad de Crohn (EC) perianal fistulizante es un fenotipo de mal pronóstico. No existen estudios en nuestro medio. Nuestro objetivo es determinar las características clínicas, sociodemográficas y tratamiento de la EC perianal fistulizante en un registro multicéntrico Colombiano.
Materiales y métodosSe realizó un estudio observacional multicéntrico, retrospectivo, con recolección prospectiva de la información, en los principales centros de referencia de Enfermedad Inflamatoria Intestinal (EII) del país. Las variables continuas se expresaron como medianas y rangos inter-cuartiles. Las variables de resultado categóricas, fueron comparados por la prueba de chi-cuadrado.
ResultadosSe documentaron 65 pacientes con EC perianal fistulizante, con una mediana de edad de aparición de fistula perianal de 31.0 años (Rango: 24–42.), predominando en hombres (61.5%, Razón H: M, 1.4:1). Las fístulas perianales complejas fueron más frecuentes que las simples (75.35% vs 24.6%). En cuanto al tratamiento médico, 66.2% de los pacientes recibieron antibióticos, 64.6% esteroides, 78.5% terapia biológica, 47.7% setones no cortantes, y 46.2% requirieron manejo quirúrgico, diferente a la colocación de setones. En solo 29.2% se logró remisión completa de la fístula, y 9.2% de los pacientes terminaron en colostomía definitiva. Los pacientes con EC con fístulas complejas recibieron más terapia biológica, comparado con pacientes con EC y fístulas simples (84.8% vs 56.3%, P: 0.038).
ConclusionesLa EC fistulizante perianal es de mal pronóstico en nuestro medio, solo 3 de cada 10 pacientes logran remisión completa a pesar de tratamiento. Un manejo multidisciplinario es fundamental para el manejo integral de esta difícil patología.
Crohn's disease (CD) is a chronic inflammatory disease of the gastrointestinal tract of multifactorial aetiology, which primarily affects the colon and small intestine.1 In recent years, an increase in its incidence and prevalence has been detected both worldwide and in Latin America.2,3 A recent study in Colombia found that the prevalence of CD had increased from 5.04 per 100,000 patients in 2010 to 8.93 per 100,000 in 2017.4 Perianal fistula is a disabling phenotype of CD. A fistula is a tract with pus and/or granulation tissue between two epithelial surfaces covered by a fibrous wall. Primary tracts are connections between the internal and external orifice, and there are secondary tracts that are blind extensions.5 Due to a defect in the epithelial barrier, peptides associated with pathogenic bacteria enter the intestinal mucosa, initiating an inflammatory process, causing increased expression of cytokines and the transformation of intestinal epithelial cells into invasive mesenchymal cells (myofibroblasts), which leads to the formation of the fistula.6 Perianal fistulising CD is associated with medical, psychological, social and sexual complications that alter the quality of life of patients suffering from it.7 A study in the population of Olmsted County in Minnesota, USA, found that perianal fistulising involvement occurs in 12% of CD patients one year after diagnosis, increasing to 26% at 20 years, and it may be the only manifestation of CD in up to 5% of patients.8 A recent update of the same study found a cumulative incidence of perianal fistula of 24%, 30–40 years after the diagnosis of CD.9 In a recent Colombian registry study of 2,291 patients with inflammatory bowel disease (IBD), 9.8% of perianal fistulising CD was found in 456 patients with CD.10
Recently, there have been some important advances both in diagnostic methods and in the treatment of perianal fistulising CD. Antibiotics, especially ciprofloxacin and metronidazole, are used as first-line treatment in combination with thiopurines and tumour necrosis factor inhibitors (anti-TNFs) and often accompanied by surgery.11,12 Publications in Latin America are scarce, so we decided to unify experiences and carry out this study in different IBD reference centres in the country. The objective of our study was to determine the epidemiological and clinical characteristics, treatment and outcomes of this CD phenotype in Colombia.
Materials and methodsType of studyThe study was a multicentre, retrospective, observational study with prospective data collection. Data collected from patients older than 16 years diagnosed with perianal fistulising CD in the main IBD centres in the country were recorded.
Study populationAll the patients with perianal fistulising CD who attended the emergency or outpatient departments or were hospitalised for IBD from the following reference hospitals in Colombia were included: Hospital Pablo Tobón Uribe [Pablo Tobón Uribe Hospital] in Medellín, Fundación Santa Fe [Fundación Santa Fe University Hospital] in Bogotá, Cirujanos Unidos de Manizales [United Surgeons of Manizales], Clínica Colombia de Bogotá [Colombia Clinic of Bogotá] and Gastroadvanced IPS [health service provider specialising in gastrointestinal disorders] in Bogotá. Diagnoses of CD in medical records were reviewed, taking the following codes into account: K500, CD of the small intestine; K501, CD of the large intestine; K508, other types of CD; K509, unspecified CD; and K63, perianal fistula.
Diagnostic criteriaThe guidelines of the European Crohn's and Colitis Organisation (ECCO) for the diagnosis of CD state that there is no gold standard for the diagnosis of CD and that it must be established by different clinical, biomarker, imaging, endoscopic and histopathological criteria. They do not recommend the use of genetic or serological testing for diagnostic purposes.13 For the diagnosis of CD, we used the presence of two or more of the aforementioned criteria.14 The location and behaviour of the CD were determined according to the Montreal classification.15 For the classification of perianal fistulas in CD, the Parks classification was used, which describes five types of perianal fistulas: superficial, intersphincteric, transsphincteric, suprasphincteric and extrasphincteric.16 A modification to this classification was published by the American Gastroenterological Association (AGA) in 2003, which grouped them into simple (superficial) or complex (intersphincteric, transsphincteric, suprasphincteric and extrasphincteric). The simple ones are superficial, with a single external orifice, located below the dentate line, and have no complications such as abscesses, rectal or vaginal involvement and also have no anal stenosis. The complex ones are located above the dentate line, can have multiple external orifices and present complications such as abscesses.5
The diagnostic method for perianal fistulising CD was determined taking into account the recommendations of the AGA: physical examination, clinical examination under anaesthesia, magnetic resonance imaging (MRI) of the pelvis or rectal endosonography.5 It is also very important to assess whether there is endoscopic rectal involvement in all patients with perianal fistulising CD and to determine its extension in order to establish its severity, management and prognosis.17 The terminology of the Toronto consensus statement was used to define symptomatic remission, when there is no pain and drainage of the fistulous orifice with gentle pressure, and symptomatic response, when there is improvement in pain and decreased drainage by the fistulous orifice, in the absence of remission.18
Data collectionAll the investigators reviewed the related medical records and the variables to be measured were recorded in a database built in the Microsoft Excel® program. The variables collected for each patient were: (1) age at diagnosis of CD; (2) age at diagnosis of perianal fistula; (3) sex of the patient; 94) origin; (5) behaviour of the CD; (6) location of the CD; (7) specialty of the treating physician: (8) perianal fistula diagnostic method; (9) number of perianal fistulas; (10) time of fistula onset with respect to CD diagnosis; (11) cumulative medical treatment for CD (antibiotics, steroids, azathioprine, 6-mercaptopurine, methotrexate, tacrolimus, biological therapy, antibiotics); (12) type of biological therapy (adalimumab, certolizumab, infliximab, vedolizumab, ustekinumab); (13) stem cell injection; (14) topical anti-TNF injection; (15) placement of setons; (16) surgical treatment (rectal mucosa flap, ligation of fistulous tract, ileostomy, temporary colostomy, definitive colostomy + proctectomy); (17) evolution of the perianal fistula (persistence, partial remission, complete remission, recurrence, definitive colostomy), (18) frequency of hospitalisation; (19) mortality; and (20) cancer associated with perianal fistula.
Statistical analysisThe continuous variables were expressed as medians and interquartile ranges (IQRs) or simple ranges, as appropriate. The categorical variables were summarised as counts and percentages. The categorical outcome variables were compared using the chi-square test, and the odds ratio (OR) with its respective 95% confidence interval (95% CI) was estimated. All reported p-values were based on two-tailed tests with a statistical significance level set at 0.05. All the statistical analyses were performed using the SPSS Statistics® 18.0 program (IBM, Armonk, New York, USA).
Ethical considerationsThe project researchers adhered to the international principles of the Declaration of Helsinki (2013 version, Fortaleza, Brazil) and the Colombian Ministry of National Health resolution 008430 of 1993. According to this resolution, this study was a risk-free investigation since the patients' medical records were reviewed and the confidentiality and privacy of the information collected were guaranteed.
ResultsData were obtained from 65 patients with perianal fistulising CD, 40 (61.5%) of them men and 25 (38.4%) women. Of the total number of patients with CD in the different participating centres, 14.3% presented perianal fistulising involvement. The median age at study entry was 41.2 years (IQR: 32–53), the median age at diagnosis of CD was 29.0 years (IQR: 21–36), and the median age of onset of perianal fistula was 31.0 years (IQR: 24–42). In 36 patients (55.4%), the perianal fistula was diagnosed at the same time as the CD, in 27 patients (41.5%) it was after the diagnosis of CD (41.5%), and in 2 patients (3.1 %) perianal fistula was the only manifestation of CD. The median follow-up time was 11 years (IQR: 5.5−16), the minimum follow-up time was 1 year and the maximum time was 44 years (Table 1).
Demographic characteristics.
| Characteristics | Values |
|---|---|
| Age (n = 65) | |
| Median, (IQR) years | 41.2 (31.8−52.8) |
| Age at diagnosis of Crohn's disease (n = 65) | |
| Median, (IQR) years | 29.0 (21.0−36.0) |
| Age at diagnosis of perianal fistula (n = 65) | |
| Median, (IQR) years | 31.0 (24.0−42.5) |
| Mean follow-up time, (IQR) years | 11 (5.5−16) |
| Male gender, n (%) | 40 (61.5) |
| Department of origin (n = 65) | n (%) |
|---|---|
| Antioquia | 27 (41.5) |
| Bogotá | 25 (38.5) |
| Caldas | 8 (12.3) |
| Risaralda | 2 (3.07) |
| Guajira | 1 (1.53) |
| Foreign | 2 (3.07) |
IQR, interquartile range.
The distribution of CD behaviour in patients with perianal fistula was: 37 (56.9%) penetrating, 18 (27.7%) stricturing, and only 10 (15.4%) with inflammatory behaviour. Regarding the location of the CD, 34 patients (52.3%) with perianal fistula had ileocolonic involvement, 22 patients (33.8%) colonic involvement and 9 patients (13.8%) had isolated ileal involvement. In 39 patients (60.0%), perianal CD was accompanied by proctitis, 30 patients (46.2%) presented perianal abscesses, and 3 patients (4.6%) developed faecal incontinence.
The most used method for the initial diagnosis of perianal fistulising CD was: physical examination in 40 cases (61.5%); MRI of the pelvis in 18 cases (27.7%); rectal endosonography in 4 cases (6.2%); and clinical examination under anaesthesia in only 1 patient (1.5%).
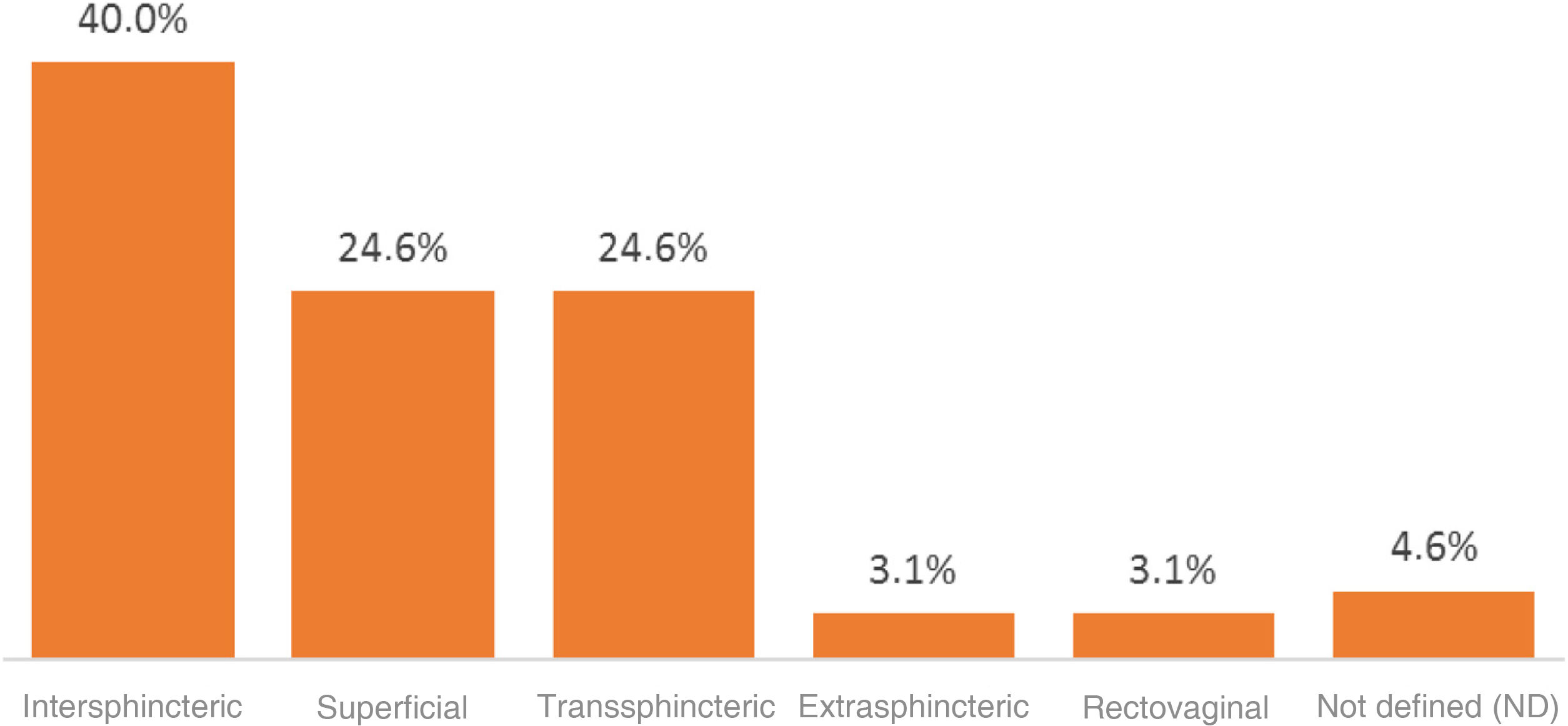
Regarding the type of perianal fistula found, 16 cases (24.6%) were superficial and 49 cases (75.3%) were complex. Within this group, 26 cases (40%) were intersphincteric, 16 cases (24.6%) were transsphincteric, 2 cases (3.1%) were extrasphincteric and 2 cases (3.1%) were recto-vaginal. In 3 patients (4.6%), the type of complex fistula could not be documented (Fig. 1). In 67.7% of the patients, the fistulas were single and in 32.2% they were multiple.
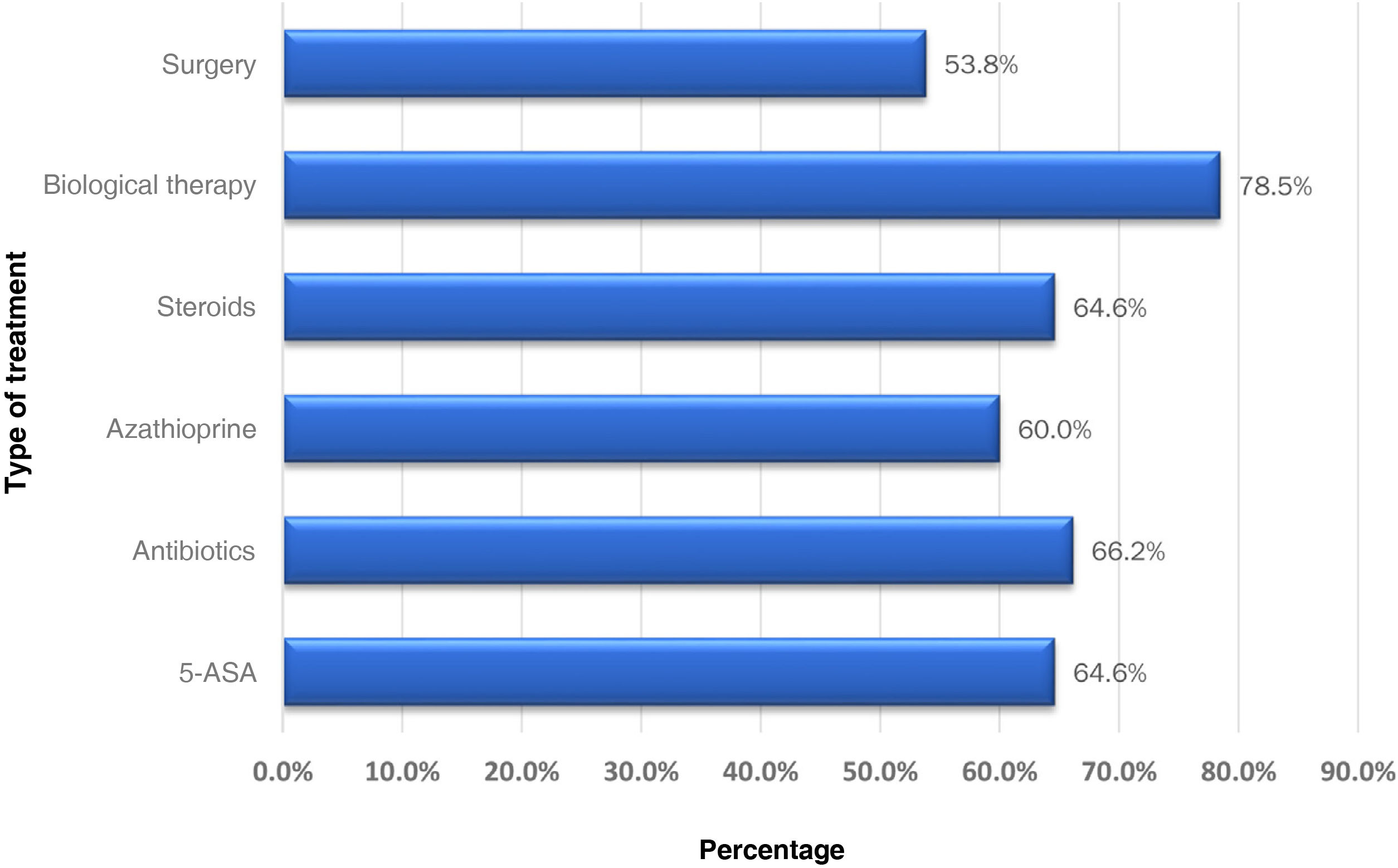
The patients were managed by a gastroenterologist in 35.4% of the cases, a coloproctologist in 26.4%, gastroenterologist surgeons in 12.3%, and only 24.6% of cases were managed jointly by a gastroenterologist and a coloproctologist. Regarding the medical treatment of patients with CD and perianal fistula, 42 patients (64.6%) received 5-ASA drugs and 43 patients (66.2%) were treated with antibiotics, most of them receiving a combination of ciprofloxacin and metronidazole (79.0%). Moreover, 42 patients (64.6%) received steroids, most of them (90%) prednisolone, and 39 patients (60%) received azathioprine. Furthermore, 51 (78.5%) of the total number of patients with perianal fistulising CD received biological therapy, 31 (60.8%) infliximab, 18 (35.3%) adalimumab and 2 (3.9%) received vedolizumab. Twenty of these patents (30.8%) received a second biologic drug, the most used agent being adalimumab in 14 patients (70%). Six (9.2%) received a third biologic, vedolizumab was used in 3 patients (50%), certolizumab in 1 patient, and ustekinumab in another (Fig. 2). Regarding the management of the 19 patients who achieved symptomatic remission, there was no significant difference when they were managed by a gastroenterologist or a coloproctologist (57.9% vs. 42.1%; p = 0.824). Neither was there any difference between the percentage of steroid use (69.6% vs. 64.7%; p = 0.746) and biological therapy (78.3% vs. 88.2%; p = 0.368) by gastroenterologists and coloproctologists for the management of patients with perianal CD.
Additionally, in patients with proctitis, steroids were used more frequently compared to those without proctitis, with this difference being significant (74.4% vs. 50.0%; p = 0.04). However, there was no significant difference regarding the use of biological therapy (84.6% vs. 69.2%; p = 0.139) or regarding the need for surgery (61.5% vs. 50.0%; p = 0.176).
Non-cutting setons were used in 31 (47.7%) of the cases, single in 18 (27.7%) and multiple in 13 (20.0%). Another type of surgery for perianal fistula was performed in 35 patients (46.2%) with perianal fistulising CD: in 12 patients (18.5%) mucosal flap surgery was performed; in 9 patients (13.8%) ileostomy; in 6 patients (6.2%) ligation of the internal tract; in 3 patients (4.6%) closure of the internal orifice; in 3 patients (4.6%) temporary colostomy; and in 3 patients (4.6%) definitive colostomy with proctectomy. Additionally, 37 of the 65 patients (56.9%) with perianal fistulising CD underwent surgery for involvement of their CD other than perianal fistulising involvement. Forty-seven (72.3%) required hospitalisation due to their CD. There were no cases of death associated with perianal fistula, and 1 patient had rectal cancer.
Regarding the evolution of the perianal fistula, complete remission was achieved in 19 patients (29.2%), partial remission in 22 patients (33.8%), and the fistula persisted in 16 patients (24.6%). Patients with simple perianal fistulas achieved more symptomatic remission than patients with complex fistulas (56.3% vs. 19.6%; p = 0.021). A total of 6 patients (9.2%) eventually had a definitive colostomy.
Patients with complex fistulas received more biological therapy than patients with simple perianal fistulas; this difference was significant (84.8% vs. 56.3%; p = 0.038), although there was no difference in surgical management (97.8% vs. 81.3%, p = 0.083).
DiscussionThis is the first multicentre study in a Latin American country to describe the epidemiological, anatomical and clinical characteristics, treatment and outcome of patients with perianal fistulising CD. First, the average age at diagnosis of patients with perianal fistulising CD (29.0 years) was lower than the age at diagnosis of CD (45.7 years) in the Colombian Registry of IBD,10 confirming that an earlier age of diagnosis of CD has a poor prognosis for this type of complications, as has been previously described.19 Additionally, in the Colombian Registry of IBD, a predominance of women was found in CD (1.4:1), while in this study the perianal fistulising CD phenotype was more frequent in men (1.6:1; 61.5%), similar to that described in a Korean registry (CONNECT) of 1,193 patients with perianal fistula, where 70.3% were men,20 and to that published in an Israeli cohort with 1,530 individuals, 65% of them male.21 Furthermore, the presence of perianal fistula was the only manifestation of CD in 3.1% of patients, similar to the 5% reported in another study in Minnesota (USA).8
In the universal literature, a reported 70%–80% of perianal fistulas in CD are complex.7 In this study, 75.3% were complex, the majority being intersphincteric and transsphincteric, similar to the result reported by Parks et al. in their study of 400 patients with perianal fistula.9 Moreover, 60% of the patients presented proctitis due to CD, while only 13.8% had isolated involvement of the small intestine due to CD, similar to that described in the universal literature.22
This study showed that only 24.6% of the cases of perianal fistulising CD were managed jointly by a gastroenterologist and a coloproctologist, which is not the ideal scenario, since the vast majority of patients present complex perianal fistulas, a manifestation that requires medical/surgical management in most cases, as recommended by different scientific associations.12,13,18
Although the international guidelines do not recommend the use of 5-ASA drugs in patients with CD,11,13 in this study it 64.6% of patients were found to receive these drugs. The administration of steroids proved to be quite similar, which were used in 64.6% of the subjects, despite the fact that studies have suggested that they are risk factors for abscess formation in individuals with perianal fistulising CD.22 78.5% of the patients required biological therapy. The best clinical evidence for the management of perianal fistulising CD has been provided by tumour necrosis factor inhibitor drugs (anti-TNFs). A recent systematic review and meta-analysis of 27 controlled studies found that anti-TNFs (mainly infliximab and adalimumab) are effective in inducing and maintaining response and remission of perianal fistulas (RR: 2.01; 95% CI: 1.36–2.97).23 However, the ideal procedure would be to monitor levels of anti-TNFs to optimise treatment, given that patients with perianal fistulising CD require higher therapeutic levels of anti-TNFs.24 In addition, new biological therapies such as vedolizumab and ustekinumab have recently shown efficacy in closing perianal fistulas in CD.25–27
Non-cutting setons were required in 47.7% of the patients, single setons in 18 patients (27.7%) and multiple setons in 13 patients (20.0%). Meanwhile, 46.2% received another type of surgical management, the most common being mucosal flap surgery, used in 18.5% of cases. Complete remission of perianal fistulas was achieved in only 29.2% of patients during follow-up.
A Spanish study found results similar to our own in 313 patients with perianal fistulising CD from 11 hospitals in Madrid, also with a predominance of males (55%), in which 80% of the fistulas were complex, 62% of patients were treated with antibiotics, 71% with thiopurines, 54% with anti-TNFs, and 66% underwent surgery.28 In a Dutch study involving 232 subjects with perianal fistulising CD, 78% of which were complex fistulas, 78.0% received medical treatment and 53.2% surgical treatment, and only 37.0% achieved complete remission of the perianal fistulas during follow-up, findings which are very similar to our own study.29
Although cases of the local injection of anti-TNFs in the fistulous tract have been described with acceptable results with infliximab and adalimumab,30 our study found no cases with this treatment. Additionally, the efficacy of mesenchymal stem cell injection therapy, almost always extracted from adipose tissue, has proven to be effective in healing perianal fistulas. A recent systematic review and meta-analysis with 11 studies showed that mesenchymal stem cell therapy was associated with a higher rate of healing of perianal fistulas compared with short-term control measures (6–24 weeks) (OR: 3.06; 95% CI: 1.05–8.90; p = 0.04) and in the long term (24–52 weeks) (OR: 2.37; 95% CI: 0.90–6.25; p = 0.08), with no increase in adverse events or in serious adverse events.31 Currently, we do not have this treatment in Colombia for patients with refractory perianal fistulising CD
A temporary stoma may be an option in refractory perianal CD. In our study, a colostomy was required in 9.2% of the patients and an ileostomy in 13.8%. A systematic review with 16 studies found an early response rate of 63.8% (95% CI: 54.1–72.5). However, the restoration success rate is very low, with 16.6% (95% CI: 11.8–22.9) and 41.6% (95% CI: 32.6–51.2) requiring proctectomy and definitive ostomy, respectively.32
This is the first multicentre Latin American study to describe the epidemiology, clinical behaviour and outcome of patients with perianal fistulising CD. Within the limitations of our study, we consider that as it is a retrospective study there may be a selection bias in the data collection. Furthermore, the participating centres are tertiary hospitals, and the patients they treat tend to be more complex cases.
ConclusionsPerianal fistulising CD is a CD phenotype with a poor prognosis in our setting, with complex fistulas being more common, similar to that reported in the universal literature, and only a quarter of cases are treated with multidisciplinary management including both a gastroenterologist and a coloproctologist. Regarding treatment, there is a high percentage of steroid use, even although the international recommendations state that they are of little use in the management of these patients; furthermore, complete symptomatic remission is only achieved in 30% of the cases. In the future, in addition to clinical remission as a therapeutic objective, radiological remission should also be taken into account. To this end, our patients should be monitored with MRI of the pelvis or rectal endosonography. Measuring anti-TNF levels in order to optimise treatment, applying anti-TNFs locally and mesenchymal stem cell therapy can all play a very important role in improving the future management of our patients with this difficult disease, which greatly compromises their quality of life and always requires multidisciplinary management.
FundingNo funding was received from any entity to conduct this study.
AuthorsF. Juliao-Baños: study design, patient recruitment and drafting of the manuscript.
L. Osorio: data collection.
J. Carvajal, G. Mosquera Klinger, Anwar Medellín, Jorge Padrón, Belén de Molano, Fabián Puentes, Gustavo Reyes and Fabio Gil: patient recruitment.
Viviana Parra Izquierdo: patient recruitment and correction of the draft manuscript.
Héctor Sánchez: statistical analysis.
Conflicts of interestNone of the study authors reported any conflict of interest.